Summary
Background
Red blood cell (RBC) transfusions in preterm neonates are associated with retinopathy of prematurity (ROP).
Methods
BORN is a multicenter randomized trial investigating whether RBC transfusions from cord blood (CB-RBCs) instead of adult donors (A-RBCs) reduce ROP severity (NCT05100212). The study was conducted between December 2021 and November 2024 in 8 hospitals sited in 8 different Italian regions. Extremely low gestational age neonates (ELGANs) were randomized 1:1 to receive A-RBCs (control) or CB-RBCs (intervention) from birth to postmenstrual age (PMA) of 29 + 6 weeks. The main outcome was severe-ROP rate at 40 weeks PMA or discharge.
Findings
By intention-to-treat-analysis, 56 patients per arm were evaluated: 16 in the control arm and 14 in the intervention arm developed severe ROP (28·6% versus 25·0%, risk difference [RD] −0·03 [95% CI −0·21% to 0·14%]; P = 0·831). Twenty-four (42·8%) patients in the intervention arm received also A-RBCs. Per-protocol-analysis included those patients receiving exclusively the assigned treatment, consisting of 38 ELGANs in the control arm and 17 in the intervention arm, with comparable characteristics. Thirteen ELGANs in the control arm developed severe ROP, and 10 required ROP treatment whilst no patients in the intervention arm developed severe ROP or treatment-requiring ROP (34·2% versus 0%, RD −0·34 [95% CI −0·51% to −0·07%] for severe ROP, P = 0·005, and 26·3% versus 0%, RD −0·28 [95% CI −0·46% to −0·02%] for treatment-requiring ROP, P = 0·022). Twenty-four patients in the control arm and 5 in the intervention arm developed moderate/severe BPD (63·2% versus 29·4%, RD −0·33 [95% CI −0·56% to −0·02%], P = 0·039).
Interpretation
CB-RBCs may protect ELGANs from severe forms of ROP and BPD.
Funding
Funded by Fresenius HemoCare Italia SRL (Prot. N 0038762/21-04/11/2021; grant number 5800134-FPG).
Keywords: Extremely low gestational age neonates, Retinopathy of prematurity, Transfusions, Fetal hemoglobin, Umbilical blood, Randomized controlled trial
Research in context.
Evidence before this study
Progressive HbF replacement by HbA has been connected with a higher incidence of retinopathy of prematurity (ROP) and bronchopulmonary dysplasia (BPD), two lifelong diseases profoundly affecting the quality of life of surviving neonates. Recent studies in preterm neonates have shown that RBC transfusions obtained from allogeneic cord blood (CB-RBCs) correct anemia without affecting the physiological HbF levels.
Added value of this study
The BORN trial suggests that the CB-RBC transfusion in extremely premature neonates has a favorable clinical impact, reducing the severity of ROP and BPD and supporting the protective role of HbF in the early postnatal life of these patients.
Implications of all the available evidence
The HbF-containing RBC transfusion strategy could revolutionize the current neonatal transfusion approach. Larger randomized trials are required, paralleled by a change in the paradigm of CB collection, which is currently centered only on the hematopoietic stem cell transplant use.
Introduction
The transition of hemoglobin (Hb) synthesis from the fetal (HbF) to the adult (HbA) form is a natural, regulated process, involving a complex interaction between transcription repressors and activators.1 It begins in the last weeks of gestation and continues for several weeks after birth, with low levels of HbF synthesis persisting in postnatal life. Since preterm birth does not affect the transition from HbF to HbA, prematurely born neonates spend their initial postnatal life with HbF comprising nearly all their hemoglobin.2
The anemia of prematurity is a more severe form of the anemia typically observed in full-term neonates. It is significantly exacerbated by the intrinsic frailty of premature birth, with a clinical course punctuated by infection, inflammation, hemorrhage, and the clinical need for frequent blood sampling.3 Most extremely preterm neonates require repeated red blood cell (RBC) transfusions during the first weeks of life, which, however, increase the risks of death and other serious complications.4,5 Recently, attention has focused on the progressive replacement of HbF by HbA due to RBC transfusions. For example, there is a strong correlation between early decreases in HbF levels and retinopathy of prematurity (ROP), a lifelong visual impairment that markedly affects the quality of life of surviving patients.6, 7, 8 This compelling evidence, combined with the persistence of HbF synthesis in premature neonates, strongly suggests that HbF plays a crucial role in safeguarding these vulnerable patients during their critical, early postnatal phase of life. In a previous proof-of-concept study, transfusing anemic preterm neonates with RBC concentrates derived from allogeneic cord blood (i.e., CB-RBCs) increased the Hb concentration while preserving the HbF level.9 The subsequent BORN trial investigated whether transfusing CB-RBCs, instead of standard RBCs from adult donors (A-RBCs), would reduce the incidence of severe ROP in extremely low gestational age neonates (ELGANs; i.e., neonates born at less than 28 weeks of gestation).10
Methods
Study design
BORN is a prospective, randomized, multi-center, double-blinded, controlled trial performed in eight Italian Neonatal Intensive Care Units (NICUs) (Supplementary File, List of participating centers). The first patient was enrolled in December 2021 and the last in November 2024. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol was subjected to the following amendments: 1) changes in investigator names on consent forms, 2) inclusion of additional participating centers, 3) enrollment of “out-born” neonates provided they were not previously transfused, 4) prolongation of the study's duration (from 18 to 36 months), and 5) modifying the timing of the intervention from 31 + 6 to 29 + 6 postmenstrual age (PMA). The last amendment was prompted by the finding emerged at interim analysis that a high number of neonates in the intervention arm had received A-RBCs due to the CB-RBC unavailability. In the attempt to increase CB unit accessibility to more premature patients and reduce the number of protocol deviations, the study group decided to shorten the intervention period.11
Participants
The BORN trial enrolled preterm neonates delivered at a gestational age (GA) between 24 + 0 and 27 + 6 weeks. Exclusion criteria included maternal-fetal immunization, hydrops fetalis, major congenital malformations associated, or not, with genetic syndromes, previous transfusions, hemorrhage at birth, congenital infections, and the health care team deeming it inappropriate to approach the infant's family for informed consent.
Ethics
The study was approved by the Ethics Committee of Fondazione Policlinico A. Gemelli IRCCS (ID 4364, Prot. N. 003590/21) and of all participating centers, and was registered at https://clinicaltrials.gov (NCT05100212). All parents of enrolled patients gave their signed informed consent before neonate inclusion in the study. The study was conducted in accordance with the Declaration of Helsinki.
Randomization and masking
Patients were randomized 1:1 to receive either standard A-RBCs (Arm A, control) or allogenic CB-RBCs (Arm B, intervention). Twins were assigned to the same arm. The allocation sequence was generated using stratifying for both center and GA (< or ≥26 weeks), and permuted blocks with random block sizes and block order, using the NCSS 2020 Statistical Software 2020 (NCSS, LLC. Kaysville, UT, USA, ncss.com/software/ncss). The allocation table was not disclosed to ensure concealment, and randomization was provided through the Research Electronic Data Capture (REDCap) (RRID: SCR_003445) electronic data capture tool hosted at Fondazione Policlinico Universitario A. Gemelli, IRCCS (https://redcap-irccs.policlinicogemelli.it/). Neonatologists enrolled patients at admission to the NICU. When a new patient was enrolled, neonatologists recorded patient data in the electronic case reporting form (eCRF) of REDCap, which simultaneously sent an email to the medical team of the corresponding blood bank. The blood bank team randomized the patient as soon as possible. The arm allocation was not visible in the CRFs, and treating neonatologists were not aware the arm to which neonates were assigned. To maintaining blinding, A-RBCs and CB-RBCs were distributed in the same type of bags (i.e., CompoFlex 4F RCC storage pediatric bags).
Procedures
At the time of RBC transfusion request, if any, patients allocated to the control arm received standard A-RBC units and patients allocated to the intervention arm received CB-RBC units. Both A-RBC and CB-RBC units were matched for ABO/RhD blood group antigens. If compatible CB-RBC units were not available, A-RBC units were administered. RBCs were transfused according to the pertinent arm allocation until 29 + 6 weeks PMA. CB-RBCs were obtained from public cord blood banks belonging to the Italian Cord Blood Bank Network. CB-RBCs were prepared in all centers according to a prespecified protocol. Briefly, CB units were processed within 48 h of collection, filtered to remove leukocytes (BioR Flex filters, Fresenius Kabi), fractionated into RBC concentrates using a Compomat G5 cell separator (Fresenius Kabi), and suspended in SAG-M (Saline, Adenine, Glucose, Mannitol) additive solution in a 2:1 ratio. Quality standards for CB-RBCs complied with those required by European regulations for RBC concentrates, and all units were γ-irradiated immediately prior to transfusion. Transfusion therapy was managed according to Italian transfusion guidelines and erythropoietin was administered as per each center's procedure.12
Outcomes
The primary outcome was the rate of severe ROP (defined as ROP stage 3 or higher in zone I or II and/or the presence of plus disease) at discharge or at 40 weeks of PMA, whichever occurred first. Secondary outcomes were: rate of ROP requiring treatment (defined as ROP stage III in zone I or any ROPstage with plus disease; treatment consisted of laser therapy, anti-vascular endothelial growth factor [VEGF] administration, vitrectomy) at discharge or at e40 weeks of PMA, whichever occurred first; rate of bronchopulmonary dysplasia (BPD); rate of a composite outcome including any of the following: death, severe ROP, BPD, necrotizing enterocolitis (NEC); HbF threshold predicting severe ROP and BPD; interval between two consecutive transfusions; and the hematocrit (Hct) increase after CB-RBC or A-RBC transfusions.
Diagnosis and staging of ROP were determined according to the International Classification, based on an ordinal scale with higher numbers indicating a more severe outcome: 0, 1, 2, 3, 3+, 4, and 5.13 ROP was assessed weekly from the PMA of 31 weeks. ROP examination was performed by indirect ophthalmoscopy, and RetCam imaging was used to confirm the diagnosis and monitor disease status and treatment. NEC was diagnosed and staged according to the modified Bell's criteria.14,15 BPD was diagnosed as the need for oxygen use during the first 28 days after birth; mild, moderate, and severe BPD were defined using the National Institute of Child Health and Human Development criteria for preterm infants born before 32 weeks of gestation.16,17 Intraventricular hemorrhage (IVH) was assessed weekly by ultrasound scan and graded between 0 and 4 according to Papile/Bowerman criteria.18,19
Data management
Data were collected in REDCap. Baseline patient data included: demographics, placental abnormalities, obstetric pathologies, gestational age, birth weight, antenatal prophylaxis for hyaline membrane disease, clinical chorioamnionitis, post-natal steroid administration, hematological parameters (i.e., Hb concentration, Hct, and HbF assessed spectrophotometrically using point-of-care blood gas analyzers, and expressed as the percentage of total Hb), Apgar index at 1 and 5 min, Clinical Risk Index for Babies-II (CRIB-II) score, and mortality probability. During hospitalization, Hb, Hct, and HbF were assessed twice per week. The following clinical variables were recorded: hemodynamically-significant patent ductus arteriosus (hsPDA), maximal stage of ROP, ROP treatment, NEC, BPD, IVH, erythropoietin, antibiotics, steroids and caffein administration, microbiologically-documented infections, duration of oxygen therapy, ventilation support (i.e., invasive ventilation: high frequency oscillatory ventilation and/or synchronized intermittent mandatory ventilation; non-invasive ventilation: continuous positive airway pressure and/or high flow nasal cannula), surgery, and death. At each RBC transfusion, the following data were recorded: unit identifier number, date of transfusion, patient Hct before and after transfusion, post-transfusion pH, serum lactate, and serum potassium (all tested within 24 h of transfusion).
Safety monitoring
All clinical complications observed during each patient's clinical course were considered as adverse events: the severity was reported according to the Common Terminology Criteria for Adverse Events v4·0, ranging from 1 (no symptoms) to 5 (death). Imputability to previous transfusions was scored from 0 to 3 according to the European regulations on transfusion surveillance, where 0 indicates excluded/unlikely, 1 possible, 2 likely/probable, and 3 certain. Due to the innovative nature of this intervention, a prespecified interim analysis was conducted, after randomizing the first 58 patients, to confirm the safety of CB-RBC transfusion.12 The data safety monitoring board of Fondazione Policlinico A. Gemelli IRCCS could recommend early termination of the trial, if safety concerns existed.
Sample size
The sample size was calculated considering a significant reduction of the severe ROP incidence after the treatment (primary endpoint) and, therefore, the alternative hypothesis: Incidence (Severe ROP | A-RBC) > Incidence (Severe ROP | CB-RBC). In the study design, we accounted for three incidence values, according to the different gestational age strata of 38% (24 weeks), 20% (24–25 weeks), and 5% (26–27 weeks). In the treated branch, we expected a reduction in the proportion of cases to 10%, 5%, and 1% for the 24-, 25-, and 26–27-week strata, respectively. Considering these three strata, the expected reduction in the treated sample per stratum, multi-centricity, and a mortality rate of 15% during the study period, we calculated a total sample size of 146 subjects (73 per arm) with a significance level of 0·05 and a power of 0·8.
Statistics
Demographic characteristics, clinical features, and relevant baseline variables were expressed as median and interquartile range (IQR), or proportions. To investigate differences between groups, we used the Pearson's Chi-squared test or Fisher's exact test for categorical variables, and the Mann–Whitney's U test for continuous variables. Outcome were expressed as risk difference (RD) between groups, with the relative 95% confidence interval (CI). The impact of transfusion burden on the primary outcome was estimated by logistic regression analysis, using the primary outcome as a dependent variable, and adjusting for covariates selected for their clinical relevance (according to literature data) and/or significance in univariable analyses. The association was expressed as adjusted odds ratios (OR) with the relative 95% CI. The number need to treat (NNT) was calculated as the inverse of the absolute risk reduction, calculated as the difference between event rates in control and intervention arms. To explore the impact of blood transfusions on HbF levels, we calculated the area under the curve (AUC) of HbF and PMA. For each patient, we calculated the AUC at 30 weeks of PMA and then normalized this value by the days of life (normalized AUC30, i.e., AUC at 30 weeks of PMA divided by the number of days between birth and PMA of 30 weeks). Unless otherwise specified, missing data were below 5% and no imputation was applied, except for HbF values. In this case, post-transfusion missing data were calculated based on the preceding HbF determination using previously reported algorithms (Supplementary Methods).9 To account for major protocol deviations (e.g., patients in the CB-RBC arm who received A-RBC transfusions due to the unavailability of CB-RBC units), beyond the “intention-to-treat” analysis (ITT, i.e., all enrolled patients, independently if they were or not transfused) two additional sets of analysis excluding protocol deviations were planned (per protocol [PP] analysis, and as treated [AT] analysis). Two-sided tests were applied and the statistical level of significance was set at 0·05. NCSS10 Statistical Software 2015 (NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/ncss) and GraphPad Prism version 10·0·0 (GraphPad Software, Boston, Massachusetts USA, www.graphpad.com) were used for statistical analyses and illustration preparation.
Role of funding source
The study was funded by Fresenius HemoCare Italia SRL (Prot. N 0038762/21-04/11/2021; grant number 5800134-FPG). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
One hundred forty-eight ELGANs were assessed for eligibility and 145 of those who met the inclusion criteria were randomized. Three cases were subsequently excluded because of inadequate data. Thus, in the end, 142 patients, 73 in the control arm and 69 in the intervention arm, were included in the analysis (Fig. 1). Table 1 illustrates the baseline characteristics of 142 participants. Arms were well balanced for relevant clinical antenatal and neonatal characteristics except for maternal diabetes, placenta previa, absent-end-diastolic (AED) flow, and intra-uterine-growth-restriction (IUGR): neither AED flow nor IUGR was associated with severe ROP at univariable analysis. Thirty patients died, 17 in the control arm and 13 in the intervention arm (23·2% versus 18·8%, RD −0·04 [95% CI −0·18% to 0·10%]; P = 0·516). Among 112 patients reaching the primary endpoint, 69 (61·6%) developed ROP (any stage), 30 (26·8%) developed severe ROP, and 25 (22·3%) needed some treatment for ROP. The latter consisted of intravitreal anti-VEGF (9), laser therapy (4), vitrectomy (5), anti-VEGF and laser therapy (6), and anti-VEGF and vitrectomy (1). The median age at ROP diagnosis was 35·5 weeks (IQR 34·4–37·0); all but two patients had severe ROP preceded by ROP stage 1 or 2.
Fig. 1.
CONSORT diagram of the study. The diagram illustrates the number of patients included in the different sets of analysis planned in the study protocol: the intention-to-treat set, the per-protocol set, and the as-treated set. DAT indicates direct antiglobulin test.
Table 1.
Clinical characteristics at birth and relevant antenatal features of the study population and standardized mean differences (SMD) between arms.
| All patients N = 142 | Control arm N = 73 | Intervention arm N = 69 | SMD | |
|---|---|---|---|---|
| Gestational age, weeks | 26·1 (25·0–27·3) | 26·0 (25·0–27·0) | 26·4 (25·0–27·3) | 0·079 |
| Weight, g | 750 (650–911) | 720 (597–870) | 750 (650–911) | 0·315 |
| Male/female, n (%) | 76 (53·5)/66 (46·5) | 43 (58·9)/30 (41·1) | 33 (47·8)/36 (52·2) | 0·223 |
| IUGR, n (%) | 21 (14·8) | 16 (21·9) | 5 (7·2) | 0·425 |
| Twins, n (%) | 28 (19·7) | 14 (19·2) | 14 (20·3) | 0·028 |
| Apgar score 1 min | 5 (4–7) | 5 (4–6) | 6 (3–7) | 0·028 |
| Apgar score 5 min | 8 (7–8) | 8 (7–8) | 8 (7–8) | 0·061 |
| CRIB II score | 11 (10–13) | 12 (10–13) | 11 (9–13) | 0·232 |
| Probability of mortality (%) | 17·8 (12·1–34·8) | 25·4 (12·1–34·8) | 17·8 (8·1–34·8) | 0·218 |
| Hb at birth, g/dL | 15·1 (13·8–16·5) | 15·5 (13·8–16·5) | 15·0 (13·8–16·6) | 0·118 |
| Central Hct at birth (%) | 46·0 (41·0–50·7) | 45·5 (41·7–51·0) | 46·0 (41·0–50·0) | 0·288 |
| Peripheral Hct at birth (%) | 48·0 (42·0–54·0) | 48·0 (44·0–56·0) | 47·0 (41·5–53·5) | 0·158 |
| Obstetric pathology | ||||
| Preeclampsia | 17 (12·0) | 12 (16·4) | 5 (7·2) | 0·293 |
| Maternal diabetes | 12 (8·5) | 5 (6·8) | 7 (10·1) | 0·091 |
| PROM | 67 (47·2) | 30 (41·1) | 37 (53·6) | 0·253 |
| Placenta previa | 8 (5·8) | 2 (2·6) | 6 (8·7) | 0·250 |
| Placental abruption | 20 (14·3) | 7 (9·6) | 13 (18·8) | 0·258 |
| ARED flow | 12 (8·5) | 6 (8·2) | 6 (8·7) | 0·017 |
| AED flow | 19 (13·4) | 14 (19·2) | 5 (7·2) | 0·513 |
| Suspected chorioamnionitis | 43 (30·3) | 22 (30·1) | 21 (30·4) | 0·006 |
| Documented chorioamnionitis | 13 (9·2) | 6 (8·2) | 7 (10·1) | 0·066 |
| Antenatal therapy | ||||
| Steroid prophylaxis | 128 (90·1) | 67 (91·8) | 61 (88·4) | 0·161 |
| Complete steroid prophylaxis | 89 (62·7) | 43 (58·9) | 46 (66·6) | 0·063 |
| Antibiotics | 84 (59·2) | 44 (60·2) | 40 (57·9) | 0·047 |
| Complete antibiotic prophylaxisa | 40 (28·2) | 20 (20·4) | 20 (28·9) | 0·035 |
| Antibiotic therapy | 15 (10·6) | 7 (9·6) | 8 (11·6) | 0·065 |
| Magnesium sulfate | 85 (59·9) | 44 (60·2) | 41 (59·4) | 0·072 |
| Time to randomization, days | 1 (0–3) | 1 (0–3) | 2 (1–5) | 0·131 |
| Death, n (%) | 30 (21·1) | 17 (23·3) | 13 (18·8) | 0·109 |
Data are given as N (%) or median (IQR).
IUGR: intra uterine growth restriction; CRIB: Clinical Risk Index for Babies; Hb: hemoglobin; Hct: hematocrit; ARED: absent or reversed end diastolic; AED: absent end diastolic.
Among the 84 mothers receiving antibiotics.
Outcomes
One hundred-twelve patients, 56 per arm, were evaluated for the primary outcome. Table 2 displays the most relevant features. The two arms were comparable except for the rate of AED flow (i.e., 11 [19·6%] patients in the control arm versus 3 [5·4%] in the intervention arm; P = 0·042; data not shown) and IUGR (i.e., 10 [17·9%] patients in the control arm versus 2 [3·6%] in the intervention arm; P = 0·028). Sixteen patients in the control arm and 14 in the intervention arm developed severe ROP (28·6% versus 25·0%, RD −0·03 [95% CI −0·21% to 0·14%]; P = 0·831). ROP treatment was required in 13 patients in the control arm and 12 in the intervention arm (22·3% versus 21·4%, RD −0·01 [95% CI −0·19% to 0·15%]; P = 0·825). BPD (any stage) occurred in 35 patients in the control arm and 40 in the intervention arm (62·5% versus 71·4%, RD 0·08 [95% CI −0·10% to 0·28%]; P = 0·421); 26 and 24 of them, respectively, had moderate or severe BPD (46·4% versus 42·9%, RD −0·03 [95% CI −0·23% to 0·16%]; P = 0·849). Finally, the composite outcome of death, severe ROP, BPD, and NEC, occurred in 56 of 73 patients in the control arm and 57 of 69 patients in the intervention arm (i.e., 76·7% versus 82·6%, RD 0·05 [95% CI −0·08% to 0·20%]; P = 0·383). The rates of sepsis, PDA, IVH, pulmonary hypertension, or NEC were comparable.
Table 2.
Baseline characteristics of patients and clinical outcome comparison by “intention-to-treat” analysis.
| Control arm N = 56 | Intervention arm N = 56 | P | SMD | |
|---|---|---|---|---|
| Gestational age, weeks | 26·1 (25·1–27·0) | 26·4 (25·0–27·4) | 0·779 | 0·041 |
| Weight, g | 755 (663–923) | 808 (700–958) | 0·147 | 0·299 |
| Male/female, % | 34 (60·7)/22 (39·3) | 25 (44·6)/31 (55·4) | 0·129 | 0·326 |
| Twins | 12 (21·4) | 12 (21·4) | 0·999 | <0·001 |
| IUGR | 10 (17·9) | 2 (3·6) | 0·028 | 0·475 |
| Apgar score 1 min | 5 (4–7) | 6 (4–7) | 0·896 | 0·019 |
| Apgar score 5 min | 8 (7–9) | 8 (7–8) | 0·898 | 0·013 |
| CRIB II score | 11 (10·0–13·0) | 11 (9·0–12·3) | 0·226 | 0·242 |
| Hb at birth, g/dL | 15·6 (13·9–16·7) | 15·0 (13·8–16·6) | 0·513 | 0·120 |
| Transfused patients | 45 (80·4) | 49 (87·5) | 0·440 | 0·195 |
| Age at first transfusion, PMA week | 27·6 (26·2–29·0) | 27·8 (26·2–29·7) | 0·699 | 0·001 |
| RBC transfusions (<40 weeks PMA) | 3·0 (1·0–5·0) | 2 (1–6) | 0·929 | 0·067 |
| A-RBC units | 3·0 (1·0–5·0) | 1·0 (0·0–2·0) | 0·001 | 0·615 |
| CB-RBC units | 0·0 (0·0–0·0) | 1·0 (0·0–2·75) | <0·001 | 1·198 |
| RBC transfusions (<30 weeks PMA) | 1·5 (0·0–3·8) | 1·0 (0·3·8) | 0·812 | 0·083 |
| A-RBC units | 1·5 (0·0–3·8) | 0·0 (0·0–1·0) | 0·001 | 0·531 |
| CB-RBC units | 0·0 (0·0–0·0) | 1·00 (0·0–2·0) | <0·001 | 1·003 |
| Patients with PLT transfusions (%) | 7 (12·5) | 12 (21·4) | 0·314 | 0·239 |
| ROP (any stage) | 34 (60·7) | 35 (62·5) | 0·999 | 0·999 |
| Stage 1 | 6 (10·7) | 6 (10·7) | 0·996 | <0·001 |
| Stage 2 | 15 (26·8) | 16 (28·6) | 0·039 | |
| Stage 3 | 13 (23·2) | 13 (23·2) | <0·001 | |
| Plus disease | 11 (19·6) | 10 (17·9) | 0·999 | 0·046 |
| Severe ROP | 16 (28·6) | 14 (25·0) | 0·831 | 0·081 |
| Treated ROP | 13 (23·2) | 12 (21·4) | 0·999 | 0·043 |
| Hyaline membrane disease | 39 (69·4) | 44 (78·6) | 0·388 | 0·203 |
| Sepsis (suspected) | 37 (66·1) | 38 (67·9) | 0·999 | 0·038 |
| Sepsis (documented) | 34 (60·7) | 24 (42·9) | 0·088 | 0·363 |
| IVH (any stages) | 19 (33·9) | 16 (28·5) | 0·837 | 0·078 |
| IVH Stage 1/2 | 13 (23·2) | 9 (16·1) | 0·798 | 0·180 |
| IVH Stage 3/4 | 6 (10·7) | 7 (12·5) | 0·056 | |
| BPD (any stage) | 35 (62·5) | 40 (71·4) | 0·421 | 0·191 |
| Moderate or severe BPD | 26 (46·4) | 24 (42·9) | 0·849 | 0·072 |
| PDA | 24 (42·9) | 30 (53·6) | 0·344 | 0·216 |
| Pulmonary hypertension | 7 (12·5) | 9 (16·1) | 0·787 | 0·102 |
| NEC | 5 (8·9) | 5 (8·9) | 0·999 | <0·001 |
| Antibiotic therapy (days) | 20·5 (14·0–35·3) | 20·0 (12·0–34·0) | 0·709 | 0·036 |
| Antifungal therapy (days) | 14·0 (9·5–24·0) | 11·0 (7·0–34·3) | 0·458 | 0·078 |
| Inotropic drugs (%) | 16 (28·6) | 21 (37·5) | 0·421 | 0·191 |
| Inotropic drugs (days) | 4 (1·5–8·0) | 4·0 (2·0–9·0) | 0·492 | 0·363 |
| Invasive ventilation (%) | 33 (58·9) | 28 (50·0) | 0·224 | 0·180 |
| Invasive ventilation (days) | 4 (0–14) | 5 (0–21) | 0·787 | 0·089 |
| Oxygen therapy (days) | 29 (11–67) | 37 (8–73) | 0·900 | 0·030 |
| Steroid therapy (%) | 28 (50·0) | 33 (58·9) | 0·448 | 0·180 |
| Erythropoietin (%) | 25 (44·6) | 21 (37·5) | 0·564 | 0·146 |
| Surfactant (%) | 41 (73·2) | 41 (73·2) | 0·999 | <0·001 |
| Caffeine (%) | 51 (91·1) | 53 (94·6) | 0·716 | 0·139 |
| Caffeine (days) | 70 (56–84) | 73 (58–85) | 0·621 | 0·152 |
SMD indicates standardized mean differences between arms.
Data are given as N (%) or median (IQR).
Invasive ventilation included high-frequency oscillatory ventilation and synchronized intermittent mandatory ventilation.
IUGR: intra-uterine growth retardation; CRIB: Clinical Risk Index for Babies; Hb: hemoglobin; RBC: Red blood cell; PLT: platelet; PMA: postmenstrual age; ROP: retinopathy of prematurity; BPD: bronchopulmonary dysplasia; IVH: intraventricular hemorrhage; PDA: patent ductus arteriosus; NEC: necrotizing enterocolitis.
As shown in Table 2, transfusions administered to patients in the intervention arm included also A-RBCs. In particular, 24 (42·8%) of 56 patients in the intervention arm received A-RBCs before 30 weeks of age: 10 received exclusively A-RBC units (1·0, IQR 1·0–2·5) and 14 received both RBC types, with more A-RBC units (2·0, IQR 1·0–3·0) than CB-RBC units (1·0, IQR 0–2·0). The high number of protocol deviations significantly affected the informative potential of the ITT analysis. We then compared control and intervention arms including exclusively patients who, before 30 weeks PMA, had received the assigned treatment (PP set). This yielded 38 patients in the control arm and 17 in the intervention arm (Table 3). The arms were well balanced for antenatal characteristics and parameters at birth, except for more IUGR neonates in the control group and higher birth weight in the intervention arm (i.e., 775 g [IQR 675–948] as compared to 705 g [IQR 600–780] in the control arm, P = 0·023). Transfusion needs before 30 weeks of age and during the entire study period were similar. Thirteen patients (34·2%) in the control arm developed severe ROP and 10 (26·3%) required ROP treatment. In contrast, no cases of severe ROP or treatment-requiring ROP, were observed in the intervention arm, with a RD between arms of −0·34 (95% CI −0·51% to −0·07%) for severe ROP (P = 0·005), and −0·28 (95% CI −0·46% to −0·02%) for treatment-requiring ROP (P = 0·022). Whereas any stage BPD rate was similar in both arms, a lower proportion of patients in the intervention arm developed moderate or severe BPD (i.e., 24 [63·2%] and 5 [29·4%] in control and intervention arms, respectively, RD −0·33 [95% CI −0·56% to −0·02%], P = 0·039). The two arms were comparable for rates of PDA, IVH, NEC, sepsis, oxygen therapy, and invasive ventilation (Table 3). No difference was observed in the composite outcome including death, severe ROP, NEC, and BPD (i.e., 47 [88·7%] of 53 patients in the control arm and 17 [77·3%] of 22 patients in the intervention arm; RD −0·11 [95% CI −0·34 to 0·11], P = 0·282). Similar findings were observed including in the PP analysis the subgroup of 13 neonates who received CB-RBCs until 32 weeks of age.
Table 3.
Baseline characteristics of patients and clinical outcomes comparison by “per protocol” analysis.
| Control arm N = 38 | Intervention arm N = 17 | P | SMD | |
|---|---|---|---|---|
| Gestational age, weeks | 25·9 (24·8–26·9) | 26·1 (24·7–27·0) | 0·883 | 0·012 |
| Weight, g | 705 (600–783) | 775 (675–947) | 0·023 | 0·782 |
| Male/female, % | 23 (60·5)/15 (39·5) | 8 (47·1)/9 (52·9) | 0·384 | 0·273 |
| Twins | 9 (23·7) | 2 (11·8) | 0·473 | 0·316 |
| IUGR | 10 (6·3) | 0 | 0·023 | 0·845 |
| Apgar score 1 min | 5 (4–7) | 6 (4–7) | 0·984 | 0·013 |
| Apgar score 5 min | 8 (7–8) | 8 (7–8) | 0·985 | 0·100 |
| CRIB II score | 12·0 (10·0–13·5) | 11·0 (10·0–13·0) | 0·395 | 0·211 |
| Hb at birth, g/dL | 14·7 (13·7–16·4) | 15·0 (23·8–16·6) | 0·628 | 0·123 |
| Age at first transfusion, PMA week | 27·4 (26·0–28·5) | 27·7 (26·0–29·1) | 0·449 | 0·157 |
| Total RBC units (40 weeks PMA) | 4·0 (2·7–6·0) | 3·0 (2·0–5·5) | 0·357 | 0·207 |
| A-RBC units | 4·0 (2·7–6·0) | 1·0 (0·0–1·5) | <0·001 | 2·112 |
| CB-RBC units | 0·0 (0·0–0·0) | 2·0 (1·0–4·5) | <0·001 | 2·000 |
| Total RBC units (<30 weeks PMA) | 2·0 (1·0–4·0) | 2·0 (1·0–3·5) | 0·251 | 0·224 |
| A-RBC units | 2·0 (1·0–4·0) | 0·0 (0·0–0·0) | <0·001 | 2·186 |
| CB-RBC units | 0·0 (0·0–0·0) | 2·0 (1·0–3·5) | <0·001 | 1·704 |
| Patients with PLT transfusions (%) | 6 (15·7) | 4 (23·5) | 0·479 | 0·200 |
| Hyaline membrane disease | 26 (68·4) | 16 (94·1) | 0·045 | 0·605 |
| ROP (any stage) | 27 (71·1) | 12 (70·6) | 0·999 | 0·010 |
| Stage 1 | 4 (10·5) | 3 (17·6) | 0·663 | 0·213 |
| Stage 2 | 12 (31·6) | 9 (52·9) | 0·148 | 0·439 |
| Stage 3 | 11 (28·9) | 0 (0·0) | 0·011 | 0·903 |
| Plus disease | 8 (21·1) | 0 (0·0) | 0·047 | 0·730 |
| Severe ROP | 13 (34·2) | 0 (0·0) | 0·005 | 1·020 |
| Treated ROP | 10 (26·3) | 0 (0·0) | 0·022 | 0·845 |
| Sepsis (suspected) | 30 (78·9) | 14 (82·4) | 0·999 | 0·086 |
| Sepsis (documented) | 30 (78·9) | 9 (52·9) | 0·061 | 0·571 |
| IVH (any stages) | 18 (46·2) | 8 (47·1) | 0·810 | 0·100 |
| IVH Stage 1/2 | 11 (28·9) | 4 (23·5) | 0·910 | 0·122 |
| IVH Stage 3/4 | 6 (15·4) | 4 (23·5) | 0·196 | |
| BPD (any stage) | 29 (76·3) | 12 (70·6) | 0·741 | 0·130 |
| Moderate or Severe BPD | 24 (63·2) | 5 (29·4) | 0·039 | 0·719 |
| PDA | 19 (20·0) | 10 (58·8) | 0·568 | 0·178 |
| Pulmonary hypertension | 7 (18·4) | 0 (0·0) | 0·086 | 0·552 |
| NEC | 4 (10·5) | 2 (11·8) | 0·949 | 0·039 |
| Antibiotic therapy (days) | 26 (16–46) | 23 (17–32) | 0·422 | 0·354 |
| Antifungal therapy (days) | 16 (11–24) | 11 (9–41) | 0·868 | 0·304 |
| Inotropic drugs (%) | 11 (28·9) | 7 (41·2) | 0·534 | 0·258 |
| Inotropic drugs (days) | 4 (1–7) | 2 (2–4) | 0·512 | 0·161 |
| Invasive ventilation (%) | 25 (65·8) | 12 (70·6) | 0·766 | 0·103 |
| Invasive ventilation (days) | 6 (0–21) | 4 (0–21) | 0·742 | 0·742 |
| Oxygen therapy (days) | 44 (20–75) | 32 (5–59) | 0·171 | 0·409 |
| Steroid therapy (%) | 24 (63·2) | 8 (57·1) | 0·375 | 0·328 |
| Erythropoietin (%) | 18 (47·4) | 4 (23·5) | 0·143 | 0·515 |
| Surfactant (%) | 30 (78·9) | 14 (82·4) | 0·999 | 0·086 |
| Caffeine (%) | 35 (92·1) | 15 (88·2) | 0·639 | 0·130 |
| Caffeine (days) | 76 (56–91) | 79 (67–96) | 0·574 | 0·215 |
SMD indicates standardized mean differences between arms.
Data are given as N (%) or median (IQR).
Invasive ventilation included high-frequency oscillatory ventilation and synchronized intermittent mandatory ventilation.
IUGR: intra-uterine growth retardation; CRIB: Clinical Risk Index for Babies; Hb: hemoglobin; RBC: Red blood cell; PLT: platelet; PMA: postmenstrual age; ROP: retinopathy of prematurity; BPD: bronchopulmonary dysplasia; IVH: intraventricular hemorrhage; PDA: patent ductus arteriosus; NEC: necrotizing enterocolitis.
The outcome analysis was then carried out in the AT set, including patients grouped according to the RBC type received before 30 weeks of age, independently of their arm assignment (Supplementary Table S1). In particular, 49 patients received only A-RBCs, 17 only CB-RBCs, and 15 both types of RBCs. Severe ROP occurred in 18 (36·7%) neonates receiving exclusively A-RBCs, none transfused exclusively with CB-RBCs (RD −0·36 [95% CI −0·50 to 0·14], P = 0·003), and 8 (53·3%) transfused with both RBC types (RD −0·16 [95% CI −0·12 to 0·45], P = 0·368 in comparison with neonates receiving only A-RBCs). Similar findings were observed for ROP requiring therapy. A-RBC and CB-RBC groups received similar numbers of RBC units before 30 weeks of age and until discharge, whereas the mixed group had higher transfusion needs at both endpoints. The diverging RBC type impact on the main outcome was also investigated by multivariable analysis (Supplementary Table S2). After adjusting for multiple confounders (GA at birth, birth weight, sex, IUGR, Apgar score 5' Hb at birth, documented sepsis, days on invasive ventilation, days on oxygen therapy), the A-RBC transfusions given before 30 weeks of age significantly predicted severe ROP (aOR 1·70 [95% CI 1·12–2·60]; P = 0·01) and treatment-requiring ROP (aOR 1·79 [95% CI 1·16–2·77]; P < 0·01). In contrast, no association between CB-RBCs and severe ROP or treatment-requiring ROP was found.
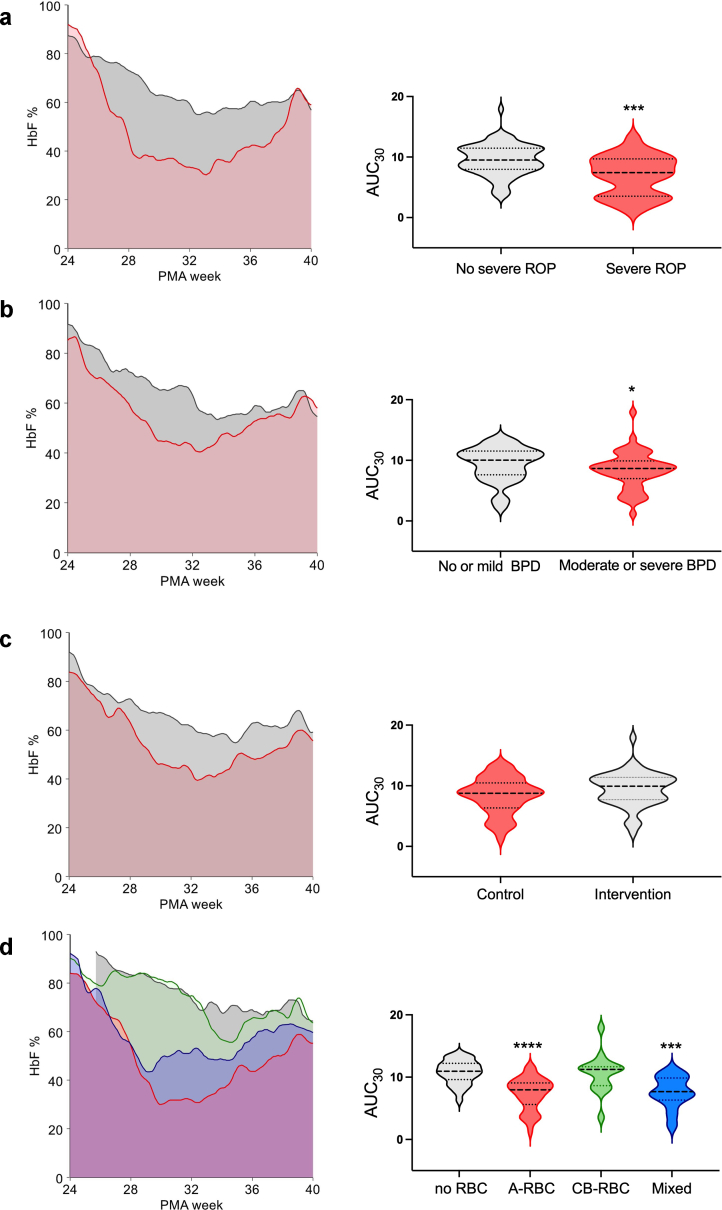
Finally, we calculated for each patient the HbF AUC from birth to 30 weeks of age and adjusted it for the number of postnatal days (Fig. 2). The adjusted HbF AUCs significantly differed by severe ROP (Fig. 2a), moderate/severe BPD (Fig. 2b), or types of transfusions received (Fig. 2d), whilst were comparable in control and intervention arms. An adjusted AUC <7·7% at 30 weeks of PMA predicted severe ROP with 57·6% sensitivity (95% CI 36·9%–76·5%) and 78·4% specificity (95% CI 67·8%–86·9%).
Fig. 2.
Area under the curve (AUC) of HbF at different postmenstrual ages (PMA) in BORN patients. The HbF AUC calculated from birth to 30 weeks PMA was adjusted for the number of postnatal days. Comparison between groups was made with Mann–Whitney's U test. a) HbF AUC of patients grouped according to the occurrence of severe ROP. Gray area refers to patients without severe ROP and red area to patients with severe ROP. ∗∗∗ denotes P < 0·001. b) HbF AUC in neonates developing or not moderate or severe BPD. Gray area indicates patients without moderate or severe BPD and red area those with moderate or severe BPD. ∗ indicates P = 0·037. c). HbF AUC of patients grouped according to arm allocation. Red area refers to control arm and gray area to the intervention arm. d) HbF AUC of patients grouped according to types of RBC products received before 30 weeks PMA. Gray area indicates non-transfused patients, red area neonates who received only A-RBCs, green area those who received only CB-RBCs, and blue area the neonates transfused with both A-RBCs and CB-RBCs. ∗∗∗ denotes P < 0·001 and ∗∗∗∗ denotes P < 0·0001 in comparison with non-transfused neonates.
Transfusion safety and efficacy
During the study period, 875 total adverse events were recorded: 792 occurred in 118 transfused neonates and 81 in 21 non-transfused neonates (6 [IQR 4–8] and 4 [IQR 2–5] per patient, in transfused and non-transfused neonates respectively; P < 0·001). No adverse event was reported as certainly related to transfusions, either of A-RBCs or CB-RBCs. Adverse events analysis by transfusion groups is reported in Supplementary Table S3. The adverse event rate was similar across groups except for urinary tract infections (less frequent in the A-RBC group) and pneumonia (more frequent in the mixed group). Regarding severity, 35 serious adverse events were recorded, without differences between groups. Comprehensively, the mixed group seemed to suffer from more severe forms of pneumonia (P = 0·001) and surgical complications (P = 0·049) than the A-RBC and CB-RBC groups. Finally, pulmonary hypertension was milder in the CB-RBC group (P = 0·003).
In total, 118 patients received 458 RBC units: 351 (76·6%) were A-RBCs and 107 (23·4%) were CB-RBCs. Lactate, pH, and potassium values recorded within 24 h post-transfusion are displayed in Supplementary Table S4 and were similar for A-RBCs and CB-RBCs. Finally, A-RBCs and CB-RBCs yielded similar post-transfusion Hct increments (i.e., 11·5% [IQR 8·0–15·6] in A-RBCs and 10·0% [IQR 8·0–13·6] in CB-RBCs, respectively, P = 0·129), along similar time intervals before the next transfusion (i.e., 7 days [IQR 3–11] for A-RBCs and 6·5 days [IQR 3–10] for CB-RBCs, respectively; P = 0·251).
Discussion
The known association between RBC transfusions and poorer outcomes in preterm neonates might result from functional differences between fetal and adult RBCs.20,21 In comparison to HbA, HbF has higher oxygen affinity, greater molecular stability, more efficient nitric oxide delivery, and does not interact with 2,3-diphosphoglycerate to modulate oxygen affinity.21 These characteristics suggest that preserving the physiological HbF reserve can be protective in the context of premature birth, which is typically characterized by an immature and less effective antioxidant response. The BORN study is the first randomized controlled trial investigating whether transfusing ELGANs with HbF-enriched CB-RBCs, instead of A-RBCs, is safe and effective. Indeed, the results of the BORN study show that CB-RBCs are at least as safe as standard A-RBC transfusions, thereby confirming our previous single-center experience and the results from the interim anlysis.9,11 During the BORN study, 107 CB-RBC transfusions were administered in eight geographically-disperse NICUs. No adverse events directly due to transfusions were reported; in particular, there no instances of hyperkalemia, unexpected acidosis, or increased lactate following CB-RBC transfusion. Hct increments and time intervals between transfusions after CB-RBC and A-RBC units were comparable. Moreover, the same protocol was used at each center to process CB units into RBC concentrates, offering a reliable foundation for further refinement.
The primary aim of the BORN study was to investigate whether CB-RBC transfusions prevent more severe forms of ROP in ELGANs. Although the ITT analysis showed no differences in severe ROP incidence between the control and intervention arms, the PP analysis demonstrated that the incidence of severe ROP and treatment-requiring ROP was significantly lower in the infants exclusively receiving CB-RBCs. The higher proportion of milder forms among ELGANs receiving CB-RBCs indicated that these may weaken ROP progression, with 2·9 and 3·8 patients needing to be treated to avoid one severe ROP or treatment-requiring ROP, respectively.
ROP initiates with the inhibition of vessel growth, followed by abnormal neovascularization stimulated by pro-angiogenic factors. Conditions exacerbating hyperoxia, such as greater oxygen delivery by HbA, can increase the risk for ROP development and progression.20 Accordingly, several studies confirmed the connection between RBC transfusions, or low HbF, and ROP, and this association is supported by a robust pathophysiological rationale in many studies.6, 7, 8,21, 22, 23, 24, 25, 26 In BORN patients, the HbF AUC recorded within 30 weeks PMA was significantly lower in neonates with severe ROP, confirming that low HbF levels may play a role. In addition, neonates receiving only CB-RBCs had similar HbF AUC values as non-transfused patients. In contrast, in the ITT set, HbF AUCs were similar between control and intervention arms. The latter resulted from by the high rate of protocol deviations, with more that 40% of patients in the intervention arm receiving A-RBCs instead of CB-RBCs, along with a small proportion of non-transfused patients in both arms. In light of these considerations, the ITT analysis appears to be less useful for assessing the effectiveness of this intervention. Moreover, the HbF monitoring evidenced that if CB-RBCs are given subsequently to repeated A-RBCs transfusions, HbF cannot recover to physiological levels, and CB-RBC transfusions fail to prevent ROP progression.
The analyses alternative to the ITT approach are not simple to interpret because of the potential loss of the benefits derived from randomization. Despite these important limitations, other aspects suggest the consistency of the aforementioned results. First, the arms in the PP set were well balanced for center (P = 0·25) and GA (< or >26 weeks, P = 0·560), the two parameters adopted to stratify patients at randomization. Second, the imbalance for IUGR was also present in the ITT set (Table 2) and could account for the higher birth weight in the intervention group in the PP set. Overall, there was no association between IUGR and severe ROP, in both univariable and multivariable analyses. In addition, the control and intervention arms in the PP set were comparable for several aspects including GA, transfusion requirements, infectious and non-infectious complications, need for inotropic drugs, and days on oxygen supplementation and invasive ventilation. Finally, multivariable logistic regression analysis confirmed that A-RBC, but not CB-RBC, transfusions increased the risk for severe ROP. Notably, the increase in risk was estimated at ∼1·7 fold for each transfused unit and because the high transfusion burden in ELGANs, the potential for CB-RBCs to mitigate against severe ROP is very encouraging.
The BORN study results also suggest that CB-RBC transfusions can influence BPD and pulmonary hypertension severity. BPD encompasses a range of pulmonary manifestations, from mild respiratory failure due to decreased alveolarization and abnormal vascular development, to more severe forms with striking disruption of lung architecture, secondary pulmonary hypertension, and need for invasive respiratory support.27 Both antenatal and postnatal factors, including invasive ventilation, oxygen supplementation, PDA, and infections, favor BPD development by promoting lung injury and impairing the development of alveoli and capillaries.27 In recent studies, BPD was associated with both repeated RBC transfusions and low HbF levels.28,29 RBC transfusions seem to favor BPD progression also in preterm neonates born >31 weeks PMA.30 Our current results prompt further studies to explore the role of CB-RBC transfusions in neonates born less prematurely than ELGANs.
CB is well recognized as a source of hematopoietic stem cells. Therefore, CB collection is intended for transplant use and is accomplished in only a minority of term deliveries at sites where CB banks operate. To this end, the CB-RBC units used in this study were recycled from CB collections that were not suitable for transplantation. Indeed, both the limited availability and short shelf-life of CB-RBCs challenged the supply of an adequate RBC transfusion support for patients in the BORN study intervention arm. Increasing the number of units processed into HbF-enriched RBC concentrates would require changing the current paradigm of CB collection, moving from only a transplant-centered approach to an approach that would satisfy both transfusion and transplantation needs. This not only has logistical implications but also needs an appropriate regulatory framework. In this regard, the BORN study results may inspire investigators and practitioners to operationalize new strategies.
In conclusion, the BORN trial is the first prospective randomized controlled trial evaluating the clinical impact of CB-RBC transfusions. The trial results suggest a significant favorable effect of CB-RBCs in preventing and/or minimizing severe forms of ROP and BPD and indicate the urgent need for larger randomized studies.
Contributors
Luciana Teofili, Patrizia Papacci, Claudio Pellegrino, Carmen Giannantonio, Tina Pasciuto, Stefano Ghirardello, and Giovanni Vento conceptualized the study. Luciana Teofili, Patrizia Papacci, Claudio Pellegrino, Francesco Cresi, Giulia Remaschi, Giulia Ansaldi, Carmen Giannantonio, Tina Pasciuto, Iolanda Mozzetta, Francesca Serrao, Velia Purcaro, Genny Raffaeli, Isabella Mondello, Paola Bergamaschi, Stefano Ghirardello, and Giovanni Vento designed the study. Luciana Teofili, Patrizia Papacci, Claudio Pellegrino, Carlo Dani, Francesco Cresi, Giulia Remaschi, Giulia Ansaldi, Carmen Giannantonio, Maria Francesca Campagnoli, Barbara Vania, Marco Fabbri, Roberta Penta de Vera d' Aragona, Anna Molisso, Enrico Beccastrini, Antonella Dragonetti, Tina Pasciuto, Sabrina Gabbriellini, Silvia Baroni, Francesca Serrao, Velia Purcaro, Genny Raffaeli, Stefania Villa, Daniele Prati, Isabella Mondello, Alessandra Falcone, Maria Letizia Patti, Tiziana Boggini, Paola Bergamaschi, Domenico Lepore, Fabrizio Gaetano Saverio Franco, Lorenzo Orazi, Antonio Baldascino, Caterina Giovanna Valentini, Emanuela Locatelli, Roberto Albiani, Federico Genzano Besso, Giulia Vanina Cantone, Alessandra Coscia, Alfonso Trimarchi, Giacomo Cavallaro, Stefano Ghirardello, and Giovanni Vento acquired the data. Luciana Teofili, Patrizia Papacci, Tina Pasciuto, and Iolanda Mozzetta have had direct access to and verified the data. Luciana Teofili, Patrizia Papacci, Claudio Pellegrino, Carmen Giannantonio, Tina Pasciuto, and Iolanda Mozzetta analyzed the data. Luciana Teofili, Patrizia Papacci, and Claudio Pellegrino wrote the original draft. All authors have read and agree to submit the final version of the manuscript.
Data sharing statement
Data supporting the findings of this study are available in anonymized form upon reasonable request to the study principal investigators.
Declaration of interests
The authors report no potential conflicts of interest, including relevant financial interests, activities, relationships, and affiliations.
Acknowledgements
BORN investigators are indebted to the personnel of NICUs and Cord Blood Banks participating in BORN, whose valuable work makes the study possible. Moreover, they express deep empathy and gratitude to parents of all ‘born too soon’ babies enrolled in BORN. S.GH. thanks Francesca Garofoli, Achille Cristian, and Micol Angelini (Fondazione IRCCS Policlinico S. Matteo, Pavia) for their invaluable contribution in supporting cord blood collection. L.T. and C.P. would like to express their heartfelt appreciation to Prof. Steven Spitalnik for his precious advice, consideration, and enthusiasm in thoughtful discussions on cord blood transfusion.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103426.
Appendix A. Supplementary data
References
- 1.Sankaran V.G., Menne T.F., Xu J., et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322(5909):1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 2.Bard H. Postnatal fetal and adult hemoglobin synthesis in early preterm newborn infants. J Clin Invest. 1973;52(8):1789–1795. doi: 10.1172/JCI107360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito-Benz M., Flanagan P., Berry M.J. Management of anaemia in pre-term infants. Br J Haematol. 2020;188(3):354–366. doi: 10.1111/bjh.16233. [DOI] [PubMed] [Google Scholar]
- 4.Ghirardello S., Dusi E., Cortinovis I., et al. Effects of red blood cell transfusions on the risk of developing complications or death: an observational study of a cohort of very low birth weight infants. Am J Perinatol. 2017;34(1):88–95. doi: 10.1055/s-0036-1584300. [DOI] [PubMed] [Google Scholar]
- 5.Vu P.T., Ohls R.K., Mayock D.E., et al. Transfusions and neurodevelopmental outcomes in extremely low gestation neonates enrolled in the PENUT Trial: a randomized clinical trial. Pediatr Res. 2021;90(1):109–116. doi: 10.1038/s41390-020-01273-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stutchfield C.J., Jain A., Odd D., Williams C., Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye (Lond) 2017;31(10):1451–1455. doi: 10.1038/eye.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiramongkolchai K., Repka M.X., Tian J., et al. Lower foetal haemoglobin levels at 31- and 34-weeks post menstrual age is associated with the development of retinopathy of prematurity: PacIFiHER Report No. 1 PacIFiHER Study Group (Preterm Infants and Fetal Haemoglobin in ROP) Eye (Lond) 2021;35(2):659–664. doi: 10.1038/s41433-020-0938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellström W., Martinsson T., Morsing E., Gränse L., Ley D., Hellström A. Low fraction of fetal haemoglobin is associated with retinopathy of prematurity in the very preterm infant. Br J Ophthalmol. 2022;106(7):970–974. doi: 10.1136/bjophthalmol-2020-318293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teofili L., Papacci P., Orlando N., et al. Allogeneic cord blood transfusions prevent fetal haemoglobin depletion in preterm neonates. Results of the CB-TrIP study. Br J Haematol. 2020;191(2):263–268. doi: 10.1111/bjh.16851. [DOI] [PubMed] [Google Scholar]
- 10.Teofili L., Papacci P., Orlando N., et al. BORN study: a multicenter randomized trial investigating cord blood red blood cell transfusions to reduce the severity of retinopathy of prematurity in extremely low gestational age neonates. Trials. 2022;23(1):1010. doi: 10.1186/s13063-022-06949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teofili L., Papacci P., Dani C., et al. Cord blood transfusions in extremely low gestational age neonates to reduce severe retinopathy of prematurity: results of a prespecified interim analysis of the randomized BORN trial. Ital J Pediatr. 2024;50(1):142. doi: 10.1186/s13052-024-01714-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girelli G., Antoncecchi S., Casadei A.M., et al. Recommendations for transfusion therapy in neonatology. Blood Transfus. 2015;13(3):484–497. doi: 10.2450/2015.0113-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Committee for the Classification of Retinopathy of Prematurity The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 14.Bell M.J., Ternberg J.L., Feigin R.D., et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliegman R.M., Walsh M.C. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17(4):213–288. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobe A.H., Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 17.Walsh M.C., Szefler S., Davis J., et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006;117(3 Pt 2):S52–S56. doi: 10.1542/peds.2005-0620I. [DOI] [PubMed] [Google Scholar]
- 18.Papile L.A., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 19.Bowerman R.A., Donn S.M., Silver T.M., Jaffe M.H. Natural history of neonatal periventricular/intraventricular hemorrhage and its complications: sonographic observations. AJR Am J Roentgenol. 1984;143(5):1041–1052. doi: 10.2214/ajr.143.5.1041. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrino C., Papacci P., Beccia F., et al. Differences in cerebral tissue oxygenation in preterm neonates receiving adult or cord blood red blood cell transfusions. JAMA Netw Open. 2023;6(11) doi: 10.1001/jamanetworkopen.2023.41643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrino C., Stone E.F., Valentini C.G., Teofili L. Fetal red blood cells: a comprehensive review of biological properties and implications for neonatal transfusion. Cells. 2024;13(22) doi: 10.3390/cells13221843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad M., Ingolfsland E.C., Christiansen S.P. Modifiable risk factors and preventative strategies for severe retinopathy of prematurity. Life (Basel) 2023;13(5) doi: 10.3390/life13051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lust C., Vesoulis Z., Jackups R., Jr., Liao S., Rao R., Mathur A.M. Early red cell transfusion is associated with development of severe retinopathy of prematurity. J Perinatol. 2019;39(3):393–400. doi: 10.1038/s41372-018-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teofili L., Papacci P., Bartolo M., et al. Transfusion-free survival predicts severe retinopathy in preterm neonates. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.814194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser K., Härtel C., Dammann O., et al. Erythrocyte transfusions are associated with retinopathy of prematurity in extremely low gestational age newborns. Acta Paediatr. 2023;112(12):2507–2515. doi: 10.1111/apa.16965. [DOI] [PubMed] [Google Scholar]
- 26.Fevereiro-Martins M., Aguiar L., Inácio Â., et al. Fetal hemoglobin as a predictive biomarker for retinopathy of prematurity: a prospective multicenter cohort study in Portugal. Biomedicines. 2025;13(1):110. doi: 10.3390/biomedicines13010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin R.J., Jobe A.H., Bancalari E. What is BPD today and in the next 50 years? Am J Physiol Lung Cell Mol Physiol. 2021;321(5):L974–L977. doi: 10.1152/ajplung.00415.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahr T.M., Snow G.L., Christensen T.R., et al. Can red blood cell and platelet transfusions have a pathogenic role in bronchopulmonary dysplasia? J Pediatr. 2024;265 doi: 10.1016/j.jpeds.2023.113836. [DOI] [PubMed] [Google Scholar]
- 29.Hellström W., Martinsson T., Hellstrom A., Morsing E., Ley D. Fetal haemoglobin and bronchopulmonary dysplasia in neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):88–92. doi: 10.1136/archdischild–2020-319181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahr T.M., Ohls R.K., Henry E., et al. The number of blood transfusions received and the incidence and severity of chronic lung disease among NICU patients born >31 weeks gestation. J Perinatol. 2025;45(2):218–223. doi: 10.1038/s41372-024-02135-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.