Highlights
-
•
This study identifies novel insights into the role of Hornerin (HRNR) gene mutations in ovarian cancer pathogenesis. While HRNR has been associated with poor prognosis in various cancers, its role in ovarian cancer has not been previously explored.
-
•
We demonstrate that HRNR expression is significantly higher in clear cell and mucinous ovarian carcinomas compared to high-grade serous carcinomas, correlating with shorter survival.
-
•
Elevated HRNR levels in late-stage patients suggest its utility as a prognostic marker.
-
•
We identified harmful genetic variants in HRNR, including a translocation, which may influence disease progression.
-
•
These findings underscore the potential of HRNR as both a prognostic biomarker and a therapeutic target, particularly for patients with HRNR genetic variants, potentially improving chemotherapy outcomes.
Keywords: Gene rearrangement, Hornerin expression, Chemoresistance, Ovarian cancer, Therapeutic marker, Hornerin mutations
Abstract
Background
Hornerin (HRNR) is part of the S100-fused protein family and has been linked to poorer prognosis in various cancers, though the mechanisms remain unclear. This study aimed to explore the role of HRNR in ovarian cancer.
Methods
We conducted proteomic analysis (n = 252) and RNA sequencing (n = 96) on primary ovarian carcinomas to evaluate HRNR expression across different histotypes, survival outcomes, and to identify genetic variants in the HRNR gene. We also assessed HRNR levels in both chemosensitive and chemoresistant ovarian cancer cell lines, as well as in serum samples at different disease stages.
Results
Our findings revealed that HRNR levels were significantly higher in clear cell and mucinous ovarian carcinomas compared to high-grade serous carcinomas, with elevated expression associated with shorter survival. Additionally, HRNR levels increased in sera from late-stage patients compared to those in early stages. We identified several potentially harmful genetic variants in exon 3 of HRNR in patients with high-grade serous carcinoma, including a translocation of a 900 bp fragment from exon 3 to a region between exons 1 and 2. Chemoresistant ovarian cancer cells exhibited higher HRNR levels than chemosensitive cells. Silencing HRNR using siRNA markedly enhanced the cytotoxic effects on all tested ovarian cancer cells.
Conclusions
HRNR plays a significant role in ovarian cancer with potential to serve as a prognostic marker. Additionally, targeting HRNR expression may offer therapeutic benefits, particularly for patients with chemoresistance or specific HRNR genetic variants, highlighting the need for further investigation in the management of ovarian cancer.
Significance
Hornerin (HRNR) is linked to poor prognosis in various cancers, but its role in ovarian cancer has been underexplored. Our study shows that HRNR expression is significantly higher in clear cell and mucinous ovarian carcinomas compared to high-grade serous carcinomas, correlating with shorter survival. Elevated HRNR levels in late-stage patients suggest its potential as a prognostic marker. We also identified harmful genetic variants in HRNR, including a novel translocation, which may affect disease progression. Targeting HRNR could enhance chemotherapy effectiveness, particularly for patients with HRNR genetic variants, positioning it as a promising biomarker and therapeutic target in ovarian cancer.
Background
Ovarian cancer is the leading cause of death from gynecological cancers in the United States, with a 5-year survival rate of 50 % in advanced stages [1]. Due to a lack of effective screening strategies and little to no warning symptoms, ovarian cancer is often diagnosed at a late stage. The combination of lack of early detection and the development of chemoresistance is mainly responsible for the observed high rate of death in ovarian cancer [2,3]. Currently, treatment options for ovarian cancers consist primarily of both cytoreductive surgery and platinum/docetaxel combination chemotherapy [4]. While many patients with advanced-stage ovarian cancers will initially achieve complete clinical response, most will recur and develop chemoresistant disease, which has been shown to correlate with a worse prognosis [5].
The molecular mechanism of the development of ovarian cancer is not well understood. Several molecules have been previously proposed as biomarkers for early detection or targets for therapy [6]. However, to date, there is no effective biomarker or treatment against this disease. Recently, we have detected altered expression of HRNR in ovarian cancer cells and blood. HRNR is a 245 kDa long member of the S100-fused protein family, with a characteristic helix-loop-helix structure [7]. HRNR is a 12,118 bp long gene located on chromosome 1q21.3 (chr1:152,212,076–152,224,193) and contains 3 exons, and an 8550 bp coding region [8]. Broadly, the S100-fused protein family plays a role in calcium binding proteins, as well as protein phosphorylation, immune and inflammatory reactions, cell proliferation, differentiation, and death [9]. Specifically, HRNR plays a role in calcium and other ion binding, organization of the cell envelope, and skin barrier formation [10]. Elevated levels of HRNR expression have been reported in several cancers, including ovarian cancer cells [11], suggesting HRNR is involved in the pathogenesis of these tumors [[12], [13], [14], [15]]. Additionally, these studies have shown that high HRNR expression is associated with overall tumor progression and worse disease prognosis [[12], [13], [14], [15], [16]].
In this study, we demonstrated that HRNR plays a significant role in ovarian cancer, with higher expression levels observed in clear cell (CCC) and mucinous carcinomas (MC) compared to high-grade (HGOSC) serous carcinomas, correlating with poorer survival outcomes. The increase in HRNR levels in late-stage patients and the identification of harmful genetic variants in exon 3 underscore its potential as a prognostic marker. Furthermore, the elevated HRNR expression in chemoresistant ovarian cancer cells highlights its possible contribution to treatment resistance. Silencing HRNR enhanced the cytotoxic effects of chemotherapy, suggesting that targeting HRNR could provide therapeutic benefits. Overall, HRNR emerges as a promising biomarker and therapeutic target, warranting further investigation in ovarian cancer management.
Materials & methods
Patient population
Human subjects
Tissues: The current study adhered to the Declaration of Helsinki and received approval from the Regional Ethical Review Board in Gothenburg, Sweden (case number 767–14), which also granted a waiver of written consent for tumor specimen use. The cohort comprised 252 primary invasive ovarian carcinoma patients diagnosed between 1993 and 2022. Fresh-frozen tumor samples were sourced from the Sahlgrenska University Hospital Oncology lab in Gothenburg, Sweden, and reclassified by certified pathologists according to current WHO diagnostic criteria for histotyping using corresponding formalin-fixed, paraffin-embedded (FFPE) samples from the Department of Clinical Pathology at the same hospital. Neoplastic cell percentage was determined in all samples via May-Grünwald Giemsa-stained touch preparation imprints. Samples with at least 50 % neoplastic cell content were selected for further analysis. Patient demographics are summarized in Table 1. Ovarian tumors were collected at the time of primary surgery. Clinicopathological and survival data were obtained from national cancer registries. Survival was stratified into four groups (i.e. 0–2 years, 2–5 years, 5–10 years and >10 years) based on overall survival, defined as the time from initial diagnosis to death of any cause.
Table 1.
HRNR Mutations from ovarian cancer patients of various histologies.
| Mutation type | Potential deleterious mutation | Gene | DNA sequence change | Amino acid change | Mutation site | chr | Genomic position | Mutated samples |
|---|---|---|---|---|---|---|---|---|
| frameshift insertion | Yes | HRNR:NM_001009931 | c.1050dupA | p.H351fs | Exon 3 | chr1 | 152,193,054–152,193,054 | P1767_360 (HGSC) |
| frameshift deletion | Yes | HRNR:NM_001009931 | c.1991delG | p.R664fs | Exon 3 | chr1 | 152,192,114–152,192,114 | P1767_306 (HGSC) |
| frameshift deletion | Yes | HRNR:NM_001009931 | c.7055_7056del | p.S2352fs | Exon 3 | chr1 | 152,187,049–152,187,050 | P1767_385 (HGSC) |
| synonymous SNV | No | HRNR:NM_001009931 | c.A2763G | p.R921R | Exon 3 | chr1 | 152,191,342–152,191,342 | P1767_360 (HGSC) |
| stopgain | Yes | HRNR:NM_001009931 | c.C1165T | p.Q389X | Exon 3 | chr1 | 152,192,940–152,192,940 | P1767_346 (HGSC) |
| synonymous SNV | No | HRNR:NM_001009931 | c.C1990A | p.R664R | Exon 3 | chr1 | 152,192,115–152,192,115 | P1767_306 (HGSC) |
| stopgain | Yes | HRNR:NM_001009931 | c.C2761T | p.R921X | Exon 3 | chr1 | 152,191,344–152,191,344 | P1767_360 (HGSC) |
| stopgain | Yes | HRNR:NM_001009931 | c.C7288T | p.Q2430X | Exon 3 | chr1 | 152,186,817–152,186,817 | P1767_363 (HGSC) |
| synonymous SNV | No | HRNR:NM_001009931 | c.C7359G | p.G2453G | Exon 3 | chr1 | 152,186,746–152,186,746 | P1767_362 (HGSC) |
| nonsynonymous SNV | No | HRNR:NM_001009931 | c.C8114A | p.S2705Y | Exon 3 | chr1 | 152,185,991–152,185,991 | P1767_306 (HGSC) |
| nonsynonymous SNV | No | HRNR:NM_001009931 | c.G3043T | p.G1015W | Exon 3 | chr1 | 152,191,062–152,191,062 | P1767_391 (HGSC) |
| nonsynonymous SNV | No | HRNR:NM_001009931 | c.G5674A | p.G1892S | Exon 3 | chr1 | 152,188,431–152,188,431 | P1767_155 (EC) |
| stopgain | Yes | HRNR:NM_001009931 | c.G6448T | p.G2150X | Exon 3 | chr1 | 152,187,657–152,187,657 | P1767_318 (HGSC) |
| synonymous SNV | No | HRNR:NM_001009931 | c.G6489T | p.G2163G | Exon 3 | chr1 | 152,187,616–152,187,616 | P1767_371 (HGSC) |
| synonymous SNV | No | HRNR:NM_001009931 | c.G7491C | p.S2497S | Exon 3 | chr1 | 152,186,614–152,186,614 | P1767_333 (EC) |
| nonsynonymous SNV | No | HRNR:NM_001009931 | c.T1072A | p.S358T | Exon 3 | chr1 | 152,193,033–152,193,033 | P1767_336 (HGSC) |
The detailed methodology for mutation and proteomic analysis has been described in Engqvist H. et al. (2018) and Werner L. et al. (in preparation), respectively. In brief, RNA sequencing data for 96 stage I-II primary invasive ovarian carcinomas were reevaluated to identify potential deleterious genetic variants (frameshift insertion, frameshift deletion, stopgain or stoploss) in the HRNR gene (GEO, accession number GSE101109). For the proteomic analysis, tissue lysates from 252 stage I-IV primary invasive ovarian carcinomas were prepared using Covaris ML230 ultrasonication. Following protein estimation, reduction, alkylation, and LysC+Trypsin digestion, peptides were purified, quantified, and analyzed on a timsTOF HT mass spectrometer. A spectral library was generated using basic reverse-phase liquid chromatography (bRP-LC) combined with label-free data-dependent acquisition (DDA)-PASEF mode. The mass spectrometry data were searched and quantified with Spectronaut against the Human Swissprot database. Data-independent acquisition (DIA) using the directDIA mode was performed for cohort analysis, followed by local normalization and filtering with Prostar to identify sufficiently detected proteins. Differential protein expression was determined using unpaired limma t-tests using the NormalyzerDE (v1.20.0) package in R/Bioconductor (v4.3.3).
Sera: Fifty-one serum samples were collected from patients diagnosed with ovarian cancer of various stages and histology by the Gynecologic Oncology Division at the Karmanos Cancer Institute. Patients provided informed consent to provide blood samples prior to chemotherapy or surgery. The samples included all stages of diagnosis, regardless of the histotype. Sera samples included in this study were collected between 2012 and 2018, under Wayne State University Human Subject Committee protocol number 027201MP2E, approval date 3/10/2011. Patient demographics are summarized in Table 2.
Table 2.
| Status |
||||
|---|---|---|---|---|
| No Cancer | Ovarian Cancer | |||
| Race | Black | Count | 10 | 10 |
| % within Race: | 50.00 % | 50.00 % | ||
| % within Status: | 34.48 % | 19.61 % | ||
| Other | Count | 0 | 3 | |
| % within Race: | 0 % | 100 % | ||
| % within Status: | 0 % | 5.88 % | ||
| White | Count | 19 | 38 | |
| % within Race: | 33.33 % | 66.67 % | ||
| % within Status: | 65.52 % | 74.51 % | ||
| Age | LE median (53) | Count | 19 | 21 |
| % within Age: | 47.50 % | 52.50 % | ||
| % within Status: | 65.52 % | 41.18 % | ||
| GT median (53) | Count | 10 | 30 | |
| % within Age: | 25.00 % | 75.00 % | ||
| % within Status: | 34.48 % | 58.82 % | ||
| Normal tissue | 29 36 % |
0 0 % |
||
| Stage | I | Count | 0 | 15 |
| % within Status: | 0 % | 29.41 % | ||
| II | Count | 0 | 6 | |
| % within Status: | 0 % | 11.76 % | ||
| III | Count | 0 | 15 | |
| % within Status: | 0 % | 29.41 % | ||
| IV | Count | 0 | 15 | |
| % within Status: | 0 % | 29.41 % | ||
| Region / Source | Karmanos Cancer Institute | Count | 29 | 51 |
| % within Status: | 100.00 % | 100.00 % | ||
| Total | Count | 29 | 51 | |
| % within Status: | 36 % | 64 % | ||
Serum samples were collected and processed as previously described [17]. Briefly, a 7 ml vial of blood obtained from each patient was allowed to form a clot over 2 h. The serum clot was collected by centrifugation with a desktop centrifuge at 2400 rpm for 10 min, and 1 ml aliquots were extracted and stored at -80 °C. Serum samples were labeled with a coded identifier.
Benign gynecologic controls: Fourteen serum samples were collected from patients with benign gynecological conditions. These included women diagnosed with ovarian cysts, peritonitis (inflammation), or uterine fibroids.
Healthy controls: Fifteen serum samples were obtained from female volunteers with no cancer history. At the time of informed consent, information about race, age, and evidence of benign gynecological conditions was recorded. We also attempted to match the racial makeup and age of healthy controls to overlap with patients with ovarian cancer and benign conditions.
Ovarian cancer staging
Ovarian cancer staging is based on tumor growth and metastatic spread, with two primary staging systems: the International Federation of Gynecology and Obstetrics (FIGO) and the American Joint Committee on Cancer (AJCC) TNM (Tumor, Nodes, Metastasis) system. The FIGO system, most widely used, categorizes stages from I to IV: Stage I denotes cancer restricted to the ovaries; Stage II indicates spread to nearby pelvic organs; Stage III shows extension to the abdomen or lymph nodes; and Stage IV indicates distant metastasis [[15], [16], [17]]. Staging criteria incorporate surgical findings, imaging, and pathology to evaluate tumor size, location, nearby tissue involvement, and distant metastases.
Immunohistochemistry (IHC): IHC was performed as previously described [6]. Paraffin blocks from ovarian cancer tissue of various stages and histology were obtained from the pathology department at Wayne State University. Briefly, 4 µM thick FFPE sections were organized on Dako FLEX IHC microscope slides and dried at 60 °C. The HRNR antibody (100 µL, S100A18 Polyclonal Antibody, ThermoFisher Scientific, Waltham, MA) was optimized using a panel containing 10 full-face tumor sections from various stages and histotypes. One of the 10 samples was used as control for the TMA hybridization experiments. Immunostaining was performed for the HRNR antibody using the optimized 1:100 dilution. The TMA sections were treated on a Dako Autostainer Plus (Agilent Technologies, Santa Clara, CA, USA) using Dako EnVision FLEX visualization systems.
Cell culture: Cell culture was performed as previously described [6]. Briefly, human EOC cell lines, MDAH-2774 (CRL-10,303) and SKOV-3 (HTB-77) were obtained from American Type Culture Collection (ATCC, Manassas, VA), and were cultured with McCoy’s 5A medium (Invitrogen, Carlsbad, CA) supplemented with fetal bovine serum (FBS, Innovative Research, Novi, MI) and penicillin/streptomycin according to the manufacturers’ protocols. Sensitive and cisplatin resistant TOV-112D EOC cells were a kind gift from Gen Sheng Wu at Wayne State University, Detroit, MI [18]. The A2780 human ovarian cancer cell line and its cisplatin resistant counterpart (1 μM) were obtained from Sigma Aldrich (St. Louis, MO) and cultured in RPMI-1640 (ThermoFisher Scientific, Waltham, MA) supplemented with FBS and penicillin/streptomycin.
Development of chemoresistance in ovarian cancer cells: Chemotherapy resistant cells were conferred as previously described [6]. Briefly, parent EOC cells MDAH-2774 and SKOV-3 were exposed to continuous culture in media containing stepwise increases in either cisplatin or docetaxel (Sigma Aldrich) over a period of 6 months with a final concentration of 1.5 μM cisplatin or 0.3 μM docetaxel. Upon reaching final concentrations, cells were grown in the absence chemotherapeutic drugs for 2 weeks, followed by replacement of the drug and verification of resistance by the Trypan blue cell viability and MTT Cell Proliferation assays. Previously published studies were consulted to determine chemotherapy dose [19,20]. Once confluent, cells were prepared as described above.
Real time RT-PCR for HRNR: Real time RT-PCR was performed as previously described [6].
Design of Hornerin PCR primers and standard: One pair of HRNR primers were selected through NCBI primer search on the human Hornerin mRNA sequence (NM_001009931.3). Primers (Sigma Aldrich, St. Louis, MO) were designed to amplify an 81 bp fragment from the human HRNR gene. The forward primer was from positions 33 to 52 located in Exon 1 and the reverse primer was from positions 94 to 113 located in Exon 2. A standard sequence was synthesized (Sigma Aldrich, St. Louis, MO) to include the HRNR upstream and downstream primer sequences, in its sequence which amplified 81 bp. The PCR standard sequence with primers locations underlined is as follows:
5′-TGTTCCTCTGGTGAGCTAGGTTACTCAAACTTGCAAAAAAAAAAATGCCTAAACTCCTACAAGGCGTCATCACTGTCATCG-‘3. A standard curve was constructed by performing real time RT-PCR on stepwise dilutions of the standard to determine the concentration of HRNR per μg RNA in the unknown samples as previously described [6].
Isolation of RNA: Total RNA was isolated as previously described [6] from human ovarian cancer cell lines and serum samples using the QuantiTect Reverse Transcription Kit (Qiagen, Ann Arbor, MI). RNA quantification of all samples was performed using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
Reverse Transcription: A 20 μl cDNA reaction volume was prepared using the SuperScript VILO Master Mix Kit (Life Technologies, Carlsbad, CA), according to the manufacturer's protocol and as previously described [6]. Measurement of the amount of cDNA in each sample was performed using a Nanodrop Spectrophotometer (ThermoFisher Scientific, Waltham, MA) [6]. Measurement of the amount of cDNA in each sample was performed using a Nanodrop Spectrophotometer (ThermoFisher Scientific, Waltham, MA). All cDNA were diluted to a final working concentration of 1 μg/μl.
Real-time RT-PCR analysis of HRNR mRNA: Quantitative real-time RT-PCR was performed as previously described [6]. Briefly, the QuantiTect SYBR Green RT-PCR kit (Qiagen, Aarhus, Denmark) and a Cepheid 1.2f Detection System (Cepheid, Sunnyvale, CA) were used [6]. An initial melting cycle was performed at 95 °C followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 63 °C for 20 s, and lastly an extension cycle at 72 °C for 30 s. To quantify each target transcript, a standard curve was constructed using a tenfold serial dilution of the standard for the target segment of HRNR. Following real-time RT-PCR, a melting curve analysis was performed to demonstrate the specificity of the PCR product as a single peak.
Gel electrophoresis: Gel electrophoresis was performed as previously described [21]. Briefly, a 2 % agarose gel was prepared by microwaving 2 g agarose and 100 ml of 1X TAE. Two microliters of ethidium bromide were added and the solution was allowed to harden. Once hard, the gel box was filled with 1X TAE buffer. DNA loading dye (R0611) was added to achieve a 1X solution for each sample (5 µl per 25 µl sample). DNA ladder and samples (30 µl) were loaded and run at 120 V. The gel was imaged on ChemiDoc (Bio-Rad Laboratories, Inc., Hercules, CA).
Genome sequencing: Gel electrophoresis products (900 kDa) were sliced and purified from the gel as previously described [22]. Samples were sent to the Wayne State Genome Sciences Core for Sanger sequencing.
Western blot: Western blot was performed as previously described [23]. Briefly, total proteins from all ovarian cancer cells and normal ovarian cells were fractionated using 8 % SDS-PAGE gel electrophoresis. Protein samples (20 µg) were mixed with 4X loading buffer, DTT, and H2O to reach a final volume of 30 µl. Protein peptides were separated under denaturing conditions. Gel proteins were then transferred to 0.45 um PVDF membranes. Membranes were washed followed by incubation with primary monoclonal antibodies overnight at 4 °C (Hornerin 1:250 anti-rabbit #NBP1–80,807 Novus Biologicals in 1 % BSA, and B-actin 1:2000 anti-rabbit #4970 Cell Signaling). Membranes were then washed and incubated with 1:2000 Donkey anti-rabbit IgG HRP #sc2313 Santa Cruz in 5 % milk for one hour. The membranes were finally washed and imaged using the ECL detection kit (Amersham Life Sciences, Amersham, U.K.).
Silencing HRNR expression in ovarian cancer cells: HRNR gene expression was silenced as previously described [6] with specific siRNA (50 nM, Ambion Silencer Select siRNA, Fisher Scientific, Waltham, MA) in both sensitive and chemoresistant ovarian cancer cell lines utilizing the GenMute™ siRNA Transfection Reagent (SignaGen Laboratories, Rockville, MD) per the manufacturer’s protocol. Briefly, ten thousand cells were seeded on a 96-well plate and allowed to incubate for 24 h. On Day 1, cells were treated with either 3 pmol or 6 pmol of HRNR siRNA (10 µM concentration, #AM16708) or scramble siRNA (5 µM concentration, #AM4611) and left to incubate for 48 h. On Day 3, MTT analysis was performed utilizing TACS MTT Cell Proliferation Assay (CAT #4890–050-K) to detect cytotoxicity of cells as previously described [21,24]. Briefly, 10 µl of MTT solution was added to each well in the 96-well plate. Samples were incubated for 2 h at 37 °C until a purple color was observed. SDS-HCl Detergent Reagent (100 µl) was added to each well before another 2-hour incubation period at 37 °C. Absorbance was read at 570 nm to determine the results of the assay.
Immunofluorescence: Immunofluorescence was performed as previously described [7]. Briefly, cells were grown in 8-well chamber slides (#354,118, Corning Inc., Glendale, AZ) until confluent. Cells were fixed with 4 % PFA and washed with PBS. Cells were blocked in 10 % donkey serum for one hour at room temperature followed by incubation overnight at 4 °C with human HRNR primary antibody 1:250 in 3 % Donkey serum (#NBP1–80,807, Novus Biologicals, rabbit). After 24 h, the cells were incubated in anti-rabbit IgG Alexa594 secondary antibody (#ab150064, Abcam, Cambridge, MA) 1:200 in 3 % donkey serum for 1 hour at room temperature in the dark. The negative control was prepared as above, but had no primary antibody added. Images were acquired with the Axiovert 25 inverted microscope (Zeiss, Thornwood, NY) using a FITC fluorescent filter.
Statistical analysis: Data are presented as Mean ± SEM. Unpaired t-test (for comparing two groups) or Ordinary One-way ANOVA (for comparing more than two groups) were used to calculate statistical significance. Boxplots were constructed using the ggpubr (v0.6.0) package in R with pairwise t-test. P values < 0.05 were considered significant. Data were graphed and analyzed using GraphPad Prism v10 or R/Bioconductor (v4.2.2).
Results
HRNR expression and genetic variants in ovarian cancer patients
Proteomics analysis of the 252 primary ovarian carcinomas from all stages revealed significantly higher HRNR levels in CCC and MC samples compared to HGOSC (Fig 1A). Moreover, elevated HRNR expression was found in short-term survivors (0–2 years) compared to patients surviving 2–5 years and >10 years, with decreasing HRNR expression over time (Fig. 1B). No difference in HRNR expression was found based on tumor stage or patient age (Fig. 1C-D). RNA sequencing analysis revealed a total of 16 unique genetic variants in exon 3 of HRNR (NM_001009931) were detected in the 96 stage I-II ovarian carcinomas, of which 7 were detected in patients with HGOSC and determined to potentially be deleterious (1 frameshift insertion: c.1050dupA:p.H351fs; 2 frameshift deletions: c.1991delG:p.R664fs and c.7055_7056del:p.S2352fs; and 4 stopgains: c.C1165T:p.Q389X, c.C2761T:p.R921X, c.C7288T:p.Q2430X, and c.G6448T:p.G2150X). Interestingly, 2/7 potentially deleterious variants were found in the same patient (frameshift insertion and stopgain), while the other genetic variants were detected in different patient samples (Table 1).
Fig. 1.
HRNR protein expression patterns in primary ovarian carcinoma using mass spectrometry. A) Elevated HRNR levels in clear cell carcinoma (CCC) and mucinous carcinoma (MC) compared to high-grade serous carcinoma (HGSC). B) Significantly higher HRNR expression in short-term survivors compared to long-term survivors (2–5 yrs and >10 yrs). C-D) No difference in HRNR expression in patient samples stratified by tumor stage or age. Paired t-test was used to calculate statistically significant differences between different patient groups; only statistically significant differences are shown. B, borderline; CCC, clear cell carcinoma; EC, endometrioid carcinoma; HGSC, high-grade serous carcinoma; LD, low-differentiated; LGSC, low-grade serous carcinoma; MC, mucinous carcinoma; SBL, serous borderline; SMBL, sero-mucinous borderline.
Overexpression of HRNR in ovarian cancer cells and plasma
Western blot analysis revealed that HRNR is expressed in the plasma obtained from all ovarian cancer stages (I-IV; Fig. 2A). Interestingly, HRNR Western blot detected three peptide fragments of 98, 64, and 50 kDa in late-stage (III-IV) ovarian cancer plasma. More importantly, the 50 kDa peptide fragment was missing in some of the early-stage plasma (I-II; Fig. 2A). However, HRNR was not detected in the plasma of healthy individuals (Fig. 2A). Immunohistochemistry (IHC) revealed that HRNR was localized to the cytoplasm of tumor cells and that most samples were HRNR-positive (98.5 %; Fig. 2B). Analysis of staining in ovarian cancer tissues using tissue microarray (TMA) revealed significantly higher (p = 0.003) HRNR expression in late-stage ovarian cancers compared to early-stage ovarian cancers (Fig. 2C).
Fig. 2.
HRNR expression in ovarian cancer. A) Representative Western blot of plasma proteins isolated from various stages of ovarian cancer patients. Samples were randomized and loaded into the gel as follows; Lane 1. Stage 4 plasma, lane 2. normal plasma, lane 3. Stage 1 plasma, lane 4. Stage 2 plasma, lane 5. Stage 3 plasma, lane 6. Stage 4 plasma, lane 7. Stage 2 plasma, lane 8. Stage 1 plasma. Western blot revealed 3 HRNR peptide fragments of 98, 64, and 50 kDa. Beta-Actin was used as an internal control. B) Representative immunohistochemistry (IHC) showing HRNR expression (gray) in high grade ovarian serous carcinoma (HGOSC) and normal liver (Control) tissues. Images were taken at 40X magnifications power using Zeiss Microscope. C) HRNR cytoplasmic staining in ovarian cancer tissues by stage (I-IV). p = 0.003.
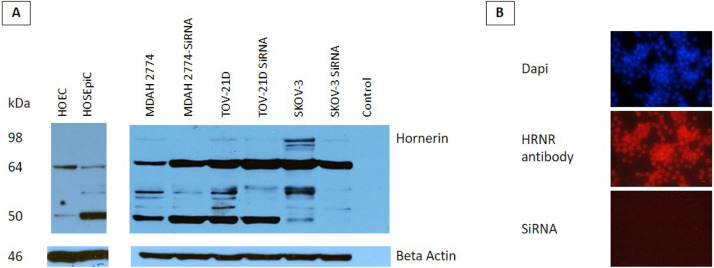
Western blots were also used to determine the expression of HRNR protein in all ovarian cancer and normal ovarian cells. Western blot analysis of normal cells revealed 2 HRNR fragments of 50 and 64 kDa (Fig. 3A). Interestingly, a new fragment at 98 kDa was detected in ovarian cancer cells (Fig. 6A). Treatment with siRNA markedly decreased the levels of this fragment in addition to other HRNR fragments in all ovarian cancer cells (Fig. 3A). Additionally, HRNR expression HRNR was confirmed in all ovarian cancer cells using immunofluorescence (Fig. 3B). Furthermore, siRNA treatment successfully eliminated the expression of HRNR in ovarian cancer cells (Fig. 3B).
Fig. 3.
Effect of HRNR gene rearrangement on protein expression. A) Representative Western blot of sensitive and chemoresistant cell lines, MDAH-2774, SKOV-3, and TOV21-D, as well as in human primary ovarian epithelial cells (HOEC), and human ovarian surface epithelial cells (HOSEpiC). HRNR is expressed differently in ovarian cancer cells indicated by banding around 98 kDa. B) Representative immunofluorescence (IF) images from the A2780 ovarian cancer cell line. Nuclear staining with DAPI was used as a control. Staining with anti-HRNR antibody confirmed ovarian cancer cells overexpress HRNR. A2780 ovarian cancer cells treated with specific siRNA showed negative HRNR expression when treated with anti-HRNR antibody.
Fig. 6.
Sequencing of real time RT-PCR products from ovarian cancer cells. A) PCR products from SKOV-3 and MDAH-2774 ovarian cancer cells were subjected to gel electrophoresis and the 900 bp product was cut from the gel, purified, and sequenced. B) Diagram of HRNR gene structure. BLAST search results of the sequenced 900 bp products from SKOV-3 and MDAH-2774 confirmed that the 900 bp sequence (labeled in red and yellow) is in Exon 3 of the HRNR gene. Translocation of the 900 bp sequence in the HRNR gene is indicated by the blue curved arrow, from Exon 3 to the location of the target sequence between Exons 1 and 2. The coding region (black) is 8550 bp long. Coding sequence (CDS).
Overexpression of HRNR in sensitive and chemoresistant ovarian cancer cells
Real time RT-PCR revealed that HRNR expression was significantly (p < 0.001) increased in all ovarian cancer cells compared to normal human ovarian surface epithelial cells (HOSEpiC) and human ovarian epithelial cells (HOEC; Fig. 4). There was a 2.6-, 1.1-, and 1.4-fold increase in HRNR mRNA levels in SKOV-3, MDAH-2774, and TOV-21 G compared to normal HOSEpiC, respectively. Similarly, there was a 4.6-, 2.3-, and 2.8-fold increase in HRNR mRNA levels in SKOV-3, MDAH-2774, and TOV-21 G compared to normal HOEC, respectively. Moreover, HRNR mRNA levels were significantly (p < 0.001) increased in all cisplatin (SKOV-3: 1.5-fold; MDAH-2774: 1.4-fold; TOV-21G: 1.2-fold) and docetaxel (SKOV-3: 2.5-fold; MDAH-2774: 2.4-fold; TOV-21G: 2.2-fold) chemoresistant cells compared to their chemosensitive counterparts (Fig. 4).
Fig. 4.
HRNR expression is elevated in sensitive and chemoresistant ovarian cancer cells. Real-time RT-PCR was used to measure mRNA levels for HRNR in multiple sensitive and chemoresistant ovarian cancer cell lines. The following sensitive ovarian cancer cell lines and their cisplatin (C) and docetaxel (F) chemoresistant counterparts were used: MDAH-2774, SKOV-3, and TOV-21 G. Additionally HRNR mRNA levels were determined in human ovarian surface epithelial cells (HOSEpiC) and human ovarian epithelial cells (HOEC). *** p < 0.001.
HRNR gene rearrangement in ovarian cancer cells
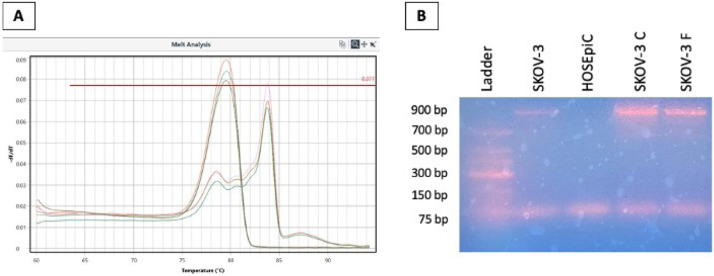
Analysis of HRNR PCR products using melting curves revealed, in addition to the expected 81 bp product, an additional PCR product in all sensitive and chemoresistant cancer cells but not in normal ovarian cells. The 81 bp product melted at 79 °C whereas the additional product melted at 84 °C (Fig. 5A). The additional PCR product was also confirmed by agarose gel electrophoresis of PCR products which revealed the size of the additional product as 900 bp (Fig. 5B).
Fig. 5.
Real-time RT-PCR products melt analysis of HRNR. A) Melt analysis of HRNR standard in serial dilution showed the 81 bp product melting temperature at 79 °C. Additional product was detected at 84 °C when amplifying all ovarian cancer cell lines. B) Representative agarose gel electrophoresis of amplified products from SKOV-3 sensitive (SKOV-3 S), SKOV-3 cisplatin resistant (SKOV-3 C), SKOV-3 docetaxel resistant (SKOV-3 F) ovarian cancer cells and normal human ovarian surface epithelial cells (HOSEpiC). The size of the additional product observed when amplifying ovarian cancer cells, but not HOSEpiC or HOEC, was estimated to be 900 bp.
The 900 bp PCR product from SKOV-3 and MDAH-2774 ovarian cancer cell lines were excised from the gel, purified, and Sanger sequenced. Intriguingly, the 900 bp product sequence from MDAH-2774 cells (Fig. 6A) was 99 % similar to a sequence in the HRNR gene at genomic position 152,223,264 to 152,224,082 located in the Exon 3 coding region (labeled in red and yellow, Fig. 6B). Similarly, the 900 bp product sequence from SKOV-3 cells (Fig. 6A) was also 99 % similar to a sequence in the HRNR gene at position 152,223,264 to 152,224,080 located in the Exon 3 coding region (labeled in red and yellow, Fig. 6B). Additionally, BLAST search comparing the 900 bp sequences from MDAH-2774 and SKOV-3 generated a 100 % match. Since our primers were designed to amplify sequences located in Exons 1 and 2, and the 900 bp product is found in Exon 3, this would indicate an insertion of the 900 bp segment from Exon 3 in the coding sequences located in Exons 1 and 2 (Fig. 6B).
Silencing of HRNR gene induced killing of ovarian cancer cells
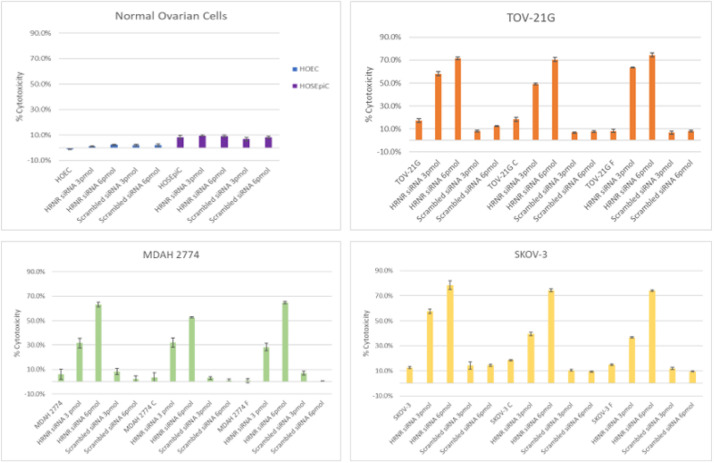
Treatment with siRNA targeting HRNR significantly (p < 0.001) induced cytotoxicity in both sensitive and chemoresistant ovarian cancer cell lines (SKOV-3, MDAH-2774, and TOV-21 G) without affecting normal ovarian cells (HOSEpiC and HOEC) (Fig. 7). When treated with 3 pmol siRNA, a 1.6-, 1.3-, and 1.6-fold increase in cytotoxicity in SKOV-3, MDAH-2774, and TOV-21 G sensitive ovarian cancer cells, respectively, compared to HOEC normal cells, and a 0.9-, 1-, and 0.9-fold increase, respectively, compared to HOSEpiC normal cells. At 6 pmol siRNA, cytotoxicity increased by 1.6-, 1.6-, and 1.7-fold in SKOV-3, MDAH-2774, and TOV-21 G cells, respectively, compared to HOEC, with a comparable increase (0.9-, 1-, and 0.9-fold, respectively) relative to HOSEpiC. Treatment with no siRNA did not produce significant cytotoxicity in any cancer cells compared to the normal controls (HOSEpiC and HOEC) (Fig. 7).
Fig. 7.
Silencing HRNR gene expression with specific siRNA. MTT assay for cytotoxicity was used to determine the effect of silencing HRNR expression in sensitive and chemoresistant cell lines MDAH-2774, SKOV-3, and TOV-21 G. Additionally, MTT assay for cytotoxicity was performed on human ovarian surface epithelial cells (HOSEpiC) and human ovarian epithelial cells (HOEC) after HRNR siRNA treatment.
Cytotoxicity was also significantly (p < 0.001) increased in chemoresistant cells treated with siRNA silencing HRNR expression. In comparing 3 pmol siRNA treatment to no siRNA, SKOV-3 cells increased in cytotoxicity by 1.1- and 1.5-fold in docetaxel- and cisplatin-resistant cells, respectively. TOV-21 G cells showed a 2.6- and 1.8-fold increase in cytotoxicity for docetaxel- and cisplatin-resistant cells, respectively. Remarkably, MDAH-2774 cells exhibited dramatic increases in cytotoxicity with 8- and 33-fold for docetaxel- and cisplatin-resistant cells, respectively (Fig 7). In comparison of 6 pmol siRNA to no siRNA treatment, SKOV-3 cells had a 3- and 4-fold increase in cytotoxicity in docetaxel- and cisplatin-resistant cells, respectively. Similarly, TOV-21 G cells showed a 3.3- and 3.0-fold increase, and MDAH-2774 cells demonstrated a 13.8- and 77.1-fold increase in docetaxel- and cisplatin-resistant cells, respectively. No significant cytotoxic effects were observed in normal ovarian cells with any treatment (Fig 7).
Survival analysis of HRNR expression in epithelial ovarian cancer
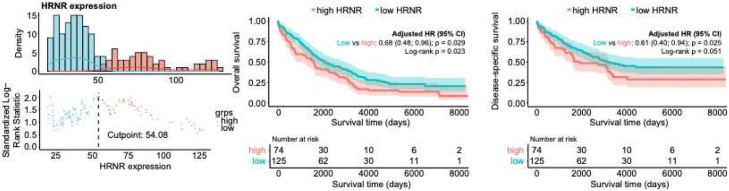
To evaluate the prognostic significance of HRNR expression in epithelial ovarian cancer, we first determined the optimal cutpoint to categorize patients into high and low HRNR expression groups using the maximally selected rank statistics method. This data-driven approach identifies the expression threshold that best separates patients based on survival outcomes. Subsequently, Kaplan–Meier survival analyses were conducted to assess the association between HRNR expression levels and patient outcomes. Patients with high HRNR expression exhibited significantly reduced overall survival (OS) compared to those with low expression (p < 0.05; Fig. 8). Similarly, a trend toward poorer disease-specific survival (DSS) was observed among patients with high HRNR expression (Fig. 8). To account for potential confounding factors, we further performed multivariable Cox proportional hazards regression analyses, adjusting for key clinical variables including patient age, disease stage, and tumor histotype. After adjustment, high HRNR expression remained significantly associated with worse OS (hazard ratio [HR], 95 % CI, p ≤ 0.05) and DSS (p ≤ 0.05), suggesting that HRNR is an independent prognostic factor in EOC. These findings support the potential utility of HRNR expression as a prognostic biomarker in ovarian cancer and warrant further investigation into its biological role in disease progression.
Fig. 8.
Survival analysis of HRNR expression in epithelial ovarian cancer. A) the optimal cutpoint for determining high and low HRNR expression. Kaplan-Meier survival analysis for the association between high and low HRNR expression. B) overall survival (OS). C) disease specific survival (DSS) in the EOC dataset. Multivariable Cox regression models were adjusted using patient age, disease stage, and histotype. High HRNR expression was significantly associated with worse OS (p < 0.05). After adjusting for covariates, high expression was associated with worse OS and DSS (p < 0.05).
Discussion
In this study, we detected HRNR expression in human ovarian tissue and plasma from cancer patients of various stages and histotypes. The expression of HRNR was significantly higher in late-stages (III and IV) as compared to early-stages (I and II), CCC and MC compared to HGOSC, and short-term survivors (0–2 years) compared to long-term survivors (2–5 years and >10 years). Additionally, high HRNR expression was significantly associated with worse OS (p < 0.05). After adjusting for covariates, high expression was associated with worse OS and DSS (p < 0.05). Intriguingly, seven potentially genetic variants in the HRNR gene were found in six patients with HGOSC. Additionally, HRNR expression was significantly higher in ovarian cancer cells compared to normal ovarian epithelial cells. Indeed, HRNR overexpression has also been reported in several cancers such as breast, hepatocellular carcinoma, and pancreatic cancers [11]. In hepatocellular carcinoma, upregulation of HRNR showed a correlation with decreased overall patient survival rate in addition to an increase in cellular proliferation, metabolism, migration, and survival of hepatocellular carcinoma cells [14]. Furthermore, overexpression of HRNR in pancreatic ductal adenocarcinoma led to increased angiogenesis, which is essential for cancer progression and further supports HRNR as a potential for a therapeutic target [15]. Remarkably, HRNR under expression in a PDX (patient-derived xenograft) model of renal cancer showed no tumor formation in vivo [25]. Moreover, HRNR expression was previously reported to be altered in endometrial cancer and suggested as part of serum protein biomarker panel for early detection of endometrial cancer [16]. Of significance, the expression of HRNR was significantly higher in chemoresistant as compared to chemosensitive ovarian cancer cells, which highlights an important role for this protein in the development of chemoresistance.
Western blot analysis of HRNR in ovarian cancer plasma and cells revealed 3 distinct peptide fragments of 50, 64, and 98 kDa. There were no detectable HRNR peptide fragments in plasma from all samples collected from healthy volunteers. Intriguingly, only 2 HRNR fragments of 50 and 64 kDa were detected but not the 98 kDa fragment in normal human ovarian surface epithelial cells. In agreement with our results, several distinct HRNR peptide fragments have been detected by Western blot in various tissues and cells [7]. In mouse skin, Western blot with anti-HRNR showing multiple peptide fragments ranging from 45 kDa to 245 kDa [10]. Multiple HRNR fragments were reported in breast cancer cells at 245, 98, 81, and 50 kDa [12]. Western blot with anti-HRNR antibodies revealed a peptide fragment at 245 kDa in most tissues and cell lines [12]. In the human epidermis, immunostaining using HRNR antibodies revealed four putative HRNR peptides in healthy epidermis as well as in the entire outer root sheath of normal human scalp hair follicles [26]. Additionally, several HRNR peptides ranging in size from 50 to 245 kDa were detected in human psoriatic skin [26].
The detection of distinct multiple immunologic HRNR peptide fragments in ovarian cancer cells and not in normal human ovarian cells indicated specific alterations of this protein in ovarian cancer. Here, we report a specific rearrangement in the HRNR gene in ovarian cancer cells. There was a translocation of a 900 bp piece of DNA located between sequence number 152,223,264 to 152,224,164 in Exon 3 to sequence number 152,212,094 to 152,212,994 between Exons 1 and 2. The cause of this HRNR gene rearrangement is unknown. However, it’s a plausible explanation for the observed overexpression of HRNR in ovarian cancer cells and thus a novel function of this protein in the pathogenesis of this disease. In fact, HRNR has been reported to be highly mutated in some cancers [27,28]. HRNR exhibited several somatic missense mutations in pancreatic adenosquamous carcinoma, which significantly correlated with lymphatic metastasis [28]. Similarly, in urinary tract cancers, missense mutations and translocation of the HRNR gene have been found and suggested to contribute to tumorigenesis [29]. Interestingly, a translocation from chromosome 1q21 to chromosome 2q37 [t(1;2) (q21;q37)] was found in a patient with acute myeloid leukemia which was speculated to play a crucial role in the development and progression of the patient’s myelodysplastic syndrome [30].
The specific alteration of HRNR gene in ovarian cancer highlights a novel role of HRNR in the pathogenesis of ovarian cancer. Thus, targeting HRNR may have potential therapeutic benefits. Indeed, we have shown that knocking down HRNR gene expression with a specific siRNA induced killing of sensitive and chemoresistant ovarian cancer cells but has no significant effects on normal ovarian cells. It has been reported that knockdown of the HRNR gene by shRNA inhibited tumor growth within a xenograft of hepatocellular carcinomas [14]. Furthermore, knockdown of HRNR gene expression in mice by specific siRNA in a subcutaneous human pancreatic ductal (PDAC) model has been shown to result in reduced endothelial tumor cell proliferation as well as a decrease in tumor size [15]. While our in vitro experiments provide compelling evidence for the role of HRNR in ovarian cancer cell proliferation, survival, and potential chemoresistance, we recognize the limitation that these findings have not yet been validated in vivo. In vitro systems, although informative, do not fully recapitulate the tumor microenvironment, immune interactions, or pharmacokinetics relevant to therapeutic targeting. Therefore, to build upon our current findings, future studies will include the use of xenograft and orthotopic ovarian cancer models to evaluate the effects of HRNR modulation on tumor growth, metastasis, and response to chemotherapy in vivo. These studies will be essential to confirm the translational relevance of HRNR as a prognostic marker and potential therapeutic target in epithelial ovarian cancer.
Although the precise molecular mechanisms by which HRNR contributes to tumor progression and chemoresistance remain to be fully elucidated, we hypothesize that HRNR may play a role in modulating cellular architecture and stress response pathways. Given its structural characteristics and known associations with cytoskeletal components in other epithelial tissues, HRNR may interact with intermediate filaments or actin-binding proteins, thereby influencing cellular adhesion, morphology, and motility. Additionally, HRNR could be involved in regulating key survival signaling pathways—such as the PI3K/AKT, MAPK, or NF-κB pathways—that are commonly activated in response to chemotherapy-induced stress. Through these interactions, HRNR may enhance the ability of ovarian cancer cells to withstand cytotoxic insults, contributing to treatment resistance and disease progression. Future studies aimed at identifying HRNR’s direct binding partners and downstream signaling effects will be critical for clarifying its mechanistic role in chemoresistance.
Interestingly, our analysis revealed that HRNR expression is higher in clear cell carcinoma (CCC) and mucinous carcinoma (MC) compared to high-grade serous ovarian cancer (HGOSC). To explore potential mechanisms underlying this histotype-specific upregulation, we examined publicly available gene expression datasets and relevant literature. One possible explanation is differential epigenetic regulation, such as DNA methylation or histone modifications, which are known to vary across ovarian cancer subtypes and may influence HRNR transcription. Additionally, distinct transcription factor networks associated with each histologic subtype could drive HRNR expression. For instance, transcription factors such as HNF1B, commonly upregulated in CCC, may play a role in the transcriptional activation of HRNR, although this hypothesis requires further experimental validation. Despite these differences, we focused our downstream analyses on HGOSC due to its high clinical prevalence and the broader availability of well-characterized experimental models, including established cell lines and clinical datasets. Nonetheless, the role of HRNR in CCC and MC remains an important area for future investigation, particularly in the context of subtype-specific pathways of tumor progression and chemoresistance.
In summary, HRNR is overexpressed in ovarian cancer cells, tissues, and plasma compared to normal, which correlates with disease stage. Additionally, HRNR expression is significantly elevated in chemoresistant ovarian cancer cells as compared to chemosensistive counterparts. A novel HRNR gene rearrangement was discovered and believed to be responsible for changes in HRNR protein expression in ovarian cancer cells. Knockdown of HRNR gene expression induced killing in all sensitive and chemoresistant ovarian cancer cells, but not in normal cells. Therefore, targeting HRNR expression may have potential future therapeutic applications for the treatment of ovarian cancer and chemoresistant ovarian cancer as well as potential for treatment of other cancers.
Ethics approval and consent to participate
N/A.
Patient consent for publication
N/A.
Data availability
All data is saved in the OneDrive @Dr. Saed Lab.
Funding
This work was supported in part by grants from the Swedish Cancer Society (23 2732 Pj 01 H), King Gustav V Jubilee Clinic Cancer Research Foundation (2022:410), the LUA/ALF-agreement in West of Sweden health care region.
CRediT authorship contribution statement
Ghassan M. Saed: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Harvey Sharma: Writing – review & editing, Methodology, Data curation. Jenna Dabaja: Writing – review & editing, Methodology. Asad Nawaz: Methodology. Ayesha Alvero: Writing – review & editing, Investigation, Formal analysis, Data curation. Robert T. Morris: Writing – review & editing, Resources, Formal analysis. Asma Basha: Writing – review & editing, Resources, Formal analysis, Data curation. Lucas Werner: Writing – review & editing, Methodology, Formal analysis, Data curation. Toshima Z. Parris: Writing – review & editing, Validation, Methodology, Formal analysis. Khalil Helou: Writing – review & editing, Validation, Resources, Methodology, Funding acquisition, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thank you to the Department of Obstetrics and Gynecology at Wayne State University School of Medicine, 48201, Detroit, MI, USA.
References
- 1.Ovarian Cancer Statistics Ovarian cancer statistics. Centers Dis. Control Prevent. 2022 [Google Scholar]
- 2.B O., RP E. Diagnosis and treatment of ovarian cancer. Hematol. Oncol. Clin. North Am. 2018;32(6):943–964. doi: 10.1016/j.hoc.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Jelovac D., Armstrong D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011;61(3):183–203. doi: 10.3322/caac.20113. Epub 2011 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim A., Ueda Y., Naka T., Enomoto T. Therapeutic strategies in epithelial ovarian cancer. J. Exp. Clin. Cancer Res. 2012;31(1):14. doi: 10.1186/1756-9966-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K., Eno M.L., Im D.D., Rosenshein N.B., Sood A.K. Clinical relevance of extent of extreme drug resistance in epithelial ovarian carcinoma. Gynecol. Oncol. 2010;116:61–65. doi: 10.1016/j.ygyno.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saed G.M., Ali-Fehmi R., Jiang Z.L., Fletcher N.M., Diamond M.P., Abu-Soud H.M., et al. Myeloperoxidase serves as a redox switch that regulates apoptosis in epithelial ovarian cancer. Gynecol. Oncol. 2010;116(2):276–281. doi: 10.1016/j.ygyno.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.T M., M T., M M., NH H. Hornerin, a novel profilaggrin-like protein and differentiation-specific marker isolated from mouse skin. J. Biol. Chem. 2001;276(50):47445–47452. doi: 10.1074/jbc.M107512200. [DOI] [PubMed] [Google Scholar]
- 8.Takaishi M., Makino T., Morohashi M. Huh N-h. Identification of Human hornerin and its expression in regenerating and psoriatic skin. J. Biol. Chem. 2005;280(6):4696–4703. doi: 10.1074/jbc.M409026200. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z., Meyer-Hoffert U., Reithmayer K., Paus R., Hansmann B., He Y., et al. Highly complex peptide aggregates of the S100 fused-type protein hornerin are present in Human skin. J. Investigative Dermatol. 2009;129(6):1446–1458. doi: 10.1038/jid.2008.370. [DOI] [PubMed] [Google Scholar]
- 10.Henry J., Hsu C.Y. FASEB; 2011. Hornerin is a Component of the Epidermal Cornified Cell Envelopes; pp. 1567–1576. al e. [DOI] [PubMed] [Google Scholar]
- 11.Hornerin; 2022. The Human Protein Atlas. [Google Scholar]
- 12.Fleming J.M., Ginsburg E., Oliver S.D., Goldsmith P., Vonderhaar B.K. BMC Cancer; 2012. Hornerin, an S100 Family protein, is Functional in Breast Cells and Aberrantly Expressed in Breast Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.J C., DI K., J K., BH K., A K. Hornerin is involved in breast cancer progression. J. Breast. Cancer. 2016:142–147. doi: 10.4048/jbc.2016.19.2.142. p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SJ F., SL S., SQ L., YP H., WJ H., B G., et al. Hornerin promotes tumor progression and is associated with poor prognosis in hepatocellular carcinoma. BMC Cancer. August. 2018;18(1):815. doi: 10.1186/s12885-018-4719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutknecht M.F., Seaman M.E., Ning B., Cornejo D.A., Mugler E., Antkowiak P.F., et al. Identification of the S100 fused-type protein hornerin as a regulator of tumor vascularity. Nat. Commun. 2017;8(1):552. doi: 10.1038/s41467-017-00488-6. Epub 20170915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarney C.M., Wang G., Bateman N.W., Conrads K.A., Ming Zhou B.L.H., Loffredo J., et al. Biomarker panel for early detection of endometrial cancer in the prostate, Lung, colorectal, and ovarian cancer screening trial. Am. J. Obstet. Gynecol. 2019;221(5) doi: 10.1016/j.ajog.2019.06.005. 472.e1-.e10. [DOI] [PubMed] [Google Scholar]
- 17.Engqvist H., Parris T.Z., Rönnerman E.W., Söderberg E.M.V., Biermann J., Mateoiu C., Sundfeldt K., Kovács A., Karlsson P., Helou K. Transcriptomic and genomic profiling of early-stage ovarian carcinomas associated with histotype and overall survival. Oncotarget. 2018;9(80):35162–35180. doi: 10.18632/oncotarget.26225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher N.M., Jiang Z., Ali-Fehmi R., Levin N.K., Belotte J., Tainsky M.A., et al. Myeloperoxidase and free iron levels: potential biomarkers for early detection and prognosis of ovarian cancer. Cancer Biomarkers. 2012;10:267–275. doi: 10.3233/CBM-2012-0255. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Zhou J.Y., Zhang L., Wu G.S. Involvement of MKP-1 and Bcl-2 in acquired cisplatin resistance in ovarian cancer cells. Cell Cycle. 2009;8:3191–3198. doi: 10.4161/cc.8.19.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AK G., A M., PJ O.D., Huang C.S., Hamilton T.C., Anderson M.E. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl. Acad. Sci. USA. 1992;89(7):3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamendola D.E., Duan Z., Yusuf R.Z., Seiden M.V. Molecular description of evolving paclitaxel resistance in the SKOV-3 human ovarian carcinoma cell line. Cancer Res. 2003;63(9):2200–2205. [PubMed] [Google Scholar]
- 22.Stein L.F., Saed G.M., Fivenson D.P. T-cell cytokine network in cutaneous lupus erythematosus. JAAD. 1997;36(2):191–196. doi: 10.1016/S0190-9622(97)70279-2. [DOI] [PubMed] [Google Scholar]
- 23.Nolly H., Saed G.M., Carretero O.A., Scicli G., Scicli A.G. Adrenal kallikrein. Hypertension. 1993;21(6 Pt 2):911–915. doi: 10.1161/01.hyp.21.6.911. [DOI] [PubMed] [Google Scholar]
- 24.Nusrat O., Belotte J., Fletcher N.M., Memaj I., Saed M.G., Diamond M.P., et al. The role of angiogenesis in the persistence of chemoresistance in epithelial ovarian cancer. Reprod. Sci. 2016;23(11):1484–1492. doi: 10.1177/1933719116645191. Epub 20160426. [DOI] [PubMed] [Google Scholar]
- 25.Saed G.M., Fletcher N.M., Diamond M.P., Morris R.T., Gomez-Lopez N., Memaj I. Novel expression of CD11b in epithelial ovarian cancer: potential therapeutic target. Gynecol. Oncol. 2018;148(3):567–575. doi: 10.1016/j.ygyno.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Dimastromatteo J., Kelly K.A. Hornerin as a novel therapeutic target for pancreatic cancer. Cancer Res. 2019;79(13_Supplement):3049. [Google Scholar]
- 27.Hsu C.Y., Gasc G., Raymond A.A., Serre G., M échin M.C., Simon M. JID; 2016. Deimination of Human Hornerin Enhances its Processing by Calpain-1 and Its Cross-Linking by Transglutaminases; pp. 422–429. [DOI] [PubMed] [Google Scholar]
- 28.Arnoff T.E., El-Deiry W.S. CDKN1A/p21WAF1, RB1, ARID1A, FLG, and HRNR mutation patterns provide insights into urinary tract environmental exposure carcinogenesis and potential treatment strategies. Am. J. Cancer Res. 2021;11(11):5452–5471. [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H., Song B., Guo S., Li G., Jin G. Identification of germline and somatic mutations in pancreatic adenosquamous carcinoma using whole exome sequencing. Cancer Biomark. 2020;27(3):389–397. doi: 10.3233/CBM-190236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.L W, YY W, Q C, Z C, SJ C. Hornerin gene was involved in a case of acute myeloid leukemia transformed from myelodysplastic syndrome with t(1;2)(q21;q37). Leukemia. 2006;20(12):2184-7. doi:10.1038/sj.leu.2404436. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is saved in the OneDrive @Dr. Saed Lab.