Abstract
Due to factors such as smoking, air pollution, and an aging population, the prevention and control of chronic respiratory diseases in China are becoming more severe. Combining current epidemiological investigations, exposure to environmental pollutants such as atmospheric pollutants, persistent organic pollutants, and heavy metals may have adverse effects on lung development. Lung differentiation models are crucial tools for elucidating the process of lung development. Through the combined effect of various cell growth factors and small molecules, pluripotent stem cells are successively induced to the definitive endoderm, the anterior foregut endoderm, lung progenitor cells, and alveolar epithelial cells and eventually form matured lung alveolar organoids, revealing the complex mechanisms of lung tissue formation. This Review compares various differentiation schemes based on in vitro culture protocols and conducts in-depth discussions for the current research on the toxicity of environmental pollutants using lung differentiation models. It aims to offer model selection criteria for scientists with different research purposes and provide scientific evidence to further research the impact of environmental pollution on respiratory diseases, as well as to provide important references for protecting public health.
Keywords: Lung differentiation model, Pluripotent stem cell, Alveolar organoids, Environmental pollution, Respiratory diseases


1. Introduction
The respiratory system is primarily responsible for delivering oxygen and expelling carbon dioxide through the nose, throat, trachea, and lungs, thus ensuring the necessary gas exchange for life activities. As the most important functional structure in the respiratory system, the lung plays a paramount role not only as the site for gas exchange but also in participating in various physiological processes including immune defense, acid–base balance, and blood circulation regulation. , The structure of the lungs is very complex, which undergo a long time and extremely precise molecular regulation from early fetal development to adult differentiation and maturation. Therefore, research on pulmonary toxicity should distinguish the use of different models based on the developmental stage of the lungs and toxicity research objectives.
In the current environment, an increasing number of epidemiological observations have identified respiratory toxicity issues. Smoking, air pollution, infections, and genetic factors can all lead to the increasing incidence of respiratory diseases, including chronic obstructive pulmonary disease (COPD), asthma, lung cancer, and others. Data released by the World Health Organization in 2020 indicate that respiratory system diseases have become one of the leading causes of death globally. The outbreak of diseases such as COVID-19, have further impacted the health and economy of billions of people worldwide and imposed a heavy burden on global public health. It is increasingly urgent to find effective treatment and prevention methods because of the complexity and diversity of these diseases. Up to now, several animal models have been used to study the development and pathogenesis of respiratory system-related organs, especially the lungs. However, the differences in the characteristics and developmental processes of human lungs compared to model animals result in experimental data not fully representing the actual toxicological processes that occur in the human body. In order to better understand the respiratory diseases and explore new therapeutic approaches, researchers have turned their attention to pluripotent stem cells (PSCs). PSCs include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). ESCs are derived from the inner cell mass of early embryos and possess the ability to self-renew and differentiate into various cell types. Thus, they are considered an ideal source for tissue regeneration and disease treatment due to their capacity to generate large numbers of specific cell types. ESCs hold extensive applications in disease modeling, drug screening, tissue engineering, and other fields. However, the application of ESCs faces challenges such as ethical controversies and immune rejection. iPSCs have provided a potential alternative to overcome these challenges. iPSCs originate from adult cells that can be reprogrammed into a pluripotent state through overexpression of four transcription factors (OCT4, SOX2, KLF4, MYC). Similar to ESCs, iPSCs also show the potential for self-renewal and multilineage differentiation, which provides more possibilities for disease modeling, drug screening, regenerative medicine, tissue engineering, and personalized medicine. , The discovery and application of iPSCs also hold significant scientific significance. Thus, using PSC models to differentiate and construct human lung cells/tissues for evaluating the respiratory toxicity of environmental pollutants has significant advantages, especially in accurately simulating the physiological state of human lungs.
Human lung differentiation is a complex and precise process involving cellular differentiation and organ formation (two-dimensional and three-dimensional) across multiple stages. Each stage of differentiation is regulated by specific signaling pathways. The process of inducing differentiation in vitro can simulate the lung development and better understand the mechanism of respiratory diseases’ occurrence. , Additionally, it can be applied to assess pulmonary toxicity of countless pollutants by simulating lung development in vitro and further elucidate the health risks of emerging pollutants. Currently, researchers have developed various lung differentiation models from the cellular level to the organ level. These models have been extensively applied in exploring respiratory diseases, drug screening, and environmental pollution assessment. Differentiation during pulmonary development allows for a more accurate assessment of environmental pollutants’ impact on lung health and supports control strategies. , This assessment approach based on cell differentiation can offer a more comprehensive understanding of the potential hazards of environmental pollutants to human health.
2. Construction of Pluripotent Stem Cell-Derived Lung Models
2.1. Differentiation of Human PSCs to Lung Progenitor Cells
Human lung development is mainly divided into five morphological stages: embryonic (4–7 weeks), pseudoglandular (5–17 weeks), canalicular (16–26 weeks), saccular (26–38 weeks), and alveolar (36 weeks–3 years). Embryonic lung cells can participate in tissue repair and lung regeneration, and exhibit significant biological importance. Embryonic lung period during real human development process in utero can be represented by the induction of PSCs into lung progenitor cells (LPCs) in vitro. , In this review, ″LPCs″ refer to early lung progenitor cells derived from PSCs that express the key early lung progenitor marker NKX2.1 and possess the ability to self-renew and differentiate into various types of lung cells, including alveolar epithelial cells, bronchial epithelial cells. According to developmental fates, PSCs can differentiate into liver, pancreas, intestine, and lung through the derived endoderm lineage. Directed differentiation to LPCs usually involves the generation of definitive endoderm (DE) and anterior foregut endoderm (AFE) by mainly manipulating TGFβ, WNT, and BMP4 pathways. The origin of the lung can be traced back to DE. High levels of Nodal signaling are crucial for the specification of DE. As members of the TGF-β superfamily, Nodal and Activin A signal through shared receptors to activate the TGF-β signaling cascade, which is essential for the induction of definitive endoderm. , As early as 2004, Atsushi Kubo and colleagues demonstrated the critical role of Activin A in inducing endoderm differentiation in mouse ESCs. Following this, in 2005, Emmanuel E Baetge and his team successfully generated human DE by adding Activin A and modulating serum concentration. Subsequently, studies performed by Adrian Kee Keong Teo and Ortwin Naujok in 2014 demonstrated that under serum-free conditions, the addition of a low concentration of the GSK3-β inhibitor CHIR-99021 can activate the WNT pathway, in conjunction with Activin A, to promote the conversion of primitive streak cells into DE. , In 2016, the introduction of the STEMdiff Definitive Endoderm Kit as a product offered a more efficient way to obtain DE cells. Anna Demchenko and colleagues simultaneously implemented both these two methods to compare efficiency in their study. For DE formation, the results indicate that the STEMdiff definitive endoderm kit exhibits comparable differentiation efficiency to that of using activin A combined with CHIR99021. Until now, the combination of CHIR-99021 and Activin A, as well as the Definitive Endoderm Kit, have become the two primary methods for DE induction in the early lung differentiation considering differentiation efficiency and cost-effectiveness. − For the generation of AFE, Michael D Green et al. in 2011 further induced the differentiation into AFE building upon the formation of DE by dual inhibition of the TGF-β (SB-431542) and the BMP4 (Noggin) signaling pathways. In 2012, Hongmei Mou and colleagues demonstrated that the addition of other BMP4 (Dorsomorphin) and TGF-β (A-83–01) inhibitors to the basal medium could also induce AFE cells expressing specific markers. This suggests that inhibiting BMP4 and TGF-β using different combinations of small molecules can effectively induce the differentiation of DE into AFE. Subsequently, in 2014, Hans-Willem Snoeck’s team proved that the combination of SB-431542 and Dorsomorphin could yield AFE cells with higher expression levels. As a result, the combined use of SB-431542 and Dorsomorphin has become the gold standard for AFE induction in lung differentiation studies in recent years. In order to further improve efficiency, the additional involvement of small molecules and proteins such as SHH and IWP2 can also promote the process of AFE formation. , The next stage is the evolution of AFE toward LPCs. Several attempts to produce LPCs from mouse have been made at first. Studies indicated that certain signaling pathways in mice play a role in specifying lung progenitors within the developing AFE. For example, the activation of WNT signaling can direct the endoderm toward a lung progenitor fate. , In a 2007 study, Wellington V. Cardoso’s team also found that retinoic acid (RA) can inhibit TGF-β signaling during mouse lung development, which is crucial for the induction and development of lung buds. In 2012, the Jayaraj Rajagopal team successfully induced LPCs from mouse and human iPSCs, demonstrating the necessity of BMP4, FGF, and WNT signaling in the transition from foregut endoderm to LPCs. , Up to now, the induction of differentiation from AFE to LPCs is achieved by adding BMP, RA, and CHIR99021 to the culture medium, ,, or by additionally including FGF family proteins such as FGF10 and FGF7 to regulate the BMP, FGF, and WNT pathways. , The above studies have indicated that the differentiation process from human PSCs to LPCs involves regulation of multiple signaling pathways. In summary, as Figure shows, the combined action of small molecules and proteins can sequentially induce the differentiation of PSCs by modulating various signaling pathways, ultimately obtained LPCs that retain their differentiation potential.
1.
By modulating signaling pathways, human embryonic stem cells are induced into lung progenitor cells to simulate early fetal lung development in utero. This model can be used to study various fetal lung abnormalities caused by exposure to environmental pollutants.
2.2. Differentiation of Lung Progenitor Cells to Alveolar Epithelial Cells
The airway and alveoli are two important components of the lung tissue. Air is introduced into the lungs through the airways and undergoes gas exchange in the alveoli, allowing the human body to obtain oxygen and discharge carbon dioxide. This process is crucial for maintaining normal function of the respiratory system. Both the airways and alveoli are composed of tightly arranged pseudostratified epithelial cells to prevent invasion from the external environment. When the number of airway or alveolar cells decreases because of damage, alveolar epithelial progenitors in vivo further differentiate into airway or alveolar epithelial cells and exert certain anti-inflammatory effects. Alveoli are the functional units of the sites for gas exchange. They are composed of tiny sacs surrounded by a dense network of capillaries, facilitating gas exchange through their thin membranes. , The alveolar epithelium is derived from the endoderm and mainly consists of two types of cells: type I alveolar epithelial cells (AT I) and type II alveolar epithelial cells (AT II). AT I is the main cellular component of the alveolar wall, being extremely thin to facilitate rapid diffusion of oxygen and carbon dioxide. It is mainly responsible for gas exchange within the alveoli. AT II can produce pulmonary surfactant; a surface-active substance that helps prevent alveolar collapse. It can reduce surface tension and maintain the expansion of alveoli. Under most conditions, AT II can self-renew and differentiate into AT I when lung damage occurs. Therefore, AT II cells are considered the facultative progenitor cells of the alveoli. − Besides gas exchange, lung epithelial cells also act as a physical barrier between the lumen and the underlying mucosa, collaborating with other immune cells to clear inhaled foreign particles, microbes, and harmful substances, protecting the respiratory system from infections and damages. −
Given that alveoli are the end point tissue of the respiratory system, the in vitro culture of AT I and AT II cells is particularly crucial. Considering the limitations of the two-dimensional cell culture systems, three-dimensional lung organoids induced from PSCs offer a closer approximation to the structure and function of natural lung tissue. , These in vitro cultured lung organoids can more accurately simulate the physiological, pathological processes, complexity, and functionality of human lung tissue. , Thus, three-dimensional lung differentiation models have been widely applied in lung basic research, clinical applications, and disease model construction. ,
As early as 2002, Linda W. Gonzales and colleagues had demonstrated that dexamethasone (Dex), isobutylmethylxanthine (IBMX), and 8-bromo-cAMP (cAMP) play critical roles in promoting surfactant production in human embryonic AT II cells. In 2007, Robert J Mason’s team pioneered the definition of the molecular phenotype of AT II cells in vitro by combining Matrigel with Dex, IBMX, and cAMP. This approach laid a solid foundation for subsequent advancements in generating AT II cells. In 2013, Sabrina Schmeckebier and colleagues built upon Robert J Mason ’s method by introducing keratinocyte growth factor (KGF) alongside the DCI (Dex, IBMX, and cAMP) combination. This modification significantly enhanced the efficiency and quality of AT II cell differentiation. Since then, the K-DCI medium has been widely recognized as the gold standard for inducing lung progenitor cells to differentiate into AT II cells and has become the most accepted protocol in the field. ,,− However, later studies suggested that KGF may not have a significant effect on epithelial cell formation, raising questions about its essential role in the protocol. , Subsequently, there are research teams beginning incorporating fibroblast growth factor 10 (FGF10) into the culture medium. Compared to previous protocols, the presence of FGF10 may reduce the time for AT II cell formation. ,, Recently, a study by Se-Ran Yang’ team demonstrated that LPCs derived from human hiPS cells can differentiate into alveolar epithelial cells after only 4 days of culture in DMEM Ham’s F12 medium supplemented with 0.25% BSA, 0.1% ITS, 1× B27, 50 nM dexamethasone, 0.1 mM IBMX, 100 ng/mL KGF, and 1% p/s. They observed downregulated expression of the proximal airway marker SOX2 but upregulation of distal lung transcription factors (SOX9, NKX2.1, GATA6, and HOPX), LPCs marker (ID2), and AT II markers (SFTPC and SFTPB) in the cells obtained. It means that even a short differentiation period is sufficient to initiate the expression of key AT II cell marker genes.
AT I cells are usually considered mature lung cells. The differentiation and regeneration of AT I are crucial for gas exchange and maintaining the function of the blood-air barrier after lung injury. AT I cells are highly susceptible to damage during lung injury. Damaged AT I potentially cause respiratory failure and severe pulmonary diseases At this time, AT II cells can proliferate and differentiate into type I phenotype, which provides a new perspective for addressing various lung injuries. ,, However, there are only a few research involving the formation of AT I cells. Carvalho and colleagues obtained an organoid capable of simultaneously expressing type II cells, type I cells, and various other airway and lung-related cell types. They first induced iPSCs into LPCs, after culturing these LPCs in expansion media containing CHIR99021, FGF7, and FGF10 for 10 days, the cells were embedded in Collagen I gel and replated into mature media supplemented with FGF10, K-DCI for additional 25 days. At this stage, both type I and type II alveolar epithelial cell markers were expressed simultaneously. Kotton’s group successfully obtained AT I cells by studying and regulating pathway changes. They initially induced iPSCs to differentiate into AT II cells and discovered that activating the YAP nuclear signaling pathway promotes a broad transcriptional transformation from AT II to AT I. For the details, they added LATS-IN-1 (activator of YAP) to the culture media while removing CHIR and KGF. They subsequently obtaining cells with both the morphology and functionality of AT I cells. Additionally, AT I cells can be detected in lung organoids generated by the Shimpei Gotoh’s team, particularly in the presence of XAV-939. This study revealed canonical WNT inhibition promotes the differentiation of AT II cells into AT I cells.
The differentiation of various lung epithelial cells under three-dimensional culture conditions demonstrates the ability of these cells to form more realistic functional airway and alveolar structures. This approach shows greater effectiveness in studying lung diseases and their impact on cell differentiation. And there are three main methods to form three-dimensional lung organoids from LPCs, including:
2.2.1. Formed by Matrigel
Single cells of LPCs were quantitatively mixed with an equal volume of Matrigel. The cell-Matrigel mixture at 50 μL volumes was added to transwell inserts to form droplets. After 30 min of solidification in the incubator, the plate of transwell was filled with induced medium containing FGF7, FGF10, CHIR99021, and EGF. After 6 days of culture, single-cell LPCs formed three-dimensional lung organoids. To obtain more mature lung organoid structures, all-trans retinoic acid and VEGF/PIGF were added to the medium for additional 6-day periods. Following this, the medium was changed to a mature formulation based on the induction medium supplemented with DCI. Ultimately, mature spherical structures that were large, transparent, and expressed alveolar markers were obtained. ,
2.2.2. Formed by coculture
This method was based on coculturing lung fibroblasts and induced LPCs/AT II derived from PSCs to generate lung organoids. In brief, a certain number of AT II were mixed with HFLF or other lung fibroblasts in K-DCI induced medium. This mixture was then seeded into transwell inserts mixed with reduced growth factor Matrigel in equal volume. Additionally, 1 mL of medium was added to the plate of transwell for 14 days. Then, a three-dimensional lung organoid model applicable for diseases or other purposes was obtained. Shimpei Gotoh’s teams continuously optimized this method and enables the long-term culture of lung organoids. −
2.2.3. Formed with Special 96-well Plates
In addition, another optimized method for forming lung organoids was implemented with special 96-well plates. In this method, induced LPCs were dissociated into single cells and then quantitatively added to 96-well round-bottom or flat-bottom ultralow attachment plates. After adding diluted Matrigel to each well, the plate was centrifuged for 5 min. Following overnight incubation, the obtained aggregates were transferred to a new plate containing fresh induced medium. Lung organoids were formed after 14 days of culture. And Se-Ran Yang’ and Seok-Ho Hong’ teams used this method to obtain three-dimensional lung organoids. ,
These methods demonstrate that nearly all the differentiation of LPCs into alveolar epithelial cells requires the addition of substances such as Dex, cAMP, IBMX, and sometime FGF7 and FGF10 (Figure ). This may be because Dex, cAMP, and FGF7 work synergistically to enhance the differentiation efficiency of alveolar epithelial cells. FGF7 is an important mediator of lung development and it plays a key role in alveolar extracellular differentiation and maturation. It is also known that the combination of glucocorticoids, cAMP, and IBMX has been proven to most effectively support the maturation and maintenance of primary fetal and adult alveolar epithelial cells. , And both IBMX and cAMP can act on the cAMP pathway and play a similar role. These established methods have successfully achieved the transformation of LPCs derived from human PSCs into alveolar epithelial cells.
2.
Lung progenitor cells (LPCs) are induced into alveolar type cells (ATs) using the CK-DCI medium. This model can be used to explore the impact of environmental pollutants exposure on postnatal lung development and the resulting childhood lung diseases.
The formation of lung organoids also involves regulation of multiple signaling pathways. Early studies have found that WNT/β-catenin signaling occupies an important position in inducing alveolar epithelial cell differentiation. Inhibition of WNT signaling can promote differentiation of AT II into AT I. Besides, BMP signaling has been shown to have a key role in proliferation and differentiation of various organs including the lung. Chung’s research indicated that activation of BMP could increase the generation of alveolar epithelial cells. Recent studies have found that Hippo-LATS-YAP signaling plays an undeniable role in the transition from AT II to AT I. , Additionally, Notch, TGFβ, and others are also important roles in the composition of multicellular lineages in organoids. ,−
2.3. Construction of Alveolar–Capillary Barrier Models
The air–blood barrier, also known as the alveolar–capillary barrier, is a biological barrier involved in gas exchange and blood flow. It typically occurs at the interface between the alveoli and the capillaries. This barrier is primarily composed of alveolar epithelial cells, the basement membrane, and endothelial cells of the blood vessels by tight connections. It controls the entry and exit of gases and other substances in the lungs, playing a crucial role in preventing the penetration of pathogens and external substances. , AT II primarily reduce surface tension at the air–liquid interface to maintain barrier integrity and prevent alveolar collapse, while endothelial cells exhibit semi permeability, allowing specific substances to pass through. Disruption of the integrity of the alveolar–capillary barrier can lead to respiratory diseases such as emphysema and inflammation. Therefore, the alveolar–capillary barrier has been increasingly recognized for its central role in pulmonary disease. − Considering that the real human lung environment is a complex system of cell–cell and cell-matrix interactions, coculture systems of alveolar epithelial and endothelial cells are necessary to more accurately investigate the threat of xenobiotic compounds to lung health, the mechanisms of disease occurrence, and drug screening and treatment for related diseases.
As early as 2004, Charles James Kirkpatrick and his team successfully established a coculture model of the air–blood barrier. They seeded alveolar epithelial and endothelial cells separately on two surfaces of a semipermeable membrane in transwell insert. Specifically, endothelial cells are plated on the inverted insert. After the cells adhere to the lower surface of the inset, alveolar epithelial cells are plated on the top surface at an appropriate density. After both cell types grow to a confluent state, the efficiency of barrier establishment is assessed by measuring transepithelial electrical resistance (TEER), with an increase in resistance indicating successful barrier formation. In addition, the protein expression and morphological composition of the coculture system were assessed using immunofluorescence staining and transmission electron microscopy (TEM). The establishment of this coculture system has provided a foundational model for multiple research studies. In coculture systems, alveolar epithelial cells are predominantly represented by A549 and NCI H441 cell lines, while endothelial cells typically include HUVEC, HPMEC, HULEC and ISO-HAS-1 cell lines. − In fact, using organoids containing AT II and/or AT I instead of alveolar epithelial cells would more realistically simulate the alveolar–capillary barrier in vivo. To further enhance the integrity and functionality of the alveolar–capillary barrier in vitro, researchers have developed triple-culture models incorporating the acute human monocytic leukemia cell line (THP-1) which can differentiate to macrophages. ,, Typically, a layer of THP-1 cells is seeded on top of the alveolar epithelial cells 4–5 days after the initiation of dual coculture. The resulting triple-culture models provide an effective tool for studying the structure, function, and regulation mechanisms of the alveolar–capillary barrier, as well as the interactions of external substances, aiding in a deeper understanding of the mechanisms underlying related diseases and the development of novel therapeutic approaches.
2.4. Emerging Technologies Building Differentiated Lung Models
In addition to traditional approaches of lung induction differentiation, several emerging technologies have recently surfaced, offering more possibilities for constructing models that better replicate in vivo lung tissue. The development of physiologically relevant lung models increasingly relies on advanced techniques that enable precise control of the cellular microenvironment and spatial organization. Among these, 3D bioprinting has emerged as a transformative tool in tissue engineering, capable of precisely fabricating complex multicellular structures that closely mimic the architecture of natural organs. By allowing the layer-by-layer deposition of lung progenitor cells embedded in bioactive hydrogels, 3D printing can recapitulate the branched geometry and cellular heterogeneity of the lung. This spatial precision helps establish region-specific cues and gradients, which are critical for directing the differentiation of cells into functional airway and alveolar epithelial lineages. , Moreover, when integrated with dynamic systems such as microfluidic chips, 3D-printed lung models can mimic temporal mechanical stimuli such as airflow and cyclic stretching, thereby providing an in vivo-like platform for studying development, disease, and toxic exposure. The ability of 3D bioprinting to precisely control cell positioning, matrix stiffness, and biochemical gradients makes it a compelling approach for investigating lung development, disease modeling, and inhalation toxicology under conditions that more closely resemble the in vivo environment.
At the same time, biomaterial-based culture systems have shown tremendous potential in supporting the growth and maturation of lung epithelial cells. By incorporating biocompatible scaffoldssuch as collagen, gelatin, or synthetic polymersinto the culture platform, researchers can better replicate the extracellular matrix (ECM) characteristics that affect cell polarity, proliferation, and differentiation. These scaffolds not only provide structural support but can also be functionalized with signaling molecules to guide lineage-specific development. Furthermore, coculture methods, in which lung epithelial cells are cultured alongside matrix, endothelial, or immune cells within these scaffolds, help recapitulate cell–cell interactions that are essential for tissue homeostasis and barrier function. The combination of 3D printing and biomaterials significantly enhances the ability to construct complex tissue models by enabling high-precision placement of cells and scaffolds. This integration improves both the multifunctionality and physiological relevance of engineered tissues. Hybrid bioprinting technologies, which merge various printing methods, allow the incorporation of renewable biomaterials across multiple scales, offering sustainable and scalable solutions for regenerative medicine and personalized therapies. Together, these technologies offer promising avenues for constructing in vitro lung models that are more physiologically relevant, effectively bridging the gap between cell cultures and in vivo systems.
3. Application of Pluripotent Stem Cell-Derived Lung Models in Evaluating the Impact of Environmental Pollutants
3.1. The Application of Human PSC Lung Progenitor Differentiation Models in Environmental Pollutant Toxicity Evaluations
More and more environmental pollutants can be frequently detected in amniotic fluid and umbilical cord blood of pregnant women. These pollutants have potential impact on lung development and differentiation during the early embryonic lung stage of the human body. Plenty of studies have confirmed that existing pollutants, such as tobacco, particulate matter, per- and polyfluoroalkyl substances (PFAS), bisphenol A (BPA) are easily detected in embryonic body fluids. − As mentioned above, inducing the differentiation of human PSCs into LPCs in vitro can to some extent simulate the development of early embryonic lungs. Therefore, multiple studies have evaluated the developmental toxicity of early embryonic lungs based on in vitro differentiation. Specifically, these pollutants that can reach the embryos have been proven to interfere with early embryonic lung development. For example, Singh et al. in 2020 found that smoking during pregnancy reduces the expression levels of H2S synthase in the human placenta. H2S is a gaseous transmitter that promotes angiogenesis, alveolarization, and has antiasthma properties. So, this reduction can increase the risk of asthma and bronchopulmonary dysplasia in newborns. In 2018, Chen et al. discovered that prenatal exposure to particulate matter leads to abnormal early lung development through a mouse model. Then in 2023, Wang et al. demonstrated that prenatal exposure to particulate matter (PM2.5) interferes with the process of fetal lung development using hESC line H9 to generate lung bud tip progenitor organoids (LPOs). Additionally, Chen’s and Willmore ’s teams respectively confirmed that prenatal exposure to PFAS and BPA affects fetal lung development and maturation. , Overall, these pollutants may interfere with human pulmonary development in the early embryonic stages, potentially leading to tracheal atresia, neonatal pulmonary hypoplasia and branching/lobation defects of the lung then increased risk of birth defects (Figure ). Besides these compounds, numerous other environmental pollutants can cross the placental barrier, posing significant health risks to early embryonic lung development.
3.2. Application of Alveolar Epithelial Cell Differentiation Models in Environmental Pollutant Toxicity Evaluations
In morphologically perspective, the induced differentiation from LPCs to alveolar epithelial cells primarily involves the pseudoglandular, canalicular, saccular, and early alveolar stages observed in human lung development. , This process roughly spans from the late embryonic stages and continues through the first few years after birth. In fact, existing literature indicates that alveolar development continues for several years, with 85% of alveolar structures forming after birth. Therefore, the neonatal period is crucial for the development of early alveoli. However, despite infants not being frequently involved in social activities, they still have the opportunity to come into contact with environmental pollutants. Infants usually get exposed to environmental pollutants through breast milk intake. Previous studies have proved that PFAS, pesticides, metals, medicines, and other environmental pollutants can be transferred to infants through breast milk. − When breastfeeding is not possible, infant formula is the most appropriate alternative. However, studies have shown the presence of various compounds such as PFAS, polychlorinated biphenyl (PCBs), heavy metals, microplastics, pesticides, bisphenol analogs, and others in infant formula. − These compounds have long been recognized as threats to embryonic development and human health. In addition to dietary intake, infants are also susceptible to exposure to pollutants through their surrounding environment. In recent years, severe air pollution has exposed pregnant women or infants to atmospheric pollutants. This exposure occurs both prenatally and postnatally and includes particulate matter, carbon monoxide (CO), sulfur and nitrogen oxides (SO2, NOx), ozone, and common tobacco smoke. Children exposed to air pollutants may experience lung development disturbances during fetal development and early postnatal periods, leading to conditions such as neonatal respiratory distress syndrome, infant or childhood lung and respiratory diseases, asthma, and even death. ,− Of course, the presence of other compounds in indoor environments can also trigger respiratory diseases in children, such as asthma and shortness of breath. − In brief, prenatal or postnatal exposure of fetuses or infants to environmental pollutants may interfere with the formation and development of infant alveolar epithelial cells (Figure ). Respiratory disease symptoms are common in childhood and can persist long-term. Adverse exposures during the prenatal period and early childhood can have lifelong impacts on respiratory health. Nowadays, several studies have explored the adverse effects of environmental pollutants (PM2.5, chromium compounds, viruses, benzo(a)pyrene, nanocarbon black, and nano-SiO2) on lung tissue by inducing alveolar epithelial cells or organoids from hPSCs. ,,, Therefore, induced differentiation process to alveoli to detect the toxicity of environmental pollutants on neonatal alveolar development can identify and mitigate harmful exposures, potentially reducing early life disease occurrences.
3.3. Application of Alveolar–Capillary Barrier Models in Environmental Toxicity Evaluations
The alveolar–capillary barrier in mature lung consists of complex structures including alveolar epithelial cells, basement membrane, and endothelial cells lining the capillaries, crucial for gas exchange. , By coculturing alveolar epithelial cells with other cell types, an alveolar–capillary barrier model was successfully established, which partially simulated the primary organ barrier function of the adult human lung. So, the promotion of this model is significant for the occurrence and development of adult lung diseases. The prominent environmental issues have led to the occurrence of various respiratory diseases. , Nowadays, one of the main risks of respiratory diseases are air pollutants. As shown in Figure , various air pollutants such as ultrafine particulate matter, gas mixture of smoking, and ozone, can cross the air–blood barrier and cause serious damage to lung health. Ultrafine particulate matter and gas mixture of smoking can penetrate deep into the lung organ, causing inflammation and respiratory diseases such as asthma, COPD, and lung cancer. − Ozone is a toxic gas that can irritate the respiratory tract. It causes symptoms of sore throat, cough, and difficulty breathing and exacerbate existing pulmonary diseases. Not only atmospheric pollutants, but other environmental risk compounds can also be inhaled into the lungs, increasing the incidence of respiratory diseases. Inhalation of harmful chemicals in pesticides may lead to pulmonary toxicity, especially for occupational populations. Exposure to metals such as As, Al, Cr, Cu, Fe, Mn, Ni, and Zn during production and processing also has shown adverse health effects on the respiratory system, manifesting as irritation, coughing, breathing difficulties, emphysema, bronchitis, pneumoconiosis, asthma, and even lung cancers.
3.
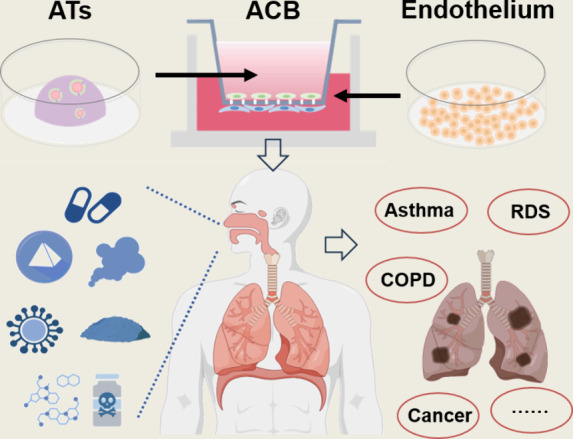
A coculture system of induced alveolar epithelial cells (ATs) and lung endothelial cells is used to establish an air–blood barrier model. This system allows the study of environmental pollutant exposure on adult lung health and its role in the development of various diseases.
Alveolar epithelial cells are critical targets for environmental pollutants entering the lung. Studies have shown that diseases such as acute respiratory distress syndrome (ARDS), COPD, emphysema, pulmonary fibrosis, and lung cancer are closely associated with the disruption of the epithelial barrier. ,,,, A coculture alveolar–capillary barrier system can provide a model for investigating the pathogenesis of acute lung injury, infectious lung diseases, and toxic lung injury, particularly focusing on the interactions between epithelial and endothelial cells. Additionally, coculture systems could be applied to optimize pulmonary drug delivery technologies, which also rely on the functionality of an intact air–blood barrier. Therefore, building a comprehensive air–blood barrier model that closely mimics the human physiological environment is crucial for advancing our understanding of the mechanisms of pollutant invasion in the lungs and their adverse effects. Moreover, such models provide essential scientific insights for the prevention and treatment of pulmonary diseases. In general, lung organ differentiation models in vitro are continuously evolving and improving, becoming essential tools for assessing the toxic effects of pollutants on the lungs at different developmental stages. These models accurately simulate the morphological characteristics of lung development, providing a critical platform for studying respiratory diseases. By analyzing the impact of pollutants on lung organs, we can gain a deeper understanding of their potential health hazards, aiding in the formulation of prevention and treatment strategies and further advancing environmental health research.
4. Conclusions and Perspective
The development of lung differentiation models in the fields of disease treatment and environmental toxicity assessment has profound implications for medical, environmental science, and societal development. With the continuous advancement of lung models, there is a deeper understanding of the pathogenesis of respiratory diseases and treatment targets. Through the simulation of lung development processes and specific disease states, we can study the molecular mechanisms of diseases, cellular signaling pathways, and gene expression patterns. This will provide a theoretical basis for the development of new drugs and treatment methods, thus accelerating progress in the treatment of respiratory diseases. From lung cancer to chronic obstructive pulmonary disease, the application of lung differentiation models will provide more precise treatment strategies for different types of respiratory diseases, thereby promoting the personalized and precise development of medicine. The application of lung differentiation models also contributes to evaluating the effects of environmental pollutants on respiratory health. By exposing in vitro cultured lung cells and tissue models to different environmental pollutants, we can simulate exposure scenarios in the real environment and assess the effects of these pollutants on pulmonary structure and function. This will help identify the toxicity mechanisms of pollutants, and implement environmental pollutant management to reduce the harm of environmental pollution to public health. The development and application of lung differentiation models not only improve the efficiency and precision of respiratory diseases treatment but also provide new ideas for environmental protection and health management. With the continuous development of technology and deepening understanding of the mechanisms, we can anticipate more innovative applications based on lung differentiation models. And new technology may provide new solutions and applications to major health and environmental challenges.
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences [grant number XDB0750300], the National Natural Science Foundation of China [grant numbers 22021003, 22476209 and 22193052], Beijing Municipal Natural Science Foundation [funding number IS23120], and Beijing Overseas Talents Center High-Level Foreign Talent Project [funding number C2022001]. Some figures were made using Figdraw.
The authors declare no competing financial interest.
References
- Basil M. C., Katzen J., Engler A. E., Guo M., Herriges M. J., Kathiriya J. J., Windmueller R., Ysasi A. B., Zacharias W. J., Chapman H. A., Kotton D. N., Rock J. R., Snoeck H. W., Vunjak-Novakovic G., Whitsett J. A., Morrisey E. E.. The Cellular and Physiological Basis for Lung Repair and Regeneration: Past, Present, and Future. Cell Stem Cell. 2020;26(4):482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C. E., Chung M. I., Fioret B., Gao X., Katsura H., Hogan B. L.. Lung organoids: current uses and future promise. Development. 2017;144(6):986–997. doi: 10.1242/dev.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikrama M., Usama M., Israr S., Humayon M.. Pulmonary fibrosis: Is stem cell therapy the way forward? J. Taibah Univ Med. Sci. 2024;19(1):82–89. doi: 10.1016/j.jtumed.2023.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The top 10 causes of death. World Health Organization, 2024. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Fernandes Q., Inchakalody V. P., Merhi M., Mestiri S., Taib N., Moustafa Abo El-Ella D., Bedhiafi T., Raza A., Al-Zaidan L., Mohsen M. O., Yousuf Al-Nesf M. A., Hssain A. A., Yassine H. M., Bachmann M. F., Uddin S., Dermime S.. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022;54(1):524–540. doi: 10.1080/07853890.2022.2031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N., Greek R., Greek J.. Are animal models predictive for humans? Philos. Ethics Humanit Med. 2009;4:2. doi: 10.1186/1747-5341-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M., Niklason L. E.. Human Pluripotent Stem Cells (iPSC) Generation, Culture, and Differentiation to Lung Progenitor Cells. Methods Mol. Biol. 2016;1576:55–92. doi: 10.1007/7651_2016_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M.. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S.. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Chandy M., Obal D., Wu J. C.. Elucidating effects of environmental exposure using human-induced pluripotent stem cell disease modeling. EMBO Mol. Med. 2022;14(11):e13260. doi: 10.15252/emmm.202013260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S.. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27(4):523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- Dye B. R., Hill D. R., Ferguson M. A., Tsai Y. H., Nagy M. S., Dyal R., Wells J. M., Mayhew C. N., Nattiv R., Klein O. D., White E. S., Deutsch G. H., Spence J. R.. In vitro generation of human pluripotent stem cell derived lung organoids. eLife. 2015;4(24):e05098. doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins F., Kotton D. N.. Embryonic and induced pluripotent stem cells for lung regeneration. Ann. Am. Thorac Soc. 2015;12:S50–3. doi: 10.1513/AnnalsATS.201410-457MG. [DOI] [PubMed] [Google Scholar]
- Lee J., Baek H., Jang J., Park J., Cha S. R., Hong S. H., Kim J., Lee J. H., Hong I. S., Wang S. J., Lee J. Y., Song M. H., Yang S. R.. Establishment of a human induced pluripotent stem cell derived alveolar organoid for toxicity assessment. Toxicol In Vitro. 2023;89:105585. doi: 10.1016/j.tiv.2023.105585. [DOI] [PubMed] [Google Scholar]
- Miller M. D., Marty M. A.. Impact of environmental chemicals on lung development. Environ. Health Perspect. 2010;118(8):1155–64. doi: 10.1289/ehp.0901856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Yin N., Faiola F.. Stem cell toxicology: a powerful tool to assess pollution effects on human health. National Science Review. 2016;3(4):430–450. doi: 10.1093/nsr/nww089. [DOI] [Google Scholar]
- Sivakumar A., Frank D. B.. Paradigms that define lung epithelial progenitor cell fate in development and regeneration. Current stem cell reports. 2019;5(4):133–144. doi: 10.1007/s40778-019-00166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackley C. R., Stripp B. R.. Building and maintaining the epithelium of the lung. J. Clin. Invest. 2012;122(8):2724–2730. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarr D. T., Morrisey E. E.. Lung Endoderm Morphogenesis: Gasping for Form and Function. Annual Review of Cell and Developmental Biology. 2015;31(1):553–573. doi: 10.1146/annurev-cellbio-100814-125249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtani Y., Azarnia M., Farrokhi A., Sharifi-Zarchi A., Aghdami N., Baharvand H.. Treatment of human embryonic stem cells with different combinations of priming and inducing factors toward definitive endoderm. Stem Cells Dev. 2013;22(9):1419–32. doi: 10.1089/scd.2012.0453. [DOI] [PubMed] [Google Scholar]
- Payne C., King J., Hay D.. The role of activin/nodal and Wnt signaling in endoderm formation. Vitam Horm. 2011;85:207–16. doi: 10.1016/B978-0-12-385961-7.00010-X. [DOI] [PubMed] [Google Scholar]
- Chen A. E., Borowiak M., Sherwood R. I., Kweudjeu A., Melton D. A.. Functional evaluation of ES cell-derived endodermal populations reveals differences between Nodal and Activin A-guided differentiation. Development. 2013;140(3):675–86. doi: 10.1242/dev.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A., Shinozaki K., Shannon J. M., Kouskoff V., Kennedy M., Woo S., Fehling H. J., Keller G.. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131(7):1651–62. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- D’Amour K. A., Agulnick A. D., Eliazer S., Kelly O. G., Kroon E., Baetge E. E.. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Teo A. K., Valdez I. A., Dirice E., Kulkarni R. N.. Comparable generation of activin-induced definitive endoderm via additive Wnt or BMP signaling in absence of serum. Stem Cell Reports. 2014;3(1):5–14. doi: 10.1016/j.stemcr.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujok O., Diekmann U., Lenzen S.. The generation of definitive endoderm from human embryonic stem cells is initially independent from activin A but requires canonical Wnt-signaling. Stem Cell Rev. Rep. 2014;10(4):480–93. doi: 10.1007/s12015-014-9509-0. [DOI] [PubMed] [Google Scholar]
- Demchenko A., Bukharova T., Kondrateva E., Tabakov V., Efremova A., Salikhova D., Goldshtein D., Balyasin M., Bulatenko N., Amelina E., Lavrov A., Smirnikhina S.. Airway and Lung Organoids from Human-Induced Pluripotent Stem Cells Can Be Used to Assess CFTR Conductance. Int. J. Mol. Sci. 2023;24(7):6293. doi: 10.3390/ijms24076293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S. K., Rana T. M.. Generation of 3D lung organoids from human induced pluripotent stem cells for modeling of lung development and viral infection. Heliyon. 2023;9(9):e19601. doi: 10.1016/j.heliyon.2023.e19601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsobaie S., Alsobaie T., Alshammary A., Mantalaris S.. Differentiation of human induced pluripotent stem cells into functional lung alveolar epithelial cells in 3D dynamic culture. Front. Bioeng. Biotechnol. 2023;11:1173149. doi: 10.3389/fbioe.2023.1173149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A., Sarmah H., Tanaka J., Hwang Y., Sawada A., Shimamura Y., Otoshi T., Kondo Y., Fang Y., Shimizu D., Ninish Z., Suer J. L., Dubois N. C., Davis J., Toyooka S., Wu J., Que J., Hawkins F. J., Lin C. S., Mori M.. Conditional blastocyst complementation of a defective Foxa2 lineage efficiently promotes the generation of the whole lung. eLife. 2023;12:e86105. doi: 10.7554/eLife.86105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess C. L., Huang J., Bawa P. S., Alysandratos K. D., Minakin K., Ayers L. J., Morley M. P., Babu A., Villacorta-Martin C., Yampolskaya M., Hinds A., Thapa B. R., Wang F., Matschulat A., Mehta P., Morrisey E. E., Varelas X., Kotton D. N.. Generation of human alveolar epithelial type I cells from pluripotent stem cells. Cell Stem Cell. 2024;31(5):657–675. doi: 10.1016/j.stem.2024.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. D., Chen A., Nostro M. C., d’Souza S. L., Schaniel C., Lemischka I. R., Gouon-Evans V., Keller G., Snoeck H. W.. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 2011;29(3):267–72. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H., Zhao R., Sherwood R., Ahfeldt T., Lapey A., Wain J., Sicilian L., Izvolsky K., Lau F. H., Musunuru K., Cowan C., Rajagopal J.. Generation of Multipotent Lung and Airway Progenitors from Mouse ESCs and Patient-Specific Cystic Fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. X., Islam M. N., O’Neill J., Hu Z., Yang Y. G., Chen Y. W., Mumau M., Green M. D., Vunjak-Novakovic G., Bhattacharya J., Snoeck H. W.. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014;32(1):84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori C., Ansari M., Angelidis I., Olmer R., Martin U., Theis F. J., Schiller H. B., Drukker M.. Human pluripotent stem cell fate trajectories toward lung and hepatocyte progenitors. iScience. 2023;26(11):108205. doi: 10.1016/j.isci.2023.108205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A. M., Tian Y., Tsukiyama T., Cohen E. D., Zhou D., Lu M. M., Yamaguchi T. P., Morrisey E. E.. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17(2):290–8. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson K. S., Domyan E. T., Vezina C. M., Sun X.. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. U. S. A. 2009;106(38):16287–92. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Desai T. J., Qian J., Niederreither K., Lu J., Cardoso W. V.. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134(16):2969–79. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Longmire T. A., Ikonomou L., Hawkins F., Christodoulou C., Cao Y., Jean J. C., Kwok L. W., Mou H., Rajagopal J., Shen S. S., Dowton A. A., Serra M., Weiss D. J., Green M. D., Snoeck H. W., Ramirez M. I., Kotton D. N.. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M., Morrisey E. E.. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141(3):502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp J. A., Morley M. P., Loebel C., Kremp M. M., Chaudhry F. N., Basil M. C., Leach J. P., Liberti D. C., Niethamer T. K., Ying Y., Jayachandran S., Babu A., Zhou S., Frank D. B., Burdick J. A., Morrisey E. E.. Genomic, epigenomic, and biophysical cues controlling the emergence of the lung alveolus. Science. 2021;371(6534):eabc3172. doi: 10.1126/science.abc3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias W. J., Frank D. B., Zepp J. A., Morley M. P., Alkhaleel F. A., Kong J., Zhou S., Cantu E., Morrisey E. E.. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555(7695):251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot L., Nathan N., Tabary O., Thouvenin G., Le Rouzic P., Corvol H., Amselem S., Clement A.. Alveolar epithelial cells: Master regulators of lung homeostasis. International Journal of Biochemistry & Cell Biology. 2013;45(11):2568–2573. doi: 10.1016/j.biocel.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Alysandratos K. D., Herriges M. J., Kotton D. N.. Epithelial Stem and Progenitor Cells in Lung Repair and Regeneration. Annu. Rev. Physiol. 2021;83:529–550. doi: 10.1146/annurev-physiol-041520-092904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A., Morley M., Hawkins F., McCauley K. B., Jean J. C., Heins H., Na C.-L., Weaver T. E., Vedaie M., Hurley K., Hinds A., Russo S. J., Kook S., Zacharias W., Ochs M., Traber K., Quinton L. J., Crane A., Davis B. R., White F. V., Wambach J., Whitsett J. A., Cole F. S., Morrisey E. E., Guttentag S. H., Beers M. F., Kotton D. N.. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell. 2017;21(4):472–488. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C. E., Cronce M. J., Rackley C. R., Bowie E. J., Keene D. R., Stripp B. R., Randell S. H., Noble P. W., Hogan B. L.. Type 2 alveolar cells are stem cells in adult lung. J. Clin Invest. 2013;123(7):3025–36. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquimia O. D., Petursdottir D. H., Periolo N., Fernandez C.. Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth. Infect. Immun. 2013;81(1):381–9. doi: 10.1128/IAI.00950-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui T., Wangpaichitr M., Schally A. V., Griswold A. J., Vidaurre I., Sha W., Jackson R. M.. Alveolar epithelial cell growth hormone releasing hormone receptor in alveolar epithelial inflammation. Exp Lung Res. 2023;49(1):152–164. doi: 10.1080/01902148.2023.2246074. [DOI] [PubMed] [Google Scholar]
- Chan M., Liu Y.. Function of epithelial stem cell in the repair of alveolar injury. Stem Cell Res. Ther. 2022;13:170. doi: 10.1186/s13287-022-02847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-W., Huang S. X., de Carvalho A. L. R. T., Ho S.-H., Islam M. N., Volpi S., Notarangelo L. D., Ciancanelli M., Casanova J.-L., Bhattacharya J., Liang A. F., Palermo L. M., Porotto M., Moscona A., Snoeck H.-W.. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 2017;19(5):542–549. doi: 10.1038/ncb3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiola F., Yin N., Yang R.. Environmental Toxicology: The Importance of Disease-Specific In Vitro Models. Environment & Health. 2024;2(2):65–67. doi: 10.1021/envhealth.3c00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel S. L., McVicar R. N., Winquist A. M., Niles W. D., Snyder E. Y.. Generation of Complete Multi-Cell Type Lung Organoids From Human Embryonic and Patient-Specific Induced Pluripotent Stem Cells for Infectious Disease Modeling and Therapeutics Validation. Curr. Protoc. Stem Cell Biol. 2020;54:e118. doi: 10.1002/cpsc.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. Y., Wu S., Wang D., Chu C., Hong Y., Tao M., Hu H., Xu M., Guo X., Liu Y.. Human organoids in basic research and clinical applications. Sig. Transduct. Target Ther. 2022;7:168. doi: 10.1038/s41392-022-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Gao J., Garcia I. M., Chen H. J., Castaldi A., Chen Y. W.. Human pluripotent stem cell-derived lung organoids: Potential applications in development and disease modeling. WIREs Dev. Biol. 2021;10(6):e399. doi: 10.1002/wdev.399. [DOI] [PubMed] [Google Scholar]
- Gonzales L. W., Guttentag S. H., Wade K. C., Postle A. D., Ballard P. L.. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am. J. Physiol Lung Cell Mol. Physiol. 2002;283(5):L940–51. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- Wang J., Edeen K., Manzer R., Chang Y., Wang S., Chen X., Funk C. J., Cosgrove G. P., Fang X., Mason R. J.. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am. J. Respir. Cell Mol. Biol. 2007;36(6):661–8. doi: 10.1165/rcmb.2006-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeckebier S., Mauritz C., Katsirntaki K., Sgodda M., Puppe V., Duerr J., Schubert S. C., Schmiedl A., Lin Q., Palecek J., Draeger G., Ochs M., Zenke M., Cantz T., Mall M. A., Martin U.. Keratinocyte growth factor and dexamethasone plus elevated cAMP levels synergistically support pluripotent stem cell differentiation into alveolar epithelial type II cells. Tissue Eng. Part A. 2013;19(7–8):938–51. doi: 10.1089/ten.tea.2012.0066. [DOI] [PubMed] [Google Scholar]
- Rodrigues Toste de Carvalho A. L., Liu H. Y., Chen Y. W., Porotto M., Moscona A., Snoeck H. W.. The in vitro multilineage differentiation and maturation of lung and airway cells from human pluripotent stem cell-derived lung progenitors in 3D. Nat. Protoc. 2021;16(4):1802–1829. doi: 10.1038/s41596-020-00476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yang R., Chen Y., Zhao X., Chen S., Yang X., Cheng Z., Hu B., Liang X., Yin N., Liu Q., Wang H., Liu S., Faiola F.. Development of human lung induction models for air pollutants’ toxicity assessment. Environ. Sci. Technol. 2021;55(4):2440–2451. doi: 10.1021/acs.est.0c05700. [DOI] [PubMed] [Google Scholar]
- Lim K., Donovan A. P. A., Tang W., Sun D., He P., Pett J. P., Teichmann S. A., Marioni J. C., Meyer K. B., Brand A. H., Rawlins E. L.. Organoid modeling of human fetal lung alveolar development reveals mechanisms of cell fate patterning and neonatal respiratory disease. Cell Stem Cell. 2023;30(1):20–37. doi: 10.1016/j.stem.2022.11.013. [DOI] [PubMed] [Google Scholar]
- Ohnishi Y., Masui A., Suezawa T., Mikawa R., Hirai T., Hagiwara M., Gotoh S.. Screening of factors inducing alveolar type 1 epithelial cells using human pluripotent stem cells. Stem Cell Rep. 2024;19:529. doi: 10.1016/j.stemcr.2024.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine K., Ohuchi H., Fujiwara M., Yamasaki M., Yoshizawa T., Sato T., Yagishita N., Matsui D., Koga Y., Itoh N., Kato S.. Fgf10 is essential for limb and lung formation. Nature genetics. 1999;21(1):138–41. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Johnson M. D., Vanderbilt J., Allen L., Gonzalez R.. The Great Big Alveolar TI Cell: Evolving Concepts and Paradigms. Cell Physiol. Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- Aspal M., Zemans R. L.. Mechanisms of ATII-to-ATI Cell Differentiation during Lung Regeneration. International Journal of Molecular Sciences. 2020;21(9):3188. doi: 10.3390/ijms21093188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Luo Y., Xiao M., Zhang Q., Luo J., Ma L., Ruan L., Lian N., Liu Y.. Mechanisms of Alveolar Type 2 Epithelial Cell Death During Acute Lung Injury. Stem Cells. 2023;41(12):1113–1132. doi: 10.1093/stmcls/sxad074. [DOI] [PubMed] [Google Scholar]
- Kanagaki S., Ikeo S., Suezawa T., Yamamoto Y., Seki M., Hirai T., Hagiwara M., Suzuki Y., Gotoh S.. Directed induction of alveolar type I cells derived from pluripotent stem cells via Wnt signaling inhibition. Stem Cells. 2021;39(2):156–169. doi: 10.1002/stem.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suezawa T., Kanagaki S., Moriguchi K., Masui A., Nakao K., Toyomoto M., Tamai K., Mikawa R., Hirai T., Murakami K., Hagiwara M., Gotoh S.. Disease modeling of pulmonary fibrosis using human pluripotent stem cell-derived alveolar organoids. Stem Cell Reports. 2021;16(12):2973–2987. doi: 10.1016/j.stemcr.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Gotoh S., Korogi Y., Seki M., Konishi S., Ikeo S., Sone N., Nagasaki T., Matsumoto H., Muro S., Ito I., Hirai T., Kohno T., Suzuki Y., Mishima M.. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods. 2017;14(11):1097–1106. doi: 10.1038/nmeth.4448. [DOI] [PubMed] [Google Scholar]
- Kim J.-H., Kim J., Kim W. J., Choi Y. H., Yang S.-R., Hong S.-H.. Diesel Particulate Matter 2.5 Induces Epithelial-to-Mesenchymal Transition and Upregulation of SARS-CoV-2 Receptor during Human Pluripotent Stem Cell-Derived Alveolar Organoid Development. International Journal of Environmental Research and Public Health. 2020;17(22):8410. doi: 10.3390/ijerph17228410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E., Shojaie S., Wang J., Tseu I., Ackerley C., Bilodeau M., Post M.. Three-Dimensional Culture and FGF Signaling Drive Differentiation of Murine Pluripotent Cells to Distal Lung Epithelial Cells. Stem Cells and Development. 2015;24(1):21–35. doi: 10.1089/scd.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard P. L., Lee J. W., Fang X., Chapin C., Allen L., Segal M. R., Fischer H., Illek B., Gonzales L. W., Kolla V., Matthay M. A.. Regulated gene expression in cultured type II cells of adult human lung. Am. J. Physiol Lung Cell Mol. Physiol. 2010;299(1):L36–50. doi: 10.1152/ajplung.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv P., Wang W., Cao Z., Zhao D., Zhao G., Li D., Qi L., Xu J.. Fsk and IBMX inhibit proliferation and proapoptotic of glioma stem cells via activation of cAMP signaling pathway. J. Cell Biochem. 2019;120(1):321–331. doi: 10.1002/jcb.27364. [DOI] [PubMed] [Google Scholar]
- Gao L., Sun Y., Zhang X., Ma D., Xie A., Wang E., Cheng L., Liu S.. Wnt3a-Loaded Extracellular Vesicles Promote Alveolar Epithelial Regeneration after Lung Injury. Adv. Sci. 2023;10(18):e2206606. doi: 10.1002/advs.202206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan A. N., Brownfield D. G., Harbury P. B., Krasnow M. A., Desai T. J.. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359(6380):1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner M., Yanuka O., Itskovitz-Eldor J., Melton D. A., Benvenisty N.. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97(21):11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. I., Bujnis M., Barkauskas C. E., Kobayashi Y., Hogan B. L. M.. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development. 2018;145(9):dev163014. doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCanna R., Liccardo D., Zhang P., Tragesser L., Wang Y., Cao T., Chapman H. A., Morrisey E. E., Shen H., Koch W. J., Kosmider B., Wolfson M. R., Tian Y.. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin Invest. 2019;129(5):2107–2122. doi: 10.1172/JCI125014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn J., Sottoriva K., Pajcini K. V., Kitajewski J. K., Chen C., Zhang W., Malik A. B., Liu Y.. Dlk1-Mediated Temporal Regulation of Notch Signaling Is Required for Differentiation of Alveolar Type II to Type I Cells during Repair. Cell Reports. 2019;26(11):2942–2954. doi: 10.1016/j.celrep.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemondy K. A., Jansing N. L., Jiang P., Redente E. F., Gillen A. E., Fu R., Miller A. J., Spence J. R., Gerber A. N., Hesselberth J. R., Zemans R. L.. Single cell RNA sequencing identifies TGFbeta as a key regenerative cue following LPS-induced lung injury. JCI Insight. 2019;4(8):e123637. doi: 10.1172/jci.insight.123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. L., Laiman V., Tsao P. N., Chen C. M., Heriyanto D. S., Chung K. F., Chuang K. J., Chuang H. C.. Diesel exhaust particles inhibit lung branching morphogenesis via the YAP/TAZ pathway. Sci. Total Environ. 2023;861:160682. doi: 10.1016/j.scitotenv.2022.160682. [DOI] [PubMed] [Google Scholar]
- Balda M. S., Matter K.. Tight junctions. Journal of Cell Science. 1998;111:541–547. doi: 10.1242/jcs.111.5.541. [DOI] [PubMed] [Google Scholar]
- Leiby K. L., Raredon M. S. B., Niklason L. E.. Bioengineering the Blood-gas Barrier. Compr. Physiol. 2020;10(2):415–452. doi: 10.1002/j.2040-4603.2020.tb00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons S., Erfinanda L., Bartz C., Kuebler W. M.. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J. Physiol. 2019;597(4):997–1021. doi: 10.1113/JP276245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N., Strilic B., Offermanns S.. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol Rev. 2019;99(3):1467–1525. doi: 10.1152/physrev.00037.2018. [DOI] [PubMed] [Google Scholar]
- Li J., Qi Z., Li D., Huang X., Qi B., Feng J., Qu J., Wang X.. Alveolar epithelial glycocalyx shedding aggravates the epithelial barrier and disrupts epithelial tight junctions in acute respiratory distress syndrome. Biomed Pharmacother. 2021;133:111026. doi: 10.1016/j.biopha.2020.111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuner E. V., Mortensen J., Møller P., Bernard A., Vinzents P., Wåhlin P., Glasius M., Loft S.. Effects of ambient air particulate exposure on blood-gas barrier permeability and lung function. Inhal. Toxicol. 2009;21(1):38–47. doi: 10.1080/08958370802304735. [DOI] [PubMed] [Google Scholar]
- von Eckardstein A., Rohrer L.. Transendothelial lipoprotein transport and regulation of endothelial permeability and integrity by lipoproteins. Curr. Opin Lipidol. 2009;20(3):197–205. doi: 10.1097/MOL.0b013e32832afd63. [DOI] [PubMed] [Google Scholar]
- Hermanns M. I., Unger R. E., Kehe K., Peters K., Kirkpatrick C. J.. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab Invest. 2004;84(6):736–52. doi: 10.1038/labinvest.3700081. [DOI] [PubMed] [Google Scholar]
- Wei M., Cong Y., Lei J., Du R., Yang M., Lu X., Jiang Y., Cao R., Meng X., Jiang Z., Song L.. The role of ROS-pyroptosis in PM(2.5) induced air-blood barrier destruction. Chem. Biol. Interact. 2023;386:110782. doi: 10.1016/j.cbi.2023.110782. [DOI] [PubMed] [Google Scholar]
- Dohle E., Singh S., Nishigushi A., Fischer T., Wessling M., Möller M., Sader R., Kasper J., Ghanaati S., Kirkpatrick C. J.. Human Co- and Triple-Culture Model of the Alveolar-Capillary Barrier on a Basement Membrane Mimic. Tissue Eng. Part C Methods. 2018;24(9):495–503. doi: 10.1089/ten.tec.2018.0087. [DOI] [PubMed] [Google Scholar]
- Valavanidis A., Vlachogianni T., Fiotakis K., Loridas S.. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health. 2013;10(9):3886–907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A., de Souza Carvalho-Wodarz C., Seabra V., Sarmento B., Lehr C. M.. Triple co-culture of human alveolar epithelium, endothelium and macrophages for studying the interaction of nanocarriers with the air-blood barrier. Acta Biomater. 2019;91:235–247. doi: 10.1016/j.actbio.2019.04.037. [DOI] [PubMed] [Google Scholar]
- Licciardello M., Sgarminato V., Ciardelli G., Tonda-Turo C.. Development of biomimetic co-culture and tri-culture models to mimic the complex structure of the alveolar-capillary barrier. Biomater Adv. 2023;154:213620. doi: 10.1016/j.bioadv.2023.213620. [DOI] [PubMed] [Google Scholar]
- Ma Y., Deng B., He R., Huang P.. Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication. Heliyon. 2024;10(3):e24593. doi: 10.1016/j.heliyon.2024.e24593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro Carpio M., Dabaghi M., Ungureanu J., Kolb M. R., Hirota J. A., Moran-Mirabal J. M.. 3D Bioprinting Strategies, Challenges, and Opportunities to Model the Lung Tissue Microenvironment and Its Function. Front Bioeng Biotechnol. 2021;9:773511. doi: 10.3389/fbioe.2021.773511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu Y., Shu C., Shen Y., Li M., Ma N., Zhao J.. 3D bioprinting of the airways and lungs for applications in tissue engineering and in vitro models. Journal of tissue engineering. 2024;15:1–22. doi: 10.1177/20417314241309183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Pati F., Choi Y. J., Rijal G., Shim J. H., Kim S. W., Ray A. R., Cho D. W., Ghosh S.. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233–46. doi: 10.1016/j.actbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- De Spirito M., Palmieri V., Perini G., Papi M.. Bridging the Gap: Integrating 3D Bioprinting and Microfluidics for Advanced Multi-Organ Models. Biomedical Research. 2024;11(7):664. doi: 10.3390/bioengineering11070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murkar R. S., Wiese-Rischke C., Weigel T., Kopp S., Walles H.. Developing human upper, lower, and deep lung airway models: Combining different scaffolds and developing complex co-cultures. J. Tissue Eng. 2025;16:1–22. doi: 10.1177/20417314241299076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Xu M., Cui X., Yin J., Wu Q.. Hybrid 3D Bioprinting of Sustainable Biomaterials for Advanced Multiscale Tissue Engineering. Small. 2025:2408947. doi: 10.1002/smll.202408947. [DOI] [PubMed] [Google Scholar]
- Prescott S. L.. Effects of early cigarette smoke exposure on early immune development and respiratory disease. Paediatr Respir Rev. 2008;9(1):3–9. doi: 10.1016/j.prrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Luyten L. J., Saenen N. D., Janssen B. G., Vrijens K., Plusquin M., Roels H. A., Debacq-Chainiaux F., Nawrot T. S.. Air pollution and the fetal origin of disease: A systematic review of the molecular signatures of air pollution exposure in human placenta. Environmental Research. 2018;166:310–323. doi: 10.1016/j.envres.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li A., Buchanan S., Liu W.. Exposure characteristics for congeners, isomers, and enantiomers of perfluoroalkyl substances in mothers and infants. Environ. Int. 2020;144:106012. doi: 10.1016/j.envint.2020.106012. [DOI] [PubMed] [Google Scholar]
- Loukas N., Vrachnis D., Antonakopoulos N., Pergialiotis V., Mina A., Papoutsis I., Iavazzo C., Fotiou A., Stavros S., Valsamakis G., Vlachadis N., Maroudias G., Mastorakos G., Iliodromiti Z., Drakakis P., Vrachnis N.. Prenatal Exposure to Bisphenol A: Is There an Association between Bisphenol A in Second Trimester Amniotic Fluid and Fetal Growth? Medicina. 2023;59(5):882. doi: 10.3390/medicina59050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. P., Devadoss D., Manevski M., Sheybani A., Ivanciuc T., Exil V., Agarwal H., Raizada V., Garofalo R. P., Chand H. S., Sopori M. L.. Gestational Exposure to Cigarette Smoke Suppresses the Gasotransmitter H2S Biogenesis and the Effects Are Transmitted Transgenerationally. Front. Immunol. 2020;11:1628. doi: 10.3389/fimmu.2020.01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Bennett E., Wheeler A. J., Lyons A. B., Woods G. M., Johnston F., Zosky G. R.. Maternal exposure to particulate matter alters early post-natal lung function and immune cell development. Environmental Research. 2018;164:625–635. doi: 10.1016/j.envres.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Wang R., Kang N., Zhang W., Chen B., Xu S., Wu L.. The developmental toxicity of PM2.5 on the early stages of fetal lung with human lung bud tip progenitor organoids. Environ. Pollut. 2023;330:121764. doi: 10.1016/j.envpol.2023.121764. [DOI] [PubMed] [Google Scholar]
- Kung Y.-P., Lin C.-C., Chen M.-H., Tsai M.-S., Hsieh W.-S., Chen P.-C.. Intrauterine exposure to per- and polyfluoroalkyl substances may harm children’s lung function development. Environmental Research. 2021;192:110178. doi: 10.1016/j.envres.2020.110178. [DOI] [PubMed] [Google Scholar]
- Mahemuti L., Chen Q., Coughlan M. C., Qiao C., Chepelev N. L., Florian M., Dong D., Woodworth R. G., Yan J., Cao X.-l., Scoggan K. A., Jin X., Willmore W. G.. Bisphenol A induces DSB-ATM-p53 signaling leading to cell cycle arrest, senescence, autophagy, stress response, and estrogen release in human fetal lung fibroblasts. Arch. Toxicol. 2018;92(4):1453–1469. doi: 10.1007/s00204-017-2150-3. [DOI] [PubMed] [Google Scholar]
- Mullassery D., Smith N. P.. Lung development. Seminars in Pediatric Surgery. 2015;24(4):152–155. doi: 10.1053/j.sempedsurg.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Xiao K., Li X., Xu N., Wang X., Hao L., Bao H., Zhang L., Shi Y., Cai Y.. Carry-over rate of per- and polyfluoroalkyl substances to raw milk and human exposure risks in different regions of China. Sci. Total Environ. 2024;944:173902. doi: 10.1016/j.scitotenv.2024.173902. [DOI] [PubMed] [Google Scholar]
- Figueiredo T. M., Santana J. D. M., Granzotto F. H. B., Anjos B. S. D., Guerra Neto D., Azevedo L. M. G., Pereira M.. Pesticide contamination of lactating mothers’ milk in Latin America: a systematic review. Rev. Saude Publica. 2024;58:19. doi: 10.11606/s1518-8787.2024058005446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumeistere L., Belusko A., Ciprovica I.. Assessment of Heavy Metals and Trace Elements in the Human Milk of Women Living in Latvia and an Evaluation of Influencing Factors. Nutrients. 2024;16(11):1568. doi: 10.3390/nu16111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decheng S., Zhanteng S., Zhiming X., Yang L., Wuyan L., Junming W., Xia F.. Trace analysis of 20 antihistamines in milk by ultrahigh performance liquid chromatography coupled with high field quadrupole orbitrap high resolution mass spectrometry followed dispersive micro solid phase extraction. J. Chromatogr A. 2024;1727:464989. doi: 10.1016/j.chroma.2024.464989. [DOI] [PubMed] [Google Scholar]
- Mendas G., Jakovljevic I., Romanic S. H., Fingler S., Jovanovic G., Saric M. M., Pehnec G., Popovic A., Stankovic D.. Presence of polycyclic aromatic hydrocarbons and persistent organochlorine pollutants in human Milk: Evaluating their levels, association with Total antioxidant capacity, and risk assessment. Sci. Total Environ. 2024;931:172911. doi: 10.1016/j.scitotenv.2024.172911. [DOI] [PubMed] [Google Scholar]
- Cheng X., Gao L., Cao X., Zhang Y., Ai Q., Weng J., Liu Y., Li J., Zhang L., Lyu B., Wu Y., Zheng M.. Identification and Prioritization of Organic Pollutants in Human Milk from the Yangtze River Delta, China. Environ. Sci. Technol. 2024;58:11935. doi: 10.1021/acs.est.4c02909. [DOI] [PubMed] [Google Scholar]
- LaKind J. S., Naiman J., Verner M. A., Leveque L., Fenton S.. Per- and polyfluoroalkyl substances (PFAS) in breast milk and infant formula: A global issue. Environ. Res. 2023;219:115042. doi: 10.1016/j.envres.2022.115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Carrasco I., Carbonero-Aguilar P., Dahiri B., Moreno I. M., Hinojosa M.. Comparison between pollutants found in breast milk and infant formula in the last decade: A review. Sci. Total Environ. 2023;875:162461. doi: 10.1016/j.scitotenv.2023.162461. [DOI] [PubMed] [Google Scholar]
- Pajurek M., Mikolajczyk S., Warenik-Bany M.. Occurrence and dietary intake of dioxins, furans (PCDD/Fs), PCBs, and flame retardants (PBDEs and HBCDDs) in baby food and infant formula. Sci. Total Environ. 2023;903:166590. doi: 10.1016/j.scitotenv.2023.166590. [DOI] [PubMed] [Google Scholar]
- Souza M. C. O., Souza J. M. O., da Costa B. R. B., Gonzalez N., Rocha B. A., Cruz J. C., Guida Y., Souza V. C. O., Nadal M., Domingo J. L., Barbosa F.. Levels of organic pollutants and metals/metalloids in infant formula marketed in Brazil: Risks to early-life health. Food Res. Int. 2023;174:113594. doi: 10.1016/j.foodres.2023.113594. [DOI] [PubMed] [Google Scholar]
- Kadac-Czapska K., Jutrzenka Trzebiatowska P., Mazurkiewicz M., Kowalczyk P., Knez E., Behrendt M., Mahlik S., Zaleska-Medynska A., Grembecka M.. Isolation and identification of microplastics in infant formulas - A potential health risk for children. Food Chem. 2024;440:138246. doi: 10.1016/j.foodchem.2023.138246. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang Y., Zhu H., Lu S., Wang Y., Xue J., Zhang T., Kannan K., Sun H.. Infantile Internal and External Exposure to Neonicotinoid Insecticides: A Comparison of Levels across Various Sources. Environ. Sci. Technol. 2023;57(13):5358–5367. doi: 10.1021/acs.est.2c09538. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Xiao J., Zhang D., Li X., Xu J., Ma J., Xiao Q., Fu J., Guo Z., Zhu Y., Ji J., Lu S.. Bisphenol analogues in infant foods in south China and implications for infant exposure. Sci. Total Environ. 2024;910:168509. doi: 10.1016/j.scitotenv.2023.168509. [DOI] [PubMed] [Google Scholar]
- Hatzidaki E., Pagkalou M., Katsikantami I., Vakonaki E., Kavvalakis M., Tsatsakis A. M., Tzatzarakis M. N.. Endocrine-Disrupting Chemicals and Persistent Organic Pollutants in Infant Formulas and Baby Food: Legislation and Risk Assessments. Foods. 2023;12(8):1697. doi: 10.3390/foods12081697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Wang J., Chen C., Song Y., Pan J.. Transcriptomic analysis of key genes and pathways in human bronchial epithelial cells BEAS-2B exposed to urban particulate matter. Environmental Science and Pollution Research. 2021;28(8):9598–9609. doi: 10.1007/s11356-020-11347-1. [DOI] [PubMed] [Google Scholar]
- Korten I., Ramsey K., Latzin P.. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. 2017;21:38–46. doi: 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Wang L., Pinkerton K. E.. Air pollutant effects on fetal and early postnatal development. Birth Defects Res. C Embryo Today. 2007;81(3):144–54. doi: 10.1002/bdrc.20097. [DOI] [PubMed] [Google Scholar]
- Johnson M., Mazur L., Fisher M., Fraser W. D., Sun L., Hystad P., Gandhi C. K.. Prenatal Exposure to Air Pollution and Respiratory Distress in Term Newborns: Results from the MIREC Prospective Pregnancy Cohort. Environ. Health Perspect. 2024;132(1):017007. doi: 10.1289/EHP12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakchi J., Rasel R. A., Shammi K. F., Ferdous S., Sultana S., Rabeya M. R.. Effect of housing construction material on childhood acute respiratory infection: a hospital based case control study in Bangladesh. Sci. Rep. 2024;14:8163. doi: 10.1038/s41598-024-57820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F., Zhong X., Ye Y., Liu X., He G., Wu C., Chen Z., Zhu Q., Yu S., Fan J., Yao H., Ma W., Dong X., Liu T.. Mutual associations of exposure to ambient air pollutants in the first 1000 days of life with asthma/wheezing in children: a prospective cohort study in Guangzhou, China. JMIR Preprints. 2024;10:e52456. doi: 10.2196/52456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukeren M.. Comparison of urine bisphenol A levels in transient tachypnea of the newborn and healthy newborns. Turk J. Pediatr. 2023;65(1):35–45. doi: 10.24953/turkjped.2022.304. [DOI] [PubMed] [Google Scholar]
- Mendy A., Percy Z., Braun J. M., Lanphear B., La Guardia M. J., Hale R., Yolton K., Chen A.. Exposure to dust organophosphate and replacement brominated flame retardants during infancy and risk of subsequent adverse respiratory outcomes. Environ. Res. 2023;235:116560. doi: 10.1016/j.envres.2023.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C. M., Riha R. L.. Environment and lung health in a rapidly changing world. Eur. Respir Rev. 2024;33(172):240057. doi: 10.1183/16000617.0057-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Wu X., Tan Z., Ren Y., Li L., Ou J., Lin Y., Song H., Feng L., Seto D., Wu J., Zhang Q., Rong Z.. Generation of Human Embryonic Stem Cell-Derived Lung Organoids for Modeling Infection and Replication Differences between Human Adenovirus Types 3 and 55 and Evaluating Potential Antiviral Drugs. J. Virol. 2023;97(5):e00209-23. doi: 10.1128/jvi.00209-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B. N.. Alveolar macrophage in the driver’s seat. Immunity. 2006;24(4):366–8. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Guo X., Lin Y., Lin Y., Zhong Y., Yu H., Huang Y., Yang J., Cai Y., Liu F., Li Y., Zhang Q. Q., Dai J.. PM2.5 induces pulmonary microvascular injury in COPD via METTL16-mediated m6A modification. Environ. Pollut. 2022;303:119115. doi: 10.1016/j.envpol.2022.119115. [DOI] [PubMed] [Google Scholar]
- Leikauf G. D., Kim S. H., Jang A. S.. Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol. Med. 2020;52(3):329–337. doi: 10.1038/s12276-020-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W., Hu S., Li C., Ma H., Wang Q., Meng G., Guo T., Zhang J.. Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer. Biomed Res. Int. 2019;2019:2025636. doi: 10.1155/2019/2025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson C. E., Kesic M. J., Hernandez M. L.. Ozone in the development of pediatric asthma and atopic disease. Immunology and Allergy Clinics of North America. 2022;42(4):701–713. doi: 10.1016/j.iac.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. A.. Pesticide exposure-Egyptian scene. Toxicology. 2004;198(1–3):91–115. doi: 10.1016/j.tox.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Lan C. H., Ou L. C., Liu H. H., Peng C. Y.. Investigation of metal concentration distribution and corresponding health exposure assessment of fabricated metal product manufacturers. Sci. Rep. 2024;14:13662. doi: 10.1038/s41598-024-64277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu Y.. Epithelial stem cells and niches in lung alveolar regeneration and diseases. Chin Med. J. Pulm Crit Care Med. 2024;2(1):17–26. doi: 10.1016/j.pccm.2023.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola H., Washington K., Selva C., Grunwell J., Tirouvanziam R., Takayama S.. A High-Throughput Distal Lung Air-Blood Barrier Model Enabled By Density-Driven Underside Epithelium Seeding. Adv. Healthc. Mater. 2021;10(15):2100879. doi: 10.1002/adhm.202100879. [DOI] [PMC free article] [PubMed] [Google Scholar]




