Abstract
Volatile methylsiloxanes (VMS) are a group of synthetic chemical compounds broadly used in industrial applications and consumer products, leading to a sharply increased global emission through diverse pathways. Consequently, human exposure to VMS through inhalation or other routes poses potential health risks. To provide insights for environmental contamination by VMS and its concerning health risks, a systematic literature search was conducted in online databases, including the Web of Science, PubMed, Elsevier ScienceDirect, and the China National Knowledge Infrastructure. This review analyzed contamination levels of VMS in various environmental matrices, including air, dust, water, and soil. It further summarized health risk assessments for different external exposure pathways. The exacerbation of VMS pollution is predominantly linked to the increase in atmospheric concentrations, resulting in health risks primarily driven by inhalation exposure. Vulnerable groups require greater attention as their daily intake levels may potentially approach the reference dose, including occupational populations and children residing in areas near factories. Currently, data on long-term and simultaneous environmental monitoring of VMS remain limited. Furthermore, there is a lack of established exposure guidance values, due to insufficient toxicity data and limited risk quantification methods. Therefore, advancing monitoring technologies and networks for VMS and their transformation products is crucial. Future efforts should prioritize integrating advanced tools, such as mixture toxicity models, to achieve more accurate quantification of health risks under complex exposure scenarios. Overall, this review sheds light on developing and revising regulatory frameworks for governing the production and handling of VMS, as well as guiding the health risk assessment.
Keywords: Volatile Methylsiloxanes (VMS), Emerging Pollutant, Environmental Fate, Exposure Assessments, Health Risk Assessment
1. Introduction
Methylsiloxanes (MS) are organo-inorganic copolymers that structurally consist of alternating silicon–oxygen (Si–O) units as the inorganic backbone and hydrophobic organic groups (methyl groups) as side chains. They are the dominant type of organosilicon compounds, accounting for the majority of the total usage (>90%). Based on the configuration of the main chain, MS can be categorized into cyclic (cMS, Dn) and linear methylsiloxanes (lMS, Ln), where n is the number of silicon atoms. So far, 38 known types of MS, including 23 cMS (D3–D25) and 15 lMS (L2–L16), have been reported. Volatile methylsiloxanes (VMS) are a subset of MS with a relatively low molecular weight (<600 AMU), comprising 6 cVMS (D3–D8) and 7 lVMS (L2–L8). Due to the low flammability, low surface tension, and excellent electrical insulation properties, VMS are highly promising for the use in coatings, sealants, antifouling agents, and other fields, , with the largest applications and sales coming from the fields of construction, industry, and personal care products (PCPs). Among them, D4 is the dominant VMS covering 99.5% of usage in the production of organosilicon polymer products, presenting a valuable role in industrial manufacturing. , VMS are an emerging pollutant, which have been greatly consumed on a global scale, serving as indispensable man-made chemical compounds for the functioning of modern society. China, America, and Europe are the primary market regions with the highest consumption rates. With the rapid development of the organosilicon industry, global production of VMS reached 800,000–1,000,000 tons in 2017, and the global cVMS market was approximately 17.2 billion USD. Since 2009, China has become the leading country of organosilicon production and consumption worldwide.
Nevertheless, VMS are a potentially hazardous pollutant, and their broad industrial applications have increasingly posed an environmental burden. The bioaccumulative properties and long-range transport potential of VMS greatly threat human health. In recent years, VMS have been classified as substances of concern (SoC) in several countries and regions (e.g., Canada, the United Kingdom, and the European Union). In 2018, the European Chemicals Agency (ECHA) has identified D4, D5, and D6 as Substances of Very High Concern (SVHC). The Stockholm Convention on Persistent Organic Pollutants in 2023 made a proposal to list D4, D5, and D6 in Annex B. Toxicological evidence has illustrated the potential of cVMS in eliciting carcinogenesis, neurotoxicity, reproductive and developmental hazards, and immunotoxicity. − D4 is especially concerned for its property as an endocrine-disrupting chemical. VMS can enter the human body through various pathways to adversely affect health. As a result, it is highly urgent and essential to assess VMS-induced health risks.
Environmental analytical methods, environmental fate, and ecotoxicity of VMS have been previously described. Homem et al. overviewed the development trends in analyzing VMS concentrations in environmental samples using gas chromatography–mass spectrometry (GC-MS) and the major challenges. Xiang et al. summarized the environmental distribution of siloxanes and modified siloxanes, along with their migration and transformation in the environment. Feng et al. reviewed the exposure pathways of MS to humans. Sun et al. particularly described the ecotoxicity of cVMS. However, there lacks a systematic review focusing on the current contamination status and health risk assessment of VMS.
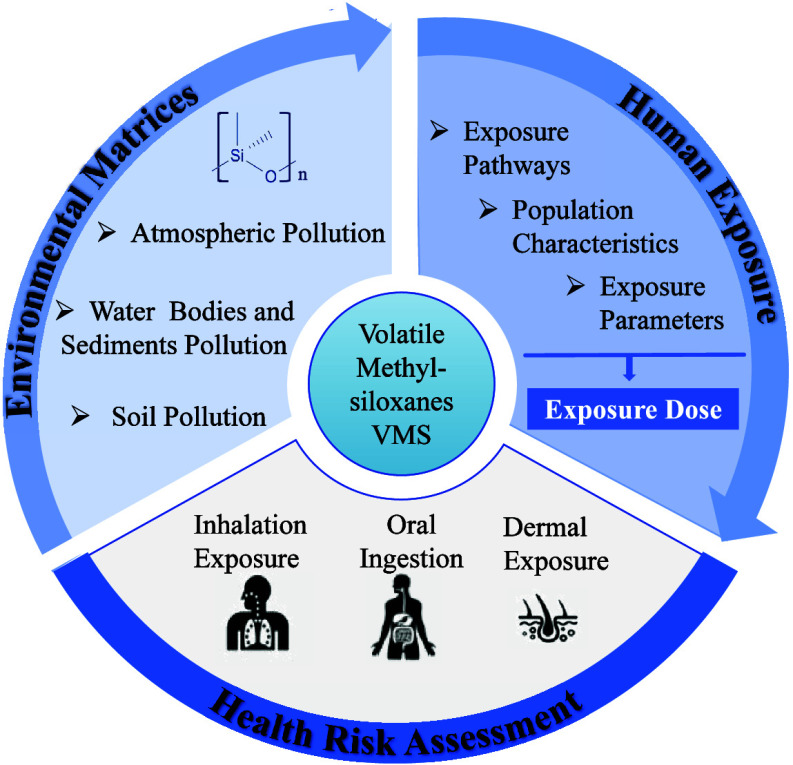
In the present study, we conducted a literature review on landscaping the environmental contamination of VMS emissions, varying levels of VMS in environmental matrices, and health risk assessment of VMS via analysis in three databases: Web of Science, Scopus, PubMed, and China National Knowledge Infrastructure. After excluding articles originated from the field of materials science, solely reporting ecotoxicity assessments or the technological advancements in VMS detection, 59 eligible research articles were finally included in our review (Figure ), involving 28 reported VMS measurements in atmospheric, 15 in water, 10 in wastewater treatment plant, and 6 in soils (Figure ). Among them, 19 studies reported environmental concentrations while simultaneously conducting health risk assessments. With a growing emphasis on human health, the volume of articles related to environmental pollution of VMS has notably increased.
1.

A flowchart of the literature screen.
2.
Temporal trends of publications about environmental pollution of MS and health risk assessments (A) and detection of VMS in different environmental matrices (B).
This review systematically examines the environmental contamination of VMS, their distribution in various environmental matrices, including air, dust and particles, water, and soil. It further provides a comprehensive and systematic description of the health risks to humans, encompassing risk assessments for different exposure routes, including inhalation, oral ingestion, and dermal contact. It advances understanding of VMS pollution and its health implications, offering valuable insights to guide future research, regulatory measures, and policy-making efforts in mitigating their environmental and health impacts.
2. Current Contamination Status of VMS
2.1. Atmospheric Pollution
Due to the hydrophobicity and volatility of VMS, the atmosphere applies the primary environmental transport and exposure pathway. Approximately 90% of VMS enters the atmosphere through volatilization, and the remaining is discharged into wastewater treatment plants (WWTPs) and landfills via wastewater and solid waste disposal, respectively. , Consequently, the atmosphere serves as the main medium favoring the transport, transformation, and migration of VMS in the environmental cycling. The sources of VMS pollution in outdoor air can be divided into direct and indirect emissions. Direct emissions of VMS include those from industrial facilities and human activities: substantial volatilization of VMS in industrial production is the chief criminal for increased environmental concentrations in industrial sites and surrounding areas compared to typical urban areas. VMS are generally consumed in PCPs, construction, and transportation industries. , Besides, VMS can be indirectly emitted into the atmosphere from the landfill gas in WWTPs and landfills or water bodies via a secondary volatilization. Indoor atmospheric pollution of VMS primarily originates from short-term emissions during cleaning and personal care activities using VMS-containing products, such as cosmetics, PCPs, household cleaners, and polishes.
The atmospheric concentrations of VMS vary with influencing factors and spatiotemporal patterns. Photochemical oxidants are dominantly responsible for the degradation of VMS in the atmosphere, and consequently, there is a significant correlation between the outdoor concentration of VMS and the abundance of hydroxyl radicals in the atmosphere. Circadianly, concentrations of VMS at night are higher than the daytime. , Seasonally, concentrations of VMS in winter are higher than other seasons.
2.1.1. VMS in the Gaseous Phase
VMS primarily exists in the gaseous phase. Due to the high volatility and long-range transport potential, VMS can be widely distributed on a global scale. − Geographically, VMS in the gaseous phase is more popular in remote regions, such as the polar areas (1.6 × 10–3–8.9 ng/m3) and the Tibetan Plateau (18.4–565.3 ng/m3). , Notably, a long-distance transport potential of VMS in influencing global environmental quality should be highly concerned. D3–D6 are predominantly responsible for the outdoor atmospheric pollution of VMS, accounting for 75%–100% in varied regions. Among them, D5 is the most frequently detected VMS presenting the highest concentration in most locations. In contrast, L3–L5 are less detected with one-tenth concentrations of D3–D6, accounting for 20%–65% of total VMS. Regional variations in the concentrations of VMS greatly increase the complexity of the environmental distribution. A multinational study of outdoor air monitoring involving 41 countries has shown that concentrations of Σ4cVMS (D3–D6) range from 1.3 ng/m3 to 452.0 ng/m3, while Σ3lVMS (L3–L5) range from the limit of detection (LOD) to 12.1 ng/m3. In urban areas of southwestern China, concentrations of Σ3cVMS (D4–D6) even inflate to 77.0–1,400 ng/m3. A broad variation but systematic decline in the concentrations of Σ4cVMS (D3–D6) is observed in Japan, with 360–518 ng/m3 in the Saitama Prefecture, 352 ng/m3 in rural areas, and 105 ng/m3 in mountainous territories. Despite the high detection frequency for several Σ6lVMS (L3–L8), exceeding 90%, their combined concentration of Σ6lVMS (L3–L8) and other Σ7lMS (L9–L15) accounts for less than 4% of the targeted compounds in all regions, further indicating a dominance of cVMS. A significant regional variation in outdoor gaseous concentrations of VMS can be attributed to differences in population density and industrial activities, leading to higher concentrations of VMS in urban atmospheres than rural and other remote areas. Therefore, industrialization and urbanization greatly challenge the environment pollution of VMS.
Xiang et al. estimated that the majority of the VMS (90%) added to PCPs is released into indoor air during the usage, making it one of the primary sources of indoor VMS. Astonishingly, there reaches a 100% detection rate of cVMS (D4–D6) in indoor air samples collected from urban households in the United States, Canada, and several European countries, with Σ3cVMS concentrations ranging from 2.2 μg/m3 to 940.0 μg/m3. ,, In contrast, gaseous concentrations of Σ3cVMS (D4–D6) in indoor air from households in some northern and southwestern Chinese cities are much lower at 4.5 × 10–2–19.0 μg/m3. , The concentration of D5 alone in households from the United Kingdom and Barcelona can reach 23.0–736.0 μg/m3, highlighting the special concern of D5 among the various VMS compounds. , Sha et al. investigated the contamination of four categories of emerging pollutants in indoor air in Sweden. They confirmed VMS as great contributors to indoor air pollution, with an average concentration of ∑3cVMS (D4–D6) ranging from 0.1 μg/m3 to 3.6 μg/m3, and surprisingly 1,000 times higher than perfluoroalkyl alcohols, brominated flame retardants, and organophosphorus flame retardants. Due to the frequent use of VMS-containing products and an enclosed space, indoor concentrations of VMS are typically higher than outdoors on a scale of magnitude from the micrograms (μg/m3) to nanograms per cubic meter (ng/m3). Furthermore, the indoor contamination of VMS even exacerbates the existing indoor air quality through the generation of oxidative products secondary organic aerosols (SOA) that increases the environmental risks under low-level nitric oxide (NO). Measurement and characterization of VMS-derived SOA, however, are challenging because of the low concentrations, lack of authentic standards, and difficulties in complex environmental matrices. , To strictly standardize the monitoring of VMS pollution in the environment, some European countries have established guidelines for Σ2cVMS (D4, D5) concentrations in indoor air.. Differences in indoor concentrations of VMS across regions may be attributed to variations in PCP formulations, usage habits, and indoor ventilation conditions. Pollution levels of VMS in the gaseous phase across regions are summarized in Table .
1. Concentrations of VMS in the Gaseous Phase across Regions .
| Site | Time | Sample Size | Region | Method | LOD (ng/m3) | Types of cVMS | ΣcVMS (μg/m3) | Types of lVMS | ΣlMS (μg/m3) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Inside the factory workshop | 2021 | 42 | WWTP in Beijing, China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.09–0.11 | D4–D6 | 1.9–17 , | | | |
| 42 | WWTP in Kunming, China | 1.7–14 , | ||||||||
| 2017 | 21 | Construction sites, and automobile and paint factories in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.17–0.37 | D4–D6 | 340–890 | L5–L8 | 0.02–13 | ||
| 2012 | 14 | Construction sites, and automobile, paint and textile factories in Northern China | Agilent 7890/5975C GC-MS | 0.14–0.36 | D4–D6 | 58.6–570 | L5–L8 | 0.05–11 | ||
| 2011 | 35 | Siloxane manufacturing plants in Shandong, China | Agilent 7890/5975C GC-MS | 0.14–0.36 | D4–D6 | 34–2,700 | L5, L6 | 1.2–14.3 | ||
| 2018 | 50 | Automobile, paint and textile factories in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.18–0.37 | D4–D6 | 240–500 | | | ||
| Inside the factory dormitory | 2017 | 105 | Construction sites, and automobile and paint factories in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.17–0.37 | D4–D6 | 170–420 | L5–L8 | 0.29–8.7 | |
| 2019–2020 | 14 | Hanoi, Vietnam | Agilent 7890 GC-MS | 0.12 | D4–D6 | 0.0279–0.657 | | | ||
| Residential premises | 2016–2017 | 75 | Hanoi, Vietnam | Agilent 7890/5977A GC-MS | 0.04–0.25 | D3–D6 | 0.0019–0.717 | L4–L9 | 0.031–0.817 | |
| 2015 | 85 | Oven-baking process in the kitchen, Munich, Germany | GCMS-QP2010 SE Single Quadrupole GC-MS | 250 | D3–D9 | Low-pollution molds:<LOD-23.6 | | | ||
| 2015 | 85 | Oven-baking process in the kitchen, Munich, Germany | GCMS-QP2010 SE Single Quadrupole GC-MS | 250 | D3–D9 | High-pollution molds:4.1–599 | | | ||
| 2014 | 60 | Albany, USA | Agilent 6890/5973 GC-MS | 0.06–0.83 | D3–D7 | 0.041–1.49 | L3–L8 | ND–0.705 | ||
| 2021 | 54 | within 1500 m of refinery, Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.30–0.35 | D4–D6 | 12–75 | | | ||
| 2017 | 68 | Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.17–0.37 | D4–D6 | 1.7- 23 | L3–L16 | ND | ||
| 2012 | 60 | Southwest China | Agilent 7890A/5975C GC-MS | 0.14–0.36 | D4–D6 | 0.045–0.14 | L4–L11 | ND | ||
| 2019–2020 | 16 | Laboratories in Hanoi, Vietnam | Agilent 7890B GC-MS | 0.12 | D4–D6 | ND-0.208 | | | ||
| (0.0467) | ||||||||||
| Other premises | 2017 | 28 | Museums in Florence, Italy | Agilent 6890N-5973 GC-TD-MS | 2.0–40 | D3–D6 | 12.6–60.3 | | | |
| 2016 | 27 | Offices and laboratories in Uppsala, Sweden | Agilent 7890B GC and 5977A Series GC/MSD | 20–42 | D4–D6 | 0.05–2.61 | | | ||
| 2018 | 50 | Universities in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.18–0.37 | D4–D6 | 110–200 in female dormitory; | L7–L12 | 0.25–0.56 | ||
| 2018 | 50 | Universities in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.18–0.37 | D4–D6 | 5.3–19 in male dormitory | L7–L12 | 0.25–0.56 | ||
| 2013–2016 | 204 | Industrial zones in Tarragona, Spain | Thermo Scientific Focus TD-GC and Thermo Scientific DSQ II MSD | 0.05–0.09 (ng) | D3–D6 | | L2–L5 | 0.7 ± 0.6–18 ± 12 | ||
| Outside the factory workplace | 2021 | 14 | Refinery in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.30–0.35 | D4–D6 | 0.077–45 | | | |
| 2017 | 21 | Construction sites, and automobile and paint factories in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.17–0.37 | D4–D6 | 160–400 | L5–L8 | 0.24–9.0 | ||
| 2022 | 52 | City neighborhood, New York, USA | Agilent 7000D GC-MS/MS | 0.05–12 (ng) | D3–D5 | 0.0188–1.95 | L5, L7 | 0.0003–0.0298 | ||
| Residential outdoor | 2019–2020 | 38 | Hanoi, Vietnam | Agilent 7890B GC | 0.12 | D4–D6 | 0.00741–0.181 | | | |
| (0.063) | ||||||||||
| 2015 | 26 | Catalonia, Spain | Thermo Scientific Focus TD-GC and Thermo Scientific DSQ II MSD | 0.05–0.09 (ng) | D3–D6 | 0.50 ± 0.43 | L2-L5 | 0.18 ± 0.13 | ||
| 2011 | 42 | Suburb, Stockholm, Sweden | TRACE GC Ultra Multichannel 800 GC | 0.0038–0.27 | D3–D6 | 0.0083–0.041 | L3–L6 | <LOD–0.00070 | ||
| 2017 | 68 | Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.17–0.37 | D4–D6 | 0.071–0.107 | | | ||
| 2017 | 68 | Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.17–0.37 | D4–D6 | 0.071–0.107 | | |
VMS, volatile methylsiloxanes; LOD, limit of detection; WWTPs, wastewater treatment plants; GC, gas chromatography; MS, mass spectrometry; ND, not detectable.
Concentration of individual target substances.
Average value.
Median.
2.1.2. VMS in Aerosol Particles
A porous structure on the surface of aerosol particles provides adsorption sites for VMS, facilitating the accumulation of VMS in the particulate phase. Compared to cVMS (122 Pa for D4, 25 Pa for D5, and 2.2 Pa for D6), a lower saturated vapor pressure of lVMS (≤0.073 Pa) favors their absorption into dust and particles.
Industrial activities (such as construction site, automobile manufacturing facility, petroleum refining facility, paint production operation, etc.), particularly silicone processing and production, are prominent contributors to VMS in aerosol particles. Cheng et al. reported the concentrations of Σ9VMS (D3–D6, L4–L8) at 0.1–3.7 μg/g in road dust near a silicone production plant in China, with D3 and L8 reaching the peak. Le et al. for the first time demonstrated that 4 cVMS (D3–D6) in ultrafine particles (PM0.1 and PM0.5) in the atmosphere of industrial areas in Vietnam tends to preferentially sorb to PM0.1. This remains the sole study to investigate cVMS in ultrafine particulate matter, and research in this field remains limited. Besides the industrial sources, traffic emissions are another important source of outdoor VMS. Yao et al. described the concentrations of VMS and the derivatives in aerosols emitted by vehicles in a tunnel ranging from 37.7 ng/m3 to 377.0 ng/m3, accounting for 0.8%–1.9% of organic components in the aerosols. These emissions primarily originate from the release of silicone polymers in automotive fuel or lubricants during the engine combustion process. Beyond industrial and transportation sectors, the contaminant of VMS is ubiquitous even in the nonindustrial settings. In dust samples collected from Hefei, China, the concentrations of Σ8VMS (D4–D6, L4–L8) range from 3.3 × 10–2 ng/g to 0.5 ng/g in urban areas and 6.1 × 10–2 ng/g to 0.2 ng/g in cultural areas. A total of 17 types of MS are identified in PM2.5 samples from the campus of Peking University in Beijing using nontargeted techniques. The current contamination status of VMS in nonindustrial areas should not be underestimated. VMS detected in street dust in nonindustrial areas may be related to the use in automotive tires, plastics, and building materials. We therefore strongly recommended comprehensive assessments of VMS in both point and diffuse sources.
Tran et al. suggested that D5 and L8 are the dominant components with the highest concentrations in particulate matter of indoor environments in New York, with a mean concentration of D5 and L8 of 2,400 (29.3–34,000) μg/g and 1,300 (LOQ–13,000) μg/g, respectively. The median concentrations of ΣMS in indoor dust from Greece, South Korea, the United States, and Vietnam are 3.0 μg/g, 1.8 μg/g, 1.2 μg/g, and 0.7 μg/g, respectively. , Particulate matter settling provides a major source of indoor dust, and therefore, VMS concentrations in particulate matter are significantly higher than those in dust. Guo et al. detected L5–L16 in indoor PM2.5 from ordinary households in urban areas of southwestern China, with total concentrations ranging from 1.6 μg/g to 2.5 μg/g. The median total concentration of cVMS (D4–D7), lVMS (L4–L8), and other lMS (L9–L14) in indoor dust samples collected in China is 0.7 μg/g. In environments where PCPs are frequently used, such as barbershops (median: 43.1 μg/g) and bathrooms (median: 5.7 μg/g), the concentrations of VMS are especially higher than the ordinary households and outdoor environments for more than tenfold. Overall, the outdoor concentrations of VMS in particulate matter are far lower than the indoor concentrations, showing a remarkable difference in the order of magnitude.
The gas–particle partitioning equilibrium affects the environmental behavior of VMS. There is a positive correlation between the concentrations of cVMS and lVMS in both the indoor gas phase and particulate phase, and they are also positively correlated with the frequency of daily usage of PCPs. It is indicated that both indoor cVMS and lVMS share the same source, in which a dynamic exchange process exists between the gas and particle phases. ,
In summary, the environmental fate and sources of air pollution are complex. Industrial activities, traffic emissions, and the use of PCPs are the primary contributors. Indoor concentrations of VMS are generally higher than outdoors, with significant variations across industrial/residential or urban/rural indoor environments. Monitoring of indoor pollution of VMS across regions is especially of importance, especially the sources in dust and particulate matter (Table ). Based on the existing literature, the total concentrations of VMS in outdoor and indoor gaseous phases range from 1.6 × 10–3–1,400 ng/m3 and 3.3 × 10–2–940.0 μg/m3, respectively, and those in outdoor and indoor aerosol particles range from 3.3 × 10–2–3,690 ng/g and 0.7–34,300 μg/g, respectively.
2. Concentrations of VMS in Particulate Matter and Dust across Regions .
| Site | Time | Sample size | Sample type | Region | Method | LOD (ng/g) | Types of cVMS | ΣcVMS (μg/g dw) | Types of lVMS | ΣlVMS (μg/g dw) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inside the factory workplace | 2017 | 21 | PM2.5 | Construction sites, and automobile and paint factories in Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 5.1–7.9 | D4–D6 | | L5–L16 | 450–15,000 | |
| 6 | dust | 0.6–0.9 | 10–37 | 220–7,200 | |||||||
| 2019–2020 | 32 | PM0.1 | Hanaka industrial park in Ninh, Vietnam | GC Trace 1310-MS TSQ 9000 | 4.0–6.0 | D4–D6 | MQL-203 ng/m3 | | | ||
| 32 | PM0.5 | MQL-507 ng/m3 | |||||||||
| 2019–2020 | 17 | dust | Waste recycling workshop, Bac Giang, Hung Yen, and Ha Tinh, Vietnam | Agilent 7890B/5977A GC-MS | 1.0–2.0 | D3–D6 | 0.329–5.890 (1.560) | | | ||
| 2018 | 50 | PM2.5 | Dormitory, Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.5–0.8 | | | L5–L16 | 850–3,100 | ||
| Residential premises | 2011, 2015, 2021 | 120 | dust | South Korea | Agilent 7890/5975C GC-MS | 0.001–2.56 | D4–D7 | 0.237–16.2 (1.46) | L3–L13 | 0.689–150 (7.01) | |
| 2016–2017 | 75 | particulate matter | Hanoi, Vietnam | Agilent 7890/5977A GC-MS | 450- 2730 | D4–D6 | 0.258–5.630 (1.350) | | | ||
| 2014 | 22 | dust | New York, USA | Agilent 6890/5973 GC-MS | 0.61–1.82 | D3–D7 | 0.069–3.660 (0.296) | L8–L14 | 0.037–4.110 (0.623) | ||
| 2014 | 28 | Patna, India | ND–0.244 (0.087) | ND-0.562 (0.0215) | |||||||
| 2014 | 28 | Athens, Greece | 0.118–25.1 (1.380) | 0.129–4.990 (0.772) | |||||||
| 2018 | 100 | dust | Henan, China | Agilent 7890B/7000D GC-MS | 0.77–3.54 | D3–D8 | 0.0127–0.567 | L5–L16 | 0.00654–0.769 | ||
| 2017 | 68 | PM2.5 | Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.5–0.8 | D4–D6 | 0.50–0.56 | L8–L14 | 2.0–2.2 | ||
| 20 | dust | 0.6–0.9 | 0.16–0.21 | 0.98–1.3 | |||||||
| Other premises | 2019–2020 | 7 | dust | Vehicle repair shop, Hanoi, Vietnam | Agilent 7890B/5977A GC-MS | 1.0–2.0 | D3–D6 | 0.126–1.950 | | | |
| 11 | University classrooms, Hanoi and Ha Tinh, Vietnam | 0.086–1.540 | |||||||||
| 2018 | 80 | dust | University, Southwest China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.5–0.8 | D4–D6 | 3.3–6.5 in female dormitory; | L5–L16 | 340–510 | ||
| 0.21–0.58 in female dormitory | 0.33–2.5 | ||||||||||
| 2021 | 74 | dust | Stores, Beijing, China | GCMS-QP2010 SE Single Quadrupole GC-MS | 0.12–0.78 | D4–D6 | 0.00741–2.39 (0.057) | L5–L16 | 0.109–49.4 (1.39) | ||
| | 36 | dust | Barber stores, Tianjin, China | GC-MS | 0.15–0.36 | D4–D6 | 0.21–68 | L4–L16 | 3.8–82 | ||
| Residential outdoor | 2019–2020 | 14 | Particulate matter | Hanoi, Vietnam | GC (Agilent 7890B) | 0.10–0.30 | D4–D6 | ND-1130 (327) | | | |
| 2016 | 50 | dust | City neighborhood, Hefei, China | GC-MS (Agilent 7890/5975) | 0.30–1.14 | D4–D6 | 0.023–0.232 (0.067) | L5–L13 | 0.127–0.924 (0.450) |
VMS, volatile methylsiloxanes; LOD, limit of detection; GC, gas chromatography; MS, mass spectrometry; MQL, minimum quantitation limit.
Median.
2.2. Pollution of VMS in Water Bodies and Sediments
There is a clear gradient in the concentrations of VMS in aquatic environments, showing higher concentrations of VMS in water bodies impacted by industrial and urban activities than natural water bodies. In addition to the elevated concentrations of VMS observed in indoor air, previous studies have also reported significantly higher levels of VMS in wastewater. Cosmetics and PCPs are the major sources of VMS in urban sewage, presenting increased concentrations during the summer due to the more frequent use of VMS-containing PCPs (e.g., deodorants, sunscreens). , Dong et al. reported that water and sludge samples collected from wastewater treatment plants in Beijing and Kunming, China, in winter and summer, respectively, exhibited significantly higher concentrations of the three VMS (D4–D6) during summer compared to winter. The measured concentrations were 3.9–5.1 μg/L in water and 3.8–6.9 μg/g dry weight in sludge during summer, whereas in winter the concentrations were 2.6–4.4 μg/L in water and 2.8–4.8 μg/L in sludge, respectively. Through the discharge into the drain and WWTPs, pollution of VMS in urban sewage can be influenced by water solubility and vapor pressure. Primarily removed through volatilization, VMS in WWTPs can also be adsorbed by sludges, hydrolyzed and biodegraded, or flowed with the effluent into nearby rivers and oceans. , Despite the high efficacy of removal (up to 99%), VMS can still be detectable in the effluent outlet of WWTPs. The concentration of Σ7VMS (D3–D6, L3–L5) in the final effluent of a large WWTPs in Tokyo, Japan, is reported at 185.0–1,170 ng/L. In Dalian, China, the annual average concentrations of D4, D5, and D6 in the effluent outlet of a WWTPs are 249.3 ng/L, 244.1 ng/L, and 210.9 ng/L, respectively. Residual VMS after processing in WWTPs may infiltrate groundwater or enter streams via wastewater discharges and sludge deposition, further posing an aquatic contamination. In addition, VMS can also be volatilized into the atmosphere, contributing to broader environmental pollution.
In marine environments, especially in coastal areas with dense industry, VMS can be found in both water and sediments. For instance, concentrations of D4–D7 and L6–L12 in sediments near industrial regions of the Incheon coast, in South Korea, ranged from a median of 10.4 (2.3–148.0) ng/g dry weight and 5.4 (1.4–19.2) ng/g dry weight, respectively. In the Tokyo Bay area, seven types of VMS (D3–D6 and L3–L5) are detected in seawater near a large WWTPs, with total concentrations ranging from the minimum detection limit to 1,700 ng/L (median: 140.0 ng/L). Among them, D5 ranks the top in concentrations of VMS. The concentration of ΣVMS in the northeastern coastal waters of China ranged from 14.4 ng/L to 130.0 ng/L (mean: 46.1 ± 27.2 ng/L). In sediments from the same area, the concentration of cVMS (D4–D7) ranged from 4.4 ng/g dry weight to 25.2 ng/g dry weight (mean: 12.4 ± 5.4 ng/g dry weight), with D5 being the dominant component, while lVMS is nondetectable. Besides, offshore VMS has a higher concentration than infralittoral VMS, showing a declined concentration from coastal area to marine regions.
VMS is also widely detectable in lakes and rivers, especially in urban areas. The lake and river systems are more susceptible to pollutants from various sources, including industrial discharges, effluents from WWTPs, agricultural runoff, and urban sewage. Higher concentrations of VMS are detected in freshwaters compared to marine environments like bays. The total concentrations of cVMS in Llobregat and Riera de Rubí rivers in Catalonia, Spain, are 22.0–58.0 ng/L and up to 203.0 ng/L, respectively. , In sediments from Catalonian rivers, concentrations of Σ3cVMS (D3–D5) range from 8.0 ng/g dry weight to 2,070 ng/g dry weight. The median total concentration of cVMS in five lakes from Hanoi, Vietnam is 282.0 (67.0–1,200) ng/L. In Dianchi Lake, the largest lake in southwestern China and the only urban wastewater-receiving body in Kunming, the average concentrations of Σ3cVMS (D4–D6) in sediments is 501.0 ng/g. Three types of cVMS (D4–D6) are detected in water samples from Dianchi Lake, and D6 presents the longest half-life and highest concentration at 20.8 ± 5.8 ng/L. An extensive distribution of VMS is found throughout the Yangtze River Basin, where the concentrations of cVMS in surface water samples typically increase to a median of 213.0 (17.3, 4,570) ng/L, particularly in the downstream regions. In the upper reaches of the Yangtze River Basin, the median concentrations of cVMS and Σ7lVMS are 117.0 ng/L and 10.6 ng/L, respectively, exhibiting minor variations across regions along the river system. VMS concentrations, pronouncedly influenced by industrial activities and effluents discharge, present a positive correlation between those in wastewater and surface water samples. Dongting Lake is a flood basin of the Yangtze River, where the concentrations of cVMS are in a range of 64.9–489.0 ng/L in surface water and 26.6–997.0 ng/g dry weight in sediments. Contamination of VMS in natural water bodies is closely related to industrial and domestic activities. The presence of VMS in drinking water sources is a potential risk to human health. So far, there lacks a definitive threshold for environmental concentrations of VMS, which is an important spotlight in the future research.
The organic carbon partition coefficient values for VMS are all greater than 3, indicating a strong tendency for VMS to partition into the sediment phase within the water–sediment system. Consequently, sediments often serve as the primary reservoir for VMS pollution in aquatic environments. Seasonal variations are observed in the contamination of VMS. For example, concentrations of VMS in Dianchi Lake water remain higher in winter than summer, probably due to the influence of temperature on the gas–water partitioning. The air/water partition coefficient values for VMS (L2–L4, D4–D5) range from 2.49 to 3.45 and increase with temperature. Overall, a greater volatilization of VMS occurs when thermal conditions favor a high temperature.
2.3. Soil Pollution of VMS
Soil pollution of VMS in agricultural, residential, and industrial regions primarily derives from the deposition of atmospheric particulate matter, application of sewage sludge from WWTPs as a biomass amendment, and leachate from waste disposal sites. Certain industrial operations particularly in oilfield environments and application of sewage sludge in agricultural practices further exacerbate soil pollution of VMS.
Soils contaminated by VMS can be influenced by the types of regions and land use. The total concentrations of D5 and D6 in soil from a heavy industrial area of Bilbao, Spain, range from 50.0 ng/g to 667.0 ng/g dry weight, which are significantly higher than urban areas (18.2–77.0 ng/g dry weight) and agricultural areas (15.0–84.0 ng/g dry weight). Notably, D4 is only detectable in the soil of industrial areas, with a concentration of 58.0 ng/g dry weight. Wang et al. detected cVMS (D4–D6) in soils from farmland amended with biosolids in Canada, with concentrations ranging from 24.0 ng/g to 949.0 ng/g dry weight. Sewage sludge is therefore validated as an important contributor to soil pollution of VMS.
The distribution of VMS in different environments has unique characteristics. In soil samples collected near the Shengli Oilfield of China, average concentrations of cVMS (D4–D6) ranging from 43.4 ng/g to 505.0 ng/g dry weight are ten times higher than those in control soils. Between 2008 and 2013, there was an annual increase in the concentrations of Σ3cVMS (D4–D6) in these soils, possibly related to the growing use of siloxanes. Additionally, chlorinated products of VMS with concentrations ranging from <LOD to 586.0 ng/g dry weight can also be detected in the oilfield soils, suggesting transformation processes of VMS in specific environments. In soil samples from an electronic-waste dismantling site in China, the average concentrations of D4, D5, and D6 (29.4 ng/g, 147.0 ng/g, and 145.0 ng/g dry weight, respectively) are 6–17 times higher than those in the control area, indicating the significance of electronic-waste processing in soil pollution of VMS. Management of electronic waste often accompanies emissions of massive waste gas, wastewater, and solid waste containing VMS that directly or indirectly contaminate the surrounding soil environment. Xu et al. consistently detected six types of VMS (D5, D6, L8–L11) in soil samples from urban residential areas in China, with average concentrations of 3.0–17.8 ng/g dry weight.
In summary, soil pollution of VMS is significantly influenced by regional industrial activities and land use practices through multiple pathways. VMS can enter soil systems primarily through atmospheric deposition, sewage sludge application, and leachate from waste disposal sites. The processes through which VMS accumulate in soil include adsorption, volatilization, hydrolysis, and bioaccumulation. Industrial operations in oilfields and electronic-waste dismantling regions especially result in increased concentrations of VMS in surrounding soils. Notably, transformation products of VMS like chlorinated siloxanes detectable in soil samples are indicative of the negative influence of ongoing chemical processes on soil pollution. Continuous monitoring and management strategies to mitigate the environmental and health risk of soil pollution of VMS are urgently needed.
3. Environmental Health Risk Assessment of VMS
During the environmental health risk assessment of VMS, a variety of influencing factors should be considered, such as human behaviors associated with exposure to environmental pollutants, toxicological properties of pollutants, and the dose–response relationship. Exposure parameters are crucial for quantifying exposure doses and assessing health risks to humans, which have been standardized in guidelines or consensus proposed by the United States Environmental Protection Agency (US EPA), , the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC), , or the Chinese Population Exposure Factors Handbook. ,
Inhalation, ingestion, and dermal contact are common human exposure pathways to VMS, with indoor inhalation as the primary route. The average daily dose (ADD) is one of fundamental indicators of health risks. Current risk assessment models primarily base their calculations on the average or maximum of the environmental concentration measurements, focusing on short-term risks while neglecting long-term impacts. Previous studies have cumulatively assessed the hazard index of VMS by comparing the exposure level with a safe reference dose (RfD), thus providing valuable references for a preliminary screen. However, RfD may diverge from the real situation because of a direct association with the calculation, in which an uncertainty factor (UF) subjectively introduced in its derivation may lead to varying assessment results.
The chronic RfD (cRfD) is calculated by the no-observed-adverse-effect level (NOAEL) divided by th UF. The ADD/cRfD ratio of less than 1 indicates no health risk. The ratio of the exposure dose to the derived no-effect level (DNEL) provided by ECHA is another common indicator used in human health risk assessment, with the ratio of less than 1 indicative of no health risk. , However, the lack of toxicological data for other types of VMS, such as D6, greatly limits comprehensive risk assessments. Existing approaches to quantifying health risks also include the use of the margin of exposure (MOE) or the tolerable daily intake (TDI), which are defined differently by various international institutions and organizations. For example, short-chain chlorinated paraffins, as an important class of substances within persistent organic pollutants, have been evaluated for exposure risks in previous studies. In these evaluations, health risk assessment of nontumor effects was often quantified using tools such as MOE and TDI. , However, the scarcity of comprehensive toxicological data has led to current assessments of VMS being primarily based on RfD.
Calculation formulas of exposure doses by various pathways in the existing literature are summarized in Table . Occupational populations (e.g., workers in silicone production factories and beauty industry professionals), female college students, and children in nonoccupational populations are high-risk populations to VMS exposures. A multiroute exposure assessment of VMS is still scant that blocks a thorough landscape of health risks of VMS to humans. By developing and utilizing physiologically based pharmacokinetic (PBPK) models, multiple exposure routes (e.g., diet, inhalation, dermal contact) can be integrated to assess total exposure. For example, in childhood pesticide exposure studies, the PBPK model has been used to simulate exposure scenarios of dietary intake and inhalation and to predict cumulative excretion of metabolites in urine, thereby quantifying the contribution of different exposure pathways. It is a valuable tool for converting exposure to estimate absorbed dose estimates. ,
3. Exposure Parameters and Formulas Used in Previous Literature.
| Exposure pathways | Formula No. | Formulas | Exposure parameters | Reference |
|---|---|---|---|---|
| Inhalation | a | ADD, average daily dose; C g+p, concentration of VMS in gaseous and aerosolized states (μg/m3); IR, inhalation rate (m3/day); EF, exposure frequency (days); ED, exposure duration (years), F uptaken, uptake fraction; BW, body weight (kg); AT, average time (day). | ||
| b | E, exposure; C, concentration in air (μg/m3); IRair, inhalation rate for adults (1.2 m3/h); AT, average time for 8 h per day spent in this industrial area (h); F uptaken, uptake fraction. | |||
| c | DED, daily exposure dose; C, concentration in air (μg/m3); IR, inhalation rate (m3/day); ED, exposure duration (years); BW, body weight (kg). | |||
| d | E, exposure; Fuptaken, uptake fraction; Cair, concentration in air (μg/m3); V air , volume of inhalation (m3/day); C PM2.5, concentration in PM2.5(μg/g); Q PM2.5, PM2.5 inhalation rate (g/d); BW, body weight (kg). | |||
| e | Inhexp, the daily inhalation exposure per capita; C air, concentration in air (μg/m3); V room, indoor air volume (m3); IR, inhalation rate (20 m3/day for adults and 5 m3/day for children); ED, exposure duration (day); BW, average body weight (kg). | |||
| Oral ingestion | f | EDI, estimated daily intakes; C, concentration in dust (ng/g); ED, exposure duration (years); IR, dust ingestion rate (20 and 50 mg/day for adults and children with a normal exposure case, 50 and 200 mg/day for adults and children with a high exposure case); EF, exposure frequency (day/year); BW, average body weight (kg); AT, average time (day). | ||
| g | ADD, average daily dose; C, concentrations of VMS (μg/g); ED, exposure duration (years); EF, exposure frequency (days); F ing, uptake fraction of the compound; Q, dust ingestion rate (g/day); BW, body weight (kg); AT, average time (day). | |||
| h | EDI, estimated daily intakes; C, concentration of VMS (ng/g); R, daily dust intake rate (g/d); P time, percentage of the time that people spent in measured regions; BW, average body weight (kg). | |||
| i | EDI, estimated daily intakes; C, concentration of VMS (ng/g); R, daily dust intake rate (g/d); IEF, indoor exposure fraction (h/day); BW, average body weight (kg). | |||
| j | E, exposure via ingestion; Cdust, concentration of VMS in indoor dust (μg/g); Q dust, dust ingestion rate (g/day). | |||
| k | EDI, estimated daily intakes; C, concentration of VMS (ng/g); W, daily intake rate (g/d); BIO, bioavailability, 1; BW, average body weight (kg). | |||
| l | E, mouthing exposure; C, concentration of VMS in children’s products (μg/g); W, weight of the entire product; Bio, bioavailability, 1; MF, VMS in the product that can migrate to saliva; BW, body weight (kg). | |||
| Dermal exposure | m | DED, dermal exposure dose; C, concentration of VMS (μg/g); ABS, dermal adsorption fraction; AF, skin adherence factor (mg/cm2); SA, exposed surface area of skin (cm2/day); EF, exposure frequency (day/year); BW, average body weight (kg); CF, conversion factor (106 mg/kg). | ||
| n | EDI, estimated daily intakes; C, concentration of VMS (μg/g); ABS, dermal adsorption fraction (0.03); AF, skin adherence factor (0.07 and 0.2 mg/cm2 for adults and children); SA, exposed surface area of skin (5700 and 2700 cm2 for adults and children, respectively); EF, exposure frequency (day/year); ED, exposure duration (years); BW, average body weight (kg); AT, average time (day). | |||
| o | ADD, average daily dose; i, type of PCPs; C PCPs, concentrations PCPs (μg/g); EF, exposure frequency (days); ED, exposure duration (years); F der, fraction of the product that may be retained on the skin surface; A, amount of product used per application (g/event); F q , frequency of application (events/day); R, retention factor; BW, body weight (kg); AT, average time (day). |
3.1. Inhalation Exposure to VMS
Previous studies have primarily focused on the illustration of exposure pathways to VMS and the corresponding contribution rates. In 2020, Guo et al. for the first time assessed population exposure risks of VMS by combining internal and external exposures and the correlation between ADD and plasma concentrations of target compounds. They found that inhalation is the predominant pathway in a VMS-rich environment, where ADD from inhalation is positively correlated with plasma concentrations. Specifically in indoor environments, ADD from inhalation primarily comes from D4–D6 in the gaseous phase, with PM2.5 contributing 5.5%–8.9% of the total exposure. Exposure rates of VMS via inhalation are strikingly 3 orders of magnitude higher than dust ingestion. cVMS in the gaseous phase is the dominant contributor to nearly the total ADD via inhalation (ADDinh) in all indoor areas, which is particularly evident in coresident family members of factory workers and female college students. Due to higher external exposure concentrations, exposure frequency, and exposure duration, occupational groups generally face greater exposure risks, with oilfield worker and WWTPs workers being at the highest risk among them. The daily intake of cVMS for oilfield workers ranged from 8,170 ng/kg/day to 202,000 ng/kg/day and for WWTPs workers ranged from 4,300 ng/kg/day to 44,000 ng/kg/day. , It is significantly higher than the exposure dose resulting from the use of high-concentration PCPs products; for example, the total daily intake of VMS for adults in bathrooms was 1,560 ng/kg/day and in salons was 80,700 ng/day. , Although the estimated ADDs for all population groups are lower than the reference values, the estimated exposure levels for children are significantly higher than those for adults. Unique physical characteristics of higher respiratory rates and lower body weights in children are responsible for the high health risks of VMS in this vulnerable population. The median daily total intake of cVMS (D4–D6) from indoor air exposure in Vietnam is 0.3 (2.9 × 10–2–2.0) in adults and 2.5 (0.3–19.6) ng/kg/day in children. In the United States, the estimated intake of VMS for adults ranges from 0.3 μg/kg/day to 3.2 μg/kg/d, which is 0.31–0.32 μg/kg/day in adults from southwestern China. Exposure levels of VMS varying across regions and populations are attributed to geographic, lifestyle, and environmental factors.
The European Union’s Scientific Committee on Consumer Safety (SCCS) has established an NOAEL of 150 mg/kg/day for the chronic inhalation toxicities of D4 and D5, with a UF of 1000 and cRfD of 0.15 mg/kg/day for simultaneous exposures. Current estimates have shown a decreased value compared to the reference, even though the ADD for inhalation exposure to VMS is estimated based on the highest environmental concentrations. However, this is not a problem we should take lightly. Existing reference values are primarily based on toxicity data for D4 and D5, and the safety thresholds for other types of VMS remain unclear. Moreover, actual exposure scenarios are complicated and involved with multiple VMS and other volatile organic compounds, presenting synergistic or antagonistic interactions to challenge the health risk assessments. Although short-term exposure may not reach significant toxicity thresholds, the long-term exposure to VMS influenced by chronic and cumulative effects is particularly important to children, women, and individuals with compromised immune systems who may require stricter exposure limits.
3.2. Oral Ingestion
Particulate matter and dust can enter the human body through oral ingestion. Daily exposures to VMS are elevated at home due to the fact that the concentrations of VMS within dust in a household are higher than those in offices and laboratory areas, leading to a high health risk at home. Exposure assessments from multiple countries reported that the total siloxane exposure dose through indoor dust ingestion is 0.3–11.9 ng/kg/day in infants and children at 1–6 years of age and 6.0 × 10–2–2.5 ng/kg/day in adults older than 19 years of age. The much higher exposure dose in infants and children can be explained by the unique behavior patterns (e.g., frequent contact with the floor and frequent hand-to-mouth activities) and metabolic characteristics. Individuals working at barbershops and female dormitories usually have increased exposure and higher concentrations of VMS. For example, the estimated exposure dose for barbers through indoor dust inhalation in China is 0.9–14.3 ng/kg/day, compared to 0.1–3.4 ng/kg/day for the general adult population.
Oral exposure to VMS can also occur through food, food packaging materials, and silicone-based products. Studies have shown that polydimethylsiloxane can degrade into VMS during high-temperature cooking. VMS residues (D4–D6 and L6–L14) have been detected in fried foods, with ADD ranging from 10.31 ng/kg/day to 241.13 ng/kg/day for fried chicken and from 4.77 ng/kg/day to 127.31 ng/kg/day for French fries, respectively. Due to their lipophilic nature, VMS are more readily absorbed by high-fat foods. Additionally, VMS maybe ingested through contaminated aquatic products via dietary intake. For instance, VMS have been detected in fish from Spain, with ADDing ranging from 40 ng/kg/day to 110 ng/kg/day from raw fish consumption; however, cooking processes may reduce VMS concentrations by over 68%. Silicone-based products, such as bakeware, can also release VMS into food, particularly in high-fat environments. For example, baked goods prepared in silicone molds have been found to contain D3–D9 and D21, although the overall migration levels are relatively low. Four types of VMS (D7, D9, D10, and D12) have been identified in foods packaged with paper-based materials. However, studies have yet to evaluate the risks of oral exposure to these compounds. Furthermore, small amounts of VMS have been detected in silicone-based pacifiers and children’s toys, but oral exposure through these products is not considered a major pathway. , Overall, research on VMS residues in food, and their potential health risks remain limited.
According to the toxicological data of ECHA and route-to-route extrapolation, the DNEL is 1.35 × 109 ng/kg/year for D4 and 1.83 × 109 ng/kg/year for D5. , DNEL for D6 is not available. The cRfD for oral ingestion of D4 and D5 is 64 μg/kg/day. Previous estimates of daily exposure doses of VMS are approximately 1,000 times lower than the cRfD, indicating relatively low health risks brought by oral ingestion of VMS. A continuous monitoring of VMS via oral ingestion and health risk assessments are still important to high-risk populations like infants and children.
3.3. Dermal Exposure
VMS is widely used in cosmetics, PCPs, and household items, leading to a direct dermal contact. Horii and Kannan analyzed the presence of VMS in 76 types of consumer products, and more than half of them contain D4, D5, or D6, with D5 reaching a maximum concentration of 81.8 mg/g. Dudzina et al. consistently reported that D5 is the dominant type of VMS in PCPs, while D4 is maintained at a low level. Approximately 60%–80% of D4–D6 via dermal exposure remains on the surface, , but almost all can evaporate from the skin surface at the end of the exposure. The unique characteristics of dermal exposure to VMS significantly affect the absorption efficiency of VMS and further challenge the health risk assessments.
Capela et al. reported the dermal exposure levels of 25.0 μg/kg/day in Spanish adults and 0.4 μg/kg/day in children. In southwestern China, the dermal exposure to VMS from PCPs among college students is 1.0–1.6 ng/kg/year in males and 3.6–5.4 ng/kg/year in females. Variations in the concentrations of VMS via dermal exposure are mainly attributed to the differences in VMS contents in consumer products and usage habits.
Besides the use of PCPs, dermal absorption of VMS from dust is another pathway of dermal exposure. Chen et al. assessed the exposure levels of VMS through dermal contact with dust in adults and children in Korean households. The estimated daily intake of VMS at average exposure levels is 1.7 ng/kg/year in adults and 5.5 ng/kg/year in children, consistently highlighting the risk population of children. College students in southwestern China have lower exposure levels through dermal contact with dust, with male students exposed to 5.0 × 10–4–2.0 × 10–3 ng/kg/year and female students to 5.0 × 10–4–0.7 ng/kg/year. The exposure dose of VMS through dermal contact with dust in male college students is comparable to that of the general residential population in China. ,
The cRfD of D4 and D5 via dermal absorption is 67 μg/kg/day. Previously reported exposure doses of VMS estimated in various populations are always lower than the cRfD. However, research evidence on internal exposure levels of VMS through the use of topical PCPs and relevant health risk assessments is currently scant. Existing literature reporting health risk assessments based on external exposure data of VMS via varying exposure pathways are summarized in Table .
4. Health Risk Assessment of Methylsiloxane for Different Exposure Routes .
| Exposure
range for normal exposure scenarios (ng/kg/day) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Exposure Route | Time | Region | Population | Formula | ΣcVMS | ΣlMS | Reference Value (ng/kg bw/day) | Reference |
| Inhalation | 2020–2021 | WWTPs, Portugal | Maintenance Technicians | c | 9,770 ± 1630 ng/kg/year | | cRfDs of D4 and D5:150,000 | |
| Managers | 8,010 ± 722 ng/kg/year | |||||||
| Laboratory Technicians | 5410 ± 874 ng/kg/year | |||||||
| 2021 | WWTPs, China | wastewater treatment technicians | a | 9,700–44,000 | | cRfDs of D4 and D5:150,000 | ||
| laboratory technicians | 4,300–28,000 | |||||||
| sludge treatment technicians | 4,600–25,000 | |||||||
| office staff | 4,700–24,000 | |||||||
| 2019 | Shengli oilfield, Dongying, China | Workers | b | 8,170–202,000 ng/day | 280–6,500 ng/day | | ||
| 2017 | Southwest China | Workers | d | 30,000–79,000 | 530–14,000 | | ||
| 2021 | Near a refinery in China | Children | a | 300–2,100 | | cRfDs of D4 and D5:150,000 | ||
| 2016–2017 | Hanoi, Bacninh, Thaibinh, and Tuyenquang, Vietnam | Resident adults >18 years of age | | 1.47–20.4 | 3.78–16.4 | | ||
| Adolescents at 11–17 years of age | 2.02–28.0 | 5.20–22.6 | ||||||
| Middle childhood at 6–10 years of age | 2.87–39.8 | 7.40–32.1 | ||||||
| Early childhood at 1–5 years of age | 3.35–46.5 | 8.63–37.4 | ||||||
| Infants at 6–12 months of age | 5.38–74.7 | 13.9–60.2 | ||||||
| 2016 | Uppsala, Sweden | Adults at 26–61 years of age | c | 61–440 (Home) | | RfD: 630,000 | ||
| 34–200 (Offices) | | |||||||
| 2014 | Greece | Resident adults | | 2.48 | NOAEL of D5:19,000,000 | |||
| Adolescents | 3.13 | |||||||
| Children | 5.57 | |||||||
| Toddlers | 11.9 | |||||||
| Infants | 11.1 | |||||||
| 2014 | Albany, USA | Resident adults | | 270 | | |||
| Teenagers at 11–18 years of age | 340 | |||||||
| Children at 3–11 years of age | 760 | |||||||
| Toddlers at 1–3 years of age | 1,590 | |||||||
| Infants <1 year of age | 3,180 | |||||||
| 2014 | Bathroom, Porto, Portugal | Adults | e | 160 | NOEL of D4:16,000,000; | |||
| Children | 3 | NOEL of D5:5,400,000 | ||||||
| 2021 | Southwest China City | Resident adults | a | 370–340 | | cRfDs of D4 and D5:150,000 | ||
| 2018 | Southwest China City | Residents >18 years of age | a | 210–320 | 0.030–0.032 | cRfDs of D4 and D5:150,000 | ||
| 6–17 years old | 230–420 | 0.011–0.042 | ||||||
| 3–6 years old | 380–620 | 0.018–0.062 | ||||||
| 0–3 years old | 690–720 | 0.069–0.072 | ||||||
| College students at 18–24 years of age | 220–3,600 | 0.011–680 | ||||||
| Ingestion of particulate matter and dust | 2011, 2015, 2021 | Interior of the Korean household | Resident adults | n | 0.13–1.54 | 0.59–21.1 | | |
| Children | 0.81–9.31 | 3.53–127 | ||||||
| 2021 | Beijing, China | Sales clerks | o | 0.025–2.83 | | |||
| 2018 | Henan, China | Resident adults | i | 0.35 | 0.72 | | ||
| Teenagers | 0.42 | 0.86 | ||||||
| Children | 0.69 | 1.41 | ||||||
| Toddlers | 2.10 | 4.31 | ||||||
| Infants | 1.78 | 3.65 | ||||||
| 2018 | Universities, Southwestern China | College Students | o | 75–1,200 | 150–110,000 | | ||
| 2017 | Southwest China | Workers | j | 8.9–30 | 180–5,800 | | ||
| 2016 | Road dust, Hefei, China | Adults | o | 0.008 | | |||
| Children | 0.065 | |||||||
| | Tianjin, China | Barbers | o | 2.67 | | |||
| Ingestion of food | 2016 | Barcelona, Spain (Fish) | Residents >60 years of age | | 43–46 | | ||
| 20–60 years old | 40–51 | |||||||
| 10–19 years old | 50–52 | |||||||
| 6–9 years old | 93–110 | |||||||
| 2024 | Beijing, China | Adults | k | 10.31–241.13 (Fried chicken) | | |||
| 4.77–127.31 (French fries) | | |||||||
| Oral exposure to silicone-containing products | 2017 | China | Children aged 3 and under | n | 0.44–18.05 | 0.41–20.74 | | |
| Dermal Exposure | 2014 | Porto, Portugal (PCP) | Adults | o | 25,040 | NOEL of D4:960,000,000; | ||
| Children | 350 | NOEL of D5:1,600,000,000 | ||||||
| 2011, 2015, 2021 | South Korea (Dust) | Adults | n | 0.08–0.92 | 0.35–12.6 | | ||
| Children | 0.26–3.02 | 1.14–41.3 | ||||||
| 2021 | City in Southwest China | Adults | o | 1,200–3,800 | | cRfDs of D4 and D5:67,000 | ||
| 2018 | Universities, Southwestern China | College Students | o | 1,500–5,400 (PCP) | 970–3,900 (PCP) | cRfDs of D4 and D5:67,000 | ||
| 0.00051–0.0070 (Dust) | 0.0012–0.65 (Dust) | |||||||
VMS, volatile methylsiloxanes; WWTPs, wastewater treatment plants; cRfD, chronic reference dose; NOAEL, no observed adverse effect level; NOEL, no-observed effect level.
Exposure of individual target substances.
Median.
Mean.
5th to 95th percentile.
4. Conclusions and Perspectives
The production and consumption of VMS increased with the rapid development of modern society, particularly in the use of PCPs. Consequently, environmental contamination and health risks concerning VMS have been broadly concerning. In 2020, the European Union originated the restriction on D4 and D5 usages in cosmetics, which was later promoted to other countries like the United States and Canada. , In 2018, the Chinese Ministry of Ecology and Environment initiated a project to investigate the environmental risks associated with D4, D5, and D6. Both domestic and international industry practices and regulatory measures against the production and consumption of VMS have been introduced and updated. However, direct restrictions on their production and usage are lacking in China.
The present study reviewed the current contamination status of VMS across various environmental matrices and health risk assessments. Concentrations of VMS are higher in indoor air, wastewater, and biosolids and lower in water, soil, and sediment. With the indoor and outdoor air exchange, outdoor concentrations of VMS increase in densely populated areas, although monitoring data on VMS are mainly collected from urban areas from some Western countries and East Asian countries. , So far, estimates about the contamination of VMS in outdoor air are limited for densely populated cities in economically underdeveloped regions. Additionally, atmospheric concentrations of VMS dynamically fluctuate and are extensively influenced by the temperature, wind speed, and hydroxyl radical concentration, leading to the challenge in assessing human exposure. A panel of oxidation products can be generated by VMS in both outdoor and indoor air, such as silicates, formates, silanols, and formaldehyde. , While a limited number of studies in the current literature provide repeated measurements and long-term monitoring of VMS concentrations in the environment, simultaneous monitoring of prototype and atmospheric products remains largely absent.
Plasma exposure concentrations of VMS have been previously overlooked in the environmental health risk assessments. Besides, there lacks sufficient toxicity and carcinogenesis data about various types of VMS, and so far, chronic toxicity parameters (e.g., NOAEL and DNEL values) for D4 and D5 are available. Currently, health risks are primarily quantified using the RfD, but more effective methods for risk quantification remain lacking. Considering the four pathways that VMS and the oxidative products go through in the body, including absorption, distribution, metabolism, and excretion (ADME), more efforts are required on a thorough identification of metabolites. Humans can be exposed to VMS through multiple pathways, and thus comprehensive risk assessments of either a single or combined pathways are urgently needed. Lastly, health risk assessments of VMS based on internal exposure burdens are still a weakness from the view of analyzing adverse health effects in humans.
Based on the landscape described in our review, we recommended the following aspects to be highlighted in the future research on environmental pollution and health risk assessments of VMS. First, more cross-regional monitoring stations are needed with the aim to achieve long-term real-time monitoring and data sharing of the trends and periodic patterns of VMS concentrations worldwide. This will enable a more accurate understanding of the trends and periodic variations in VMS concentrations, as well as their transformation products in environmental matrices. In addition, advancing efficient and accurate monitoring technologies for the continuous and real-time tracking of VMS and their oxidation products is crucial. Future research should focus on improving detection methods while investigating the migration, transformation, and environmental impact of VMS to better understand their cumulative effects. Second, the accuracy of detecting exposure levels of VMS and the metabolites in human matrices (e.g., serum, plasma, fat, exhaled breath) should be elevated. An integration of external exposure levels of VMS is expected to comprehensively reflect the ADME process and provide reliable data for health risk assessments. Third, to overcome the limitations of health risk assessment methodologies, the integration of advanced tools, such as mixture toxicity models and PBPK models, is essential. Additionally, the collection of more comprehensive toxicological data will be critical for improving the accuracy and reliability of risk estimates. These advancements will enable a more holistic and robust assessment of the health risks associated with VMS exposure. Fourth, analytic models are useful tools to mimic multiple exposure pathways from environmental media (e.g., simultaneous exposure to atmospheric dust through inhalation and ingestion). This approach would provide a more comprehensive and accurate assessment of the health risks associated with VMS exposure. Fifth, population characteristics serve as an important influencing factor of VMS exposure, which can be adjusted based on questionnaire surveys and biomarker testing. Beyond the general population, specific occupational exposure scenarios warrant consideration, including skincare product users, cosmetologists, hairdressers, and wastewater treatment plant workers. Furthermore, particular concerns should made on vulnerable populations like women and children. Finally, strict standardization of VMS monitoring in the environment, as exemplified by guidelines established in some European countries for indoor air VMS concentrations, should be promoted globally.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 82241091, 82273677, and 22122611).
This study has not been subjected to peer and policy review by the China CDC, and therefore does not necessarily reflect the views of the CDC, and no official endorsement should be inferred.
The authors declare no competing financial interest.
References
- Alaee M., Wang D.-G., Gouin T.. Cyclic volatile methyl siloxanes in the environment. Chemosphere. 2013;93(5):709–710. doi: 10.1016/j.chemosphere.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Bletsou A. A., Asimakopoulos A. G., Stasinakis A. S., Thomaidis N. S., Kannan K.. Mass loading and fate of linear and cyclic siloxanes in a wastewater treatment plant in Greece. Environ. Sci. Technol. 2013;47(4):1824–32. doi: 10.1021/es304369b. [DOI] [PubMed] [Google Scholar]
- Montemayor B. P., Price B. B., van Egmond R. A.. Accounting for intended use application in characterizing the contributions of cyclopentasiloxane (D5) to aquatic loadings following personal care product use: Antiperspirants, skin care products and hair care products. Chemosphere. 2013;93(5):735–740. doi: 10.1016/j.chemosphere.2012.10.043. [DOI] [PubMed] [Google Scholar]
- Molinier B., Arata C., Katz E. F., Lunderberg D. M., Liu Y., Misztal P. K., Nazaroff W. W., Goldstein A. H.. Volatile Methyl Siloxanes and Other Organosilicon Compounds in Residential Air. Environ. Sci. Technol. 2022;56(22):15427–15436. doi: 10.1021/acs.est.2c05438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari K., Singh A., Marathe D.. Cyclic volatile methyl siloxanes (D4, D5, and D6) as the emerging pollutants in environment: environmental distribution, fate, and toxicological assessments. Environ. Sci. Pollut Res. 2024;31(27):38681–38709. doi: 10.1007/s11356-023-25568-7. [DOI] [PubMed] [Google Scholar]
- Horii, Y. ; Kannan, K. , Main Uses and Environmental Emissions of Volatile Methylsiloxanes. In Volatile Methylsiloxanes in the Environment, Homem, V. ; Ratola, N. , Eds.; Springer International Publishing: Cham, 2020; pp 33–70. [Google Scholar]
- Use of Volatile Methyl Siloxanes (VMS) in industrial environments: A Toolbox For Minimising Environmental Emissions. 2019. https://globalsilicones.org/wp-content/uploads/2020/11/VMS_Toolbox_Version_2019-09-02.pdf.
- Asimakopoulos A., Bletsou A., Thomaidis N.. Emerging contaminants: A tutorial mini-review. Global Nest Journal. 2019;14:72–79. doi: 10.30955/gnj.000823. [DOI] [Google Scholar]
- Socio-economic evaluation of the global siloxanes industry. 2017. https://sehsc.americanchemistry.com/Socio-Economic-Evaluation-of-the-Global-Siloxanes-Industry-Final-Report.pdf.
- Zhi L., Xu L., He X., Zhang C., Cai Y.. Distribution of methylsiloxanes in benthic mollusks from the Chinese Bohai Sea. J. Environ. Sci. 2019;76:199–207. doi: 10.1016/j.jes.2018.04.026. [DOI] [PubMed] [Google Scholar]
- Candidate List of substances of very high concern for Authorisation. European Chemicals Agency (ECHA): 2024. [Google Scholar]
- List of substances proposed as POPs. European Chemicals Agency (ECHA): 2024. [Google Scholar]
- Lee J., Kim K., Park S.-M., Kwon J.-S., Jeung E.-B.. Effects of Decamethylcyclopentasiloxane on Reproductive Systems in Female Rats. Toxics. 2023;11(4):302. doi: 10.3390/toxics11040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D. N., Park S. M., Jung E. M., Jeung E. B.. Prenatal Octamethylcyclotetrasiloxane Exposure Impaired Proliferation of Neuronal Progenitor, Leading to Motor, Cognition, Social and Behavioral Functions. Int. J. Mol. Sci. 2021;22(23):12949. doi: 10.3390/ijms222312949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D., Kim K., Lee M., Jung E. M., Jeung E. B.. Effects of Maternal Exposure to Decamethylcyclopentasiloxane on the Alternations in Offspring Behaviors in Mice. Biomedicines. 2023;11(1):35. doi: 10.3390/biomedicines11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen A., Greene T., Van Landingham C., Gentry R.. Toxicology of octamethylcyclotetrasiloxane (D4) Toxicol. Lett. 2017;279:2–22. doi: 10.1016/j.toxlet.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Homem, V. ; Ratola, N. , Analytical Methods for Volatile Methylsiloxanes Quantification: Current Trends and Challenges. In Volatile Methylsiloxanes in the Environment, Homem, V. ; Ratola, N. , Eds. Springer International Publishing: Cham, 2020; pp 71–118. [Google Scholar]
- Xiang X., Liu N., Xu L., Cai Y.. Review of recent findings on occurrence and fates of siloxanes in environmental compartments. Ecotox Environ. Safe. 2021;224:112631. doi: 10.1016/j.ecoenv.2021.112631. [DOI] [PubMed] [Google Scholar]
- Feng D., Zhang X., Qi D.. Human exposure pathways of methylsiloxanes: A review of recent studies. Environ. Chem. 2018;37(5):1022–1036. [Google Scholar]
- Sun H., Li D., Xu L., Qiu C., Wang S., Liu N., Sun L.. Research progress on the distribution, behavior and effects of cyclic volatile methylsiloxanes in organisms. Environ. Chem. 2022;41(1):193–204. [Google Scholar]
- Cheng Y., Shoeib M., Ahrens L., Harner T., Ma J.. Wastewater treatment plants and landfills emit volatile methyl siloxanes (VMSs) to the atmosphere: investigations using a new passive air sampler. Environ. Pollut. 2011;159(10):2380–6. doi: 10.1016/j.envpol.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Pascual C., Cantera S., Lebrero R.. Volatile Siloxanes Emissions: Impact and Sustainable Abatement Perspectives. Trends Biotechnol. 2021;39(12):1245–1248. doi: 10.1016/j.tibtech.2021.05.003. [DOI] [PubMed] [Google Scholar]
- Dharini, T. ; Krishnamoorthy, A. ; Kuppusami, P. , Silicone-Based Coatings for High-Temperature Applications. In Coatings for High-Temperature Environments: Anti-Corrosion and Anti-Wear Applications, Pakseresht, A. ; Amirtharaj Mosas, K. K. , Eds.; Cham, Springer Nature Switzerland, 2024; pp 385–401. [Google Scholar]
- Alton M. W., Browne E. C.. Atmospheric Chemistry of Volatile Methyl Siloxanes: Kinetics and Products of Oxidation by OH Radicals and Cl Atoms. Environ. Sci. Technol. 2020;54(10):5992–5999. doi: 10.1021/acs.est.0c01368. [DOI] [PubMed] [Google Scholar]
- Yucuis R. A., Stanier C. O., Hornbuckle K. C.. Cyclic siloxanes in air, including identification of high levels in Chicago and distinct diurnal variation. Chemosphere. 2013;92(8):905–10. doi: 10.1016/j.chemosphere.2013.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton M. W., Johnson V. L., Sharma S., Browne E. C.. Volatile Methyl Siloxane Atmospheric Oxidation Mechanism from a Theoretical PerspectiveHow is the Siloxanol Formed? J. Phys. Chem. A. 2023;127(48):10233–10242. doi: 10.1021/acs.jpca.3c06287. [DOI] [PubMed] [Google Scholar]
- Wania F., Warner N. A., McLachlan M. S., Durham J., Miøen M., Lei Y. D., Xu S.. Seasonal and latitudinal variability in the atmospheric concentrations of cyclic volatile methyl siloxanes in the Northern Hemisphere. Environ. Sci. Proc. Imp. 2023;25(3):496–506. doi: 10.1039/D2EM00467D. [DOI] [PubMed] [Google Scholar]
- Buser A. M., Kierkegaard A., Bogdal C., MacLeod M., Scheringer M., Hungerbühler K.. Concentrations in Ambient Air and Emissions of Cyclic Volatile Methylsiloxanes in Zurich, Switzerland. Environ. Sci. Technol. 2013;47(13):7045–7051. doi: 10.1021/es3046586. [DOI] [PubMed] [Google Scholar]
- Anh H. Q., Nguyen H. M. N., Do T. Q., Tran K. Q., Minh T. B., Tran T. M.. Air pollution caused by phthalates and cyclic siloxanes in Hanoi, Vietnam: Levels, distribution characteristics, and implications for inhalation exposure. Sci. Total Environ. 2021;760:143380. doi: 10.1016/j.scitotenv.2020.143380. [DOI] [PubMed] [Google Scholar]
- Guo J., Zhou Y., Cui J., Zhang B., Zhang J.. Assessment of volatile methylsiloxanes in environmental matrices and human plasma. Sci. Total Environ. 2019;668:1175–1182. doi: 10.1016/j.scitotenv.2019.03.092. [DOI] [PubMed] [Google Scholar]
- Tran T. M., Kannan K.. Occurrence of cyclic and linear siloxanes in indoor air from Albany, New York, USA, and its implications for inhalation exposure. Sci. Total Environ. 2015;511:138–144. doi: 10.1016/j.scitotenv.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Rauert C., Shoieb M., Schuster J. K., Eng A., Harner T.. Atmospheric concentrations and trends of poly- and perfluoroalkyl substances (PFAS) and volatile methyl siloxanes (VMS) over 7 years of sampling in the Global Atmospheric Passive Sampling (GAPS) network. Environ. Pollut. 2018;238:94–102. doi: 10.1016/j.envpol.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Wang X., Schuster J., Jones K., Gong P.. Occurrence and spatial distribution of neutral perfluoroalkyl substances and cyclic volatile methylsiloxanes in the atmosphere of the Tibetan Plateau. Atmos Chem. Phys. 2018;18:8745–8755. doi: 10.5194/acp-18-8745-2018. [DOI] [Google Scholar]
- Jiang Y., Guo J., Zhou Y., Dong K., Zhang B., Han J., Wang Y., Chen Y.. Assessment of the internal and external exposure risks to methylsiloxanes in communities near a petroleum refinery. Sci. Total Environ. 2023;900:166314. doi: 10.1016/j.scitotenv.2023.166314. [DOI] [PubMed] [Google Scholar]
- Horii Y., Ohtsuka N., Minomo K., Takemine S., Motegi M., Hara M.. Distribution characteristics of methylsiloxanes in atmospheric environment of Saitama, Japan: Diurnal and seasonal variations and emission source apportionment. Sci. Total Environ. 2021;754:142399. doi: 10.1016/j.scitotenv.2020.142399. [DOI] [PubMed] [Google Scholar]
- Zhu J., Wong S. L., Cakmak S.. Nationally Representative Levels of Selected Volatile Organic Compounds in Canadian Residential Indoor Air: Population-Based Survey. Environ. Sci. Technol. 2013;47(23):13276–13283. doi: 10.1021/es403055e. [DOI] [PubMed] [Google Scholar]
- Pieri F., Katsoyiannis A., Martellini T., Hughes D., Jones K. C., Cincinelli A.. Occurrence of linear and cyclic volatile methyl siloxanes in indoor air samples (UK and Italy) and their isotopic characterization. Environ. Int. 2013;59:363–371. doi: 10.1016/j.envint.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Guo J., Zhou Y., Wang Y., Zhang B., Zhang J.. Assessment of internal exposure to methylsiloxanes in children and associated non-dietary exposure risk. Environ. Int. 2021;154:106672. doi: 10.1016/j.envint.2021.106672. [DOI] [PubMed] [Google Scholar]
- Xu L., Shi Y., Liu N., Cai Y.. Methyl siloxanes in environmental matrices and human plasma/fat from both general industries and residential areas in China. Sci. Total Environ. 2015;505:454–63. doi: 10.1016/j.scitotenv.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Companioni-Damas E. Y., Santos F. J., Galceran M. T.. Linear and cyclic methylsiloxanes in air by concurrent solvent recondensation–large volume injection–gas chromatography–mass spectrometry. Talanta. 2014;118:245–252. doi: 10.1016/j.talanta.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Gabriel M. F., Felgueiras F., Batista R., Ribeiro C., Ramos E., Mourão Z., de Oliveira Fernandes E.. Indoor environmental quality in households of families with infant twins under 1 year of age living in Porto. Environ. Res. 2021;198:110477. doi: 10.1016/j.envres.2020.110477. [DOI] [PubMed] [Google Scholar]
- Sha B., Dahlberg A.-K., Wiberg K., Ahrens L.. Fluorotelomer alcohols (FTOHs), brominated flame retardants (BFRs), organophosphorus flame retardants (OPFRs) and cyclic volatile methylsiloxanes (cVMSs) in indoor air from occupational and home environments. Environ. Pollut. 2018;241:319–330. doi: 10.1016/j.envpol.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Meepage J. N., Welker J. K., Meyer C. M., Mohammadi S., Stanier C. O., Stone E. A.. Advances in the Separation and Detection of Secondary Organic Aerosol Produced by Decamethylcyclopentasiloxane (D5) in Laboratory-Generated and Ambient Aerosol. ACS ES&T Air. 2024;1(5):365–375. doi: 10.1021/acsestair.3c00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Xie H.-B., Elm J., Guo X., Fu Z., Chen J.. Formation of Low-Volatile Products and Unexpected High Formaldehyde Yield from the Atmospheric Oxidation of Methylsiloxanes. Environ. Sci. Technol. 2020;54(12):7136–7145. doi: 10.1021/acs.est.0c01090. [DOI] [PubMed] [Google Scholar]
- Fromme H., Debiak M., Sagunski H., Röhl C., Kraft M., Kolossa-Gehring M.. The German approach to regulate indoor air contaminants. Int. J. Hyg and Environ. Health. 2019;222(3):347–354. doi: 10.1016/j.ijheh.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Wang D.-G., Norwood W., Alaee M., Byer J. D., Brimble S.. Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment. Chemosphere. 2013;93(5):711–725. doi: 10.1016/j.chemosphere.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Surita S. C., Tansel B.. Emergence and fate of cyclic volatile polydimethylsiloxanes (D4, D5) in municipal waste streams: Release mechanisms, partitioning and persistence in air, water, soil and sediments. Sci. Total Environ. 2014;468–469:46–52. doi: 10.1016/j.scitotenv.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cheng J., Tang Z., Ma Y., Yin H., Meng T., Sun J.. Methyl siloxanes in road dust from a large silicone manufacturing site in China: implications of human exposure. Environ. Sci. Pollut Res. Int. 2021;28(13):16054–16064. doi: 10.1007/s11356-020-11773-1. [DOI] [PubMed] [Google Scholar]
- Le T. M., Le Quang H., Tran A. H., Quang M. B., Vu N. D., Thi H. N., Khanh H. V., Kannan K., Tran T. M.. Co-occurrence of phthalic acid esters (PAEs) and cyclic volatile methylsiloxanes (cVMSs) in fine particulate matter (PM0.5 and PM0.1) collected from an industrial area in Vietnam. Environ. Res. 2023;237:117018. doi: 10.1016/j.envres.2023.117018. [DOI] [PubMed] [Google Scholar]
- Yao P., Holzinger R., Materić D., Oyama B. S., de Fátima Andrade M., Paul D., Ni H., Noto H., Huang R.-J., Dusek U.. Methylsiloxanes from Vehicle Emissions Detected in Aerosol Particles. Environ. Sci. Technol. 2023;57(38):14269–14279. doi: 10.1021/acs.est.3c03797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T., Su S., Cheng J., Zhong F., Tang Z.. Methylsiloxanes in street dust from Hefei, China: Distribution, sources, and human exposure. Environ. Res. 2021;201:111513. doi: 10.1016/j.envres.2021.111513. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Qiu X., Shi X., Zhu T.. Identification of organosiloxanes in ambient fine particulate matters using an untargeted strategy via gas chromatography and time-of-flight mass spectrometry. Environ. Pollut. 2021;271:116128. doi: 10.1016/j.envpol.2020.116128. [DOI] [PubMed] [Google Scholar]
- Brandt, B. ; Kletzer, E. ; Pilz, H. ; Hadzhiyska, D. ; Seizov, P. . Silicon-Chemistry Carbon Balance: An assessment of Greenhouse Gas Emissions and Reductions; 2012.
- Tran T. M., Abualnaja K. O., Asimakopoulos A. G., Covaci A., Gevao B., Johnson-Restrepo B., Kumosani T. A., Malarvannan G., Minh T. B., Moon H. B., Nakata H., Sinha R. K., Kannan K.. A survey of cyclic and linear siloxanes in indoor dust and their implications for human exposures in twelve countries. Environ. Int. 2015;78:39–44. doi: 10.1016/j.envint.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Hoang A. Q., Trinh H. T., Nguyen H. M. N., Nguyen T. Q., Nguyen T. X., Duc T. V., Nguyen T. T., Do T. Q., Minh T. B., Tran T. M.. Assessment of cyclic volatile methyl siloxanes (CVMSs) in indoor dust from different micro-environments in northern and central Vietnam. Environmental Geochemistry and Health. 2023;45(5):1711–1722. doi: 10.1007/s10653-022-01298-6. [DOI] [PubMed] [Google Scholar]
- Guo J., Zhou Y., Sun M., Cui J., Zhang B., Zhang J.. Methylsiloxanes in plasma from potentially exposed populations and an assessment of the associated inhalation exposure risk. Environ. Int. 2020;143:105931. doi: 10.1016/j.envint.2020.105931. [DOI] [PubMed] [Google Scholar]
- Lu Y., Yuan T., Yun S. H., Wang W., Wu Q., Kannan K.. Occurrence of cyclic and linear siloxanes in indoor dust from China, and implications for human exposures. Environ. Sci. Technol. 2010;44(16):6081–7. doi: 10.1021/es101368n. [DOI] [PubMed] [Google Scholar]
- Liu N., Xu L., Cai Y.. Methyl siloxanes in barbershops and residence indoor dust and the implication for human exposures. Sci. Total Environ. 2018;618:1324–1330. doi: 10.1016/j.scitotenv.2017.09.250. [DOI] [PubMed] [Google Scholar]
- Capela D., Ratola N., Alves A., Homem V.. Volatile methylsiloxanes through wastewater treatment plants – A review of levels and implications. Environ. Int. 2017;102:9–29. doi: 10.1016/j.envint.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Dong K., Zhou Y., Guo J., Jiang Y., Zhang B., Wang Y., Chen Y.. Cyclic methylsiloxanes in wastewater treatment plants: Occurrence, emissions, environmental distributions, and occupational exposure. Sci. Total Environ. 2024;951:175524. doi: 10.1016/j.scitotenv.2024.175524. [DOI] [PubMed] [Google Scholar]
- Bernardo F., Ratola N., Sánchez-Soberón F., Alves A., Homem V.. Presence, behaviour, and risk assessment of volatile methylsiloxanes in wastewater: A year-long comprehensive study within a wastewater treatment plant. Sci. Total Environ. 2024;951:175486. doi: 10.1016/j.scitotenv.2024.175486. [DOI] [PubMed] [Google Scholar]
- Wang D.-G., Aggarwal M., Tait T., Brimble S., Pacepavicius G., Kinsman L., Theocharides M., Smyth S. A., Alaee M.. Fate of anthropogenic cyclic volatile methylsiloxanes in a wastewater treatment plant. Water Res. 2015;72:209–217. doi: 10.1016/j.watres.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Horii Y., Nojiri K., Minomo K., Motegi M., Kannan K.. Volatile methylsiloxanes in sewage treatment plants in Saitama, Japan: Mass distribution and emissions. Chemosphere. 2019;233:677–686. doi: 10.1016/j.chemosphere.2019.05.247. [DOI] [PubMed] [Google Scholar]
- Lan Y., L J., Qian C., Gong F., Geng H., Liu Z., Wu J., Li Q.. Seasonal variation of cVMS concentrations in the influents and effluents of wastewater treatment plants. Environ. Chem. 2019;38(05):1171–1179. [Google Scholar]
- Chen W., Lee S., Moon H.-B.. Cyclic and linear siloxane contamination in sediment and invertebrates around a thermal power plant in Korea: Source impact, distribution, seasonal variation, and potential for bioaccumulation. Chemosphere. 2024;349:140779. doi: 10.1016/j.chemosphere.2023.140779. [DOI] [PubMed] [Google Scholar]
- Horii Y., Minomo K., Ohtsuka N., Motegi M., Nojiri K., Kannan K.. Distribution characteristics of volatile methylsiloxanes in Tokyo Bay watershed in Japan: Analysis of surface waters by purge and trap method. Sci. Total Environ. 2017;586:56–65. doi: 10.1016/j.scitotenv.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Hong W.-J., Jia H., Liu C., Zhang Z., Sun Y., Li Y.-F.. Distribution, source, fate and bioaccumulation of methyl siloxanes in marine environment. Environ. Pollut. 2014;191:175–181. doi: 10.1016/j.envpol.2014.04.033. [DOI] [PubMed] [Google Scholar]
- Sanchís J., Martínez E., Ginebreda A., Farré M., Barceló D.. Occurrence of linear and cyclic volatile methylsiloxanes in wastewater, surface water and sediments from Catalonia. Sci. Total Environ. 2013;443:530–538. doi: 10.1016/j.scitotenv.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Companioni-Damas E. Y., Santos F. J., Galceran M. T.. Analysis of linear and cyclic methylsiloxanes in water by headspace-solid phase microextraction and gas chromatography–mass spectrometry. Talanta. 2012;89:63–69. doi: 10.1016/j.talanta.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Nu Nguyen H. M., Khieu H. T., Ta N. A., Le H. Q., Nguyen T. Q., Do T. Q., Hoang A. Q., Kannan K., Tran T. M.. Distribution of cyclic volatile methylsiloxanes in drinking water, tap water, surface water, and wastewater in Hanoi. Vietnam. Environ. Pollut. 2021;285:117260. doi: 10.1016/j.envpol.2021.117260. [DOI] [PubMed] [Google Scholar]
- Guo J., Zhou Y., Zhang B., Zhang J.. Distribution and evaluation of the fate of cyclic volatile methyl siloxanes in the largest lake of southwest China. Sci. Total Environ. 2019;657:87–95. doi: 10.1016/j.scitotenv.2018.11.454. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yin G., Sheng G. D., Yu Z., Yin D.. Distribution and spatial variation of volatile methylsiloxanes in surface water and wastewater from the Yangtze River Basin, China. Sci. Total Environ. 2024;929:172541. doi: 10.1016/j.scitotenv.2024.172541. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shen M., Tian Y., Zeng G.. Cyclic volatile methylsiloxanes in sediment, soil, and surface water from Dongting Lake, China. J. Soils and Sediments. 2018;18(5):2063–2071. doi: 10.1007/s11368-018-1948-9. [DOI] [Google Scholar]
- Xu S., Kozerski G., Mackay D.. Critical Review and Interpretation of Environmental Data for Volatile Methylsiloxanes: Partition Properties. Environ. Sci. Technol. 2014;48(20):11748–11759. doi: 10.1021/es503465b. [DOI] [PubMed] [Google Scholar]
- Xu L., Xu S., Zhi L., He X., Zhang C., Cai Y.. Methylsiloxanes Release from One Landfill through Yearly Cycle and Their Removal Mechanisms (Especially Hydroxylation) In Leachates. Environ. Sci. Technol. 2017;51(21):12337–12346. doi: 10.1021/acs.est.7b03624. [DOI] [PubMed] [Google Scholar]
- Sánchez-Brunete C., Miguel E., Albero B., Tadeo J. L.. Determination of cyclic and linear siloxanes in soil samples by ultrasonic-assisted extraction and gas chromatography–mass spectrometry. J. Chromatogr. A. 2010;1217(45):7024–7030. doi: 10.1016/j.chroma.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Wang D.-G., Steer H., Tait T., Williams Z., Pacepavicius G., Young T., Ng T., Smyth S. A., Kinsman L., Alaee M.. Concentrations of cyclic volatile methylsiloxanes in biosolid amended soil, influent, effluent, receiving water, and sediment of wastewater treatment plants in Canada. Chemosphere. 2013;93(5):766–773. doi: 10.1016/j.chemosphere.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Xu S., Warner N., Bohlin-Nizzetto P., Durham J., McNett D.. Long-range transport potential and atmospheric persistence of cyclic volatile methylsiloxanes based on global measurements. Chemosphere. 2019;228:460–468. doi: 10.1016/j.chemosphere.2019.04.130. [DOI] [PubMed] [Google Scholar]
- Shi Y., Xu S., Xu L., Cai Y.. Distribution, Elimination, and Rearrangement of Cyclic Volatile Methylsiloxanes in Oil-Contaminated Soil of the Shengli Oilfield. China. Environ. Sci. Technol. 2015;49(19):11527–11535. doi: 10.1021/acs.est.5b03197. [DOI] [PubMed] [Google Scholar]
- Xu L., Huang Z., Zhang Q., Xiang X., Zhang S., Cai Y.. Methylsiloxanes and Their Brominated Products in One e-Waste Recycling Area in China: Emission, Environmental Distribution, and Elimination. Environ. Sci. Technol. 2020;54(7):4267–4274. doi: 10.1021/acs.est.0c00056. [DOI] [PubMed] [Google Scholar]
- Duan Xiaoli H. N., Wang B., Zhao X., Nie J., Qian Y., Wang X., Zhang J.. Development of Exposure Factors Research Methods in Environmental Health Risk Assessment. J. Environ. Health. 2012;29(2):6. [Google Scholar]
- U.S. EPA . Exposure Factors Handbook, 2011. Edition (Final Report). https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NCEA&dirEntryId=236252.
- U.S. EPA . Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part a) (No. EPA/540/1-89/002). https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part.
- Jiang Y., Guo J., Zhou Y., Dong K., Zhang B., Han J., Wang Y., Chen Y.. Assessment of the internal and external exposure risks to methylsiloxanes in communities near a petroleum refinery. Sci. Total Environ. 2023;900:166314. doi: 10.1016/j.scitotenv.2023.166314. [DOI] [PubMed] [Google Scholar]
- ECETOC . Derivation of assessment factors for human health risk assessment. Technical report No. 86. ECETOC (European Center for Ecotoxicology and Toxicology of chemicals), Belgium. https://www.ecetoc.org/wp-content/uploads/2014/08/ECETOC-TR-086.pdf. [Google Scholar]
- Duan, X. , Zhao, X. , Wang, B. , Chen, Y. , Cao, S. . Highlights of the Chinese Exposure Factors Handbook (Adults). 2015. [Google Scholar]
- Cheng X., Zhang Y., Gao Y., Tian Y.. Progress in cumulative risk assessment of human health from combined exposure to environmental pollutants. Journal of Shanghai Jiao Tong University (Medical Science) 2024;44(8):1037–1043. [Google Scholar]
- European chemicals Agency. Toxicological information: toxicokinetics, metabolism and distribution. https://echa.europa.eu/pt/brief-profile/-/briefprofile/100.007.969.
- Ferreira T., Homem V., Cereceda-Balic F., Fadic X., Alves A., Ratola N.. Are volatile methylsiloxanes in downcycled tire microplastics? Levels and human exposure estimation in synthetic turf football fields. Environ. Sci. Pollut Res. Int. 2024;31(8):11950–11967. doi: 10.1007/s11356-024-31832-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lam J. C. W., Zhu M., Wang F., Zhou W., Du B., Zeng L., Zeng E. Y.. Combined Effects of Dust and Dietary Exposure of Occupational Workers and Local Residents to Short- and Medium-Chain Chlorinated Paraffins in a Mega E-Waste Recycling Industrial Park in South China. Environ. Sci. Technol. 2018;52(20):11510–11519. doi: 10.1021/acs.est.8b02625. [DOI] [PubMed] [Google Scholar]
- Fan X., Wang Z., Li Y., Wang H., Fan W., Dong Z.. Estimating the dietary exposure and risk of persistent organic pollutants in China: A national analysis. Environ. Pollut. 2021;288:117764. doi: 10.1016/j.envpol.2021.117764. [DOI] [PubMed] [Google Scholar]
- Lu C., Holbrook C. M., Andres L. M.. The Implications of Using a Physiologically Based Pharmacokinetic (PBPK) Model for Pesticide Risk Assessment. Environ. Health Perspect. 2010;118(1):125–130. doi: 10.1289/ehp.0901144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepika D., Sharma R. P., Schuhmacher M., Kumar V.. Risk Assessment of Perfluorooctane Sulfonate (PFOS) using Dynamic Age Dependent Physiologically based Pharmacokinetic Model (PBPK) across Human Lifetime. Environ. Res. 2021;199:111287. doi: 10.1016/j.envres.2021.111287. [DOI] [PubMed] [Google Scholar]
- Liu N., Sun H., Xu L., Cai Y.. Methylsiloxanes in petroleum refinery facility: Their sources, emissions, environmental distributions and occupational exposure. Environ. Int. 2021;152:106471. doi: 10.1016/j.envint.2021.106471. [DOI] [PubMed] [Google Scholar]
- Capela D., Alves A., Homem V., Santos L.. From the shop to the drain Volatile methylsiloxanes in cosmetics and personal care products. Environ. Int. 2016;92–93:50–62. doi: 10.1016/j.envint.2016.03.016. [DOI] [PubMed] [Google Scholar]
- EFSA Panel of Food Additives and Flavourings; Younes M., Aquilina G., Castle L., Engel K.-H., Fowler P., Frutos Fernandez M. J., Furst P., Gurtler R., Gundert-Remy U., Husøy T., Manco M., Mennes W., Passamonti S., Shah R., Waalkens-Berendsen D. H., Wolfle D., Wright M., Boon P., Tobback P., Giarola A., Rincon A. M., Tard A., Moldeus P.. Re-evaluation of dimethyl polysiloxane (E 900) as a food additive. EFSA Journal. 2020;18(5):e06107. doi: 10.2903/j.efsa.2020.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Zhang J., Guo Y., Zhang X., Lan S., Feng D.. Residue Analysis and Exposure Assessment of Methylsiloxane Oligomers in Fried Chicken and French Fries. Science and Technology of Food Industry. 2024;45(01):216–223. [Google Scholar]
- Sanchís J., Llorca M., Picó Y., Farré M., Barceló D.. Volatile dimethylsiloxanes in market seafood and freshwater fish from the Xúquer River. Spain. Sci. Total Environ. 2016;545–546:236–243. doi: 10.1016/j.scitotenv.2015.12.032. [DOI] [PubMed] [Google Scholar]
- Fromme H., Witte M., Fembacher L., Gruber L., Hagl T., Smolic S., Fiedler D., Sysoltseva M., Schober W.. Siloxane in baking moulds, emission to indoor air and migration to food during baking with an electric oven. Environ. Int. 2019;126:145–152. doi: 10.1016/j.envint.2019.01.081. [DOI] [PubMed] [Google Scholar]
- Sapozhnikova Y.. Non-targeted screening of chemicals migrating from paper-based food packaging by GC-Orbitrap mass spectrometry. Talanta. 2021;226:122120. doi: 10.1016/j.talanta.2021.122120. [DOI] [PubMed] [Google Scholar]
- Zhang K., Wong J. W., Begley T. H., Hayward D. G., Limm W.. Determination of siloxanes in silicone products and potential migration to milk, formula and liquid simulants. Food Addit Contam Part A Chem. Anal Control Expo Risk Assess. 2012;29(8):1311–21. doi: 10.1080/19440049.2012.684891. [DOI] [PubMed] [Google Scholar]
- Xu L., Shi Y., Wang T., Dong Z., Su W., Cai Y.. Methyl siloxanes in environmental matrices around a siloxane production facility, and their distribution and elimination in plasma of exposed population. Environ. Sci. Technol. 2012;46(21):11718–26. doi: 10.1021/es3023368. [DOI] [PubMed] [Google Scholar]
- Guo J., Zhou Y., Wang Y., Chen Y., Zhang B., Zhang J.. Methylsiloxanes risk assessment combining external and internal exposure for college students. Sci. Total Environ. 2022;845:157379. doi: 10.1016/j.scitotenv.2022.157379. [DOI] [PubMed] [Google Scholar]
- Horii Y., Kannan K.. Survey of Organosilicone Compounds, Including Cyclic and Linear Siloxanes, in Personal-Care and Household Products. Arch. Environ. Contam. Toxicol. 2008;55(4):701–710. doi: 10.1007/s00244-008-9172-z. [DOI] [PubMed] [Google Scholar]
- Dudzina T., von Goetz N., Bogdal C., Biesterbos J. W. H., Hungerbühler K.. Concentrations of cyclic volatile methylsiloxanes in European cosmetics and personal care products: Prerequisite for human and environmental exposure assessment. Environ. Intern. 2014;62:86–94. doi: 10.1016/j.envint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Krenczkowska D., Mojsiewicz-Pieńkowska K., Wielgomas B., Bazar D., Jankowski Z.. Ex Vivo Human Skin is not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure. Pharmaceutics. 2020;12(6):586. doi: 10.3390/pharmaceutics12060586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenczkowska D., Mojsiewicz-Pieńkowska K., Wielgomas B., Cal K., Bartoszewski R., Bartoszewska S., Jankowski Z.. The consequences of overcoming the human skin barrier by siloxanes (silicones) Part 1. Penetration and permeation depth study of cyclic methyl siloxanes. Chemosphere. 2019;231:607–623. doi: 10.1016/j.chemosphere.2018.09.154. [DOI] [PubMed] [Google Scholar]
- Reddy M. B., Looney R. J., Utell M. J., Plotzke K. P., Andersen M. E.. Modeling of human dermal absorption of octamethylcyclotetrasiloxane (D(4)) and decamethylcyclopentasiloxane (D(5)) Toxicol. Sci. 2007;99(2):422–31. doi: 10.1093/toxsci/kfm174. [DOI] [PubMed] [Google Scholar]
- Capela D., Alves A., Homem V., Santos L.. From the shop to the drain - Volatile methylsiloxanes in cosmetics and personal care products. Environ. Int. 2016;92–93:50–62. doi: 10.1016/j.envint.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Chen W., Oh J.-S., Lim J.-E., Moon H.-B.. Occurrence, time trends, and human exposure of siloxanes and synthetic musk compounds in indoor dust from Korean homes. Ecotox Environ. Safe. 2023;266:115538–115545. doi: 10.1016/j.ecoenv.2023.115538. [DOI] [PubMed] [Google Scholar]
- Temeltaş, E. , Getting Ahead of the Trend for Silicone-free Cosmetics. SOFW Journal 2022. [Google Scholar]
- Bettenhausen C.. Swapping Out Silicone in Our Skin and Hair Products. Chem. Eng. News. 2023;101(17):22. doi: 10.47287/cen-10117-feature2. [DOI] [Google Scholar]
- Tender Announcement for the Sub-project of Research on Environmental Risks and Domestic and International Industry Management and Control of D4, D5, and D6. Foreign Economic Cooperation Office, FECO: 2018. [Google Scholar]
- Brunet C. E., Marek R. F., Stanier C. O., Hornbuckle K. C.. Concentrations of Volatile Methyl Siloxanes in New York City Reflect Emissions from Personal Care and Industrial Use. Environ. Sci. Technol. 2024;58(20):8835–8845. doi: 10.1021/acs.est.3c10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Shi Y., Wang T., Dong Z., Su W., Cai Y.. Methyl Siloxanes in Environmental Matrices around a Siloxane Production Facility, and Their Distribution and Elimination in Plasma of Exposed Population. Environ. Sci. Technol. 2012;46(21):11718–11726. doi: 10.1021/es3023368. [DOI] [PubMed] [Google Scholar]
- Tran T. M., Le H. T., Vu N. D., Minh Dang G. H., Minh T. B., Kannan K.. Cyclic and linear siloxanes in indoor air from several northern cities in Vietnam: Levels, spatial distribution and human exposure. Chemosphere. 2017;184:1117–1124. doi: 10.1016/j.chemosphere.2017.06.092. [DOI] [PubMed] [Google Scholar]
- Martellini T., Berlangieri C., Dei L., Carretti E., Santini S., Barone A., Cincinelli A.. Indoor levels of volatile organic compounds at Florentine museum environments in Italy. Indoor Air. 2020;30(5):900–913. doi: 10.1111/ina.12659. [DOI] [PubMed] [Google Scholar]
- Gallego E., Perales J. F., Roca F. J., Guardino X., Gadea E.. Volatile methyl siloxanes (VMS) concentrations in outdoor air of several Catalan urban areas. Atmos. Environ. 2017;155:108. doi: 10.1016/j.atmosenv.2017.02.013. [DOI] [Google Scholar]
- Kierkegaard A., McLachlan M. S.. Determination of linear and cyclic volatile methylsiloxanes in air at a regional background site in Sweden. Atmos. Environ. 2013;80:322–329. doi: 10.1016/j.atmosenv.2013.08.001. [DOI] [Google Scholar]
- Niu H., Su X., Li Q., Zhao J., Hou M., Dong S., Yan X., Sun J., Feng J.. Dimethylsiloxanes in dust from nine indoor microenvironments of Henan Province: Occurrence and human exposure assessment. Sci. Total Environ. 2023;903:166546. doi: 10.1016/j.scitotenv.2023.166546. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Tang Z., He Y., Wang F., Lyu Y.. Occurrence of methylsiloxanes in indoor store dust in China and potential human exposure. Environ. Res. 2023;218:114969. doi: 10.1016/j.envres.2022.114969. [DOI] [PubMed] [Google Scholar]
- Liu N., Xu L., Cai Y.. Methyl siloxanes in barbershops and residence indoor dust and the implication for human exposures. Sci. Total Environ. 2018;618:1324–1330. doi: 10.1016/j.scitotenv.2017.09.250. [DOI] [PubMed] [Google Scholar]
- Xu L., Zhi L., Cai Y.. Methylsiloxanes in children silicone-containing products from China: Profiles, leaching, and children exposure. Environ. Int. 2017;101:165–172. doi: 10.1016/j.envint.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Sánchez-Soberón F., Ratola N.. Seasonal occurrence, concentrations, and occupational exposure to VMSs in different environments of a WWTP. Environ. Pollut. 2022;315:120423. doi: 10.1016/j.envpol.2022.120423. [DOI] [PubMed] [Google Scholar]