Abstract
The amplitude-frequency histogram of spontaneous miniature endplate potentials follows a Gaussian distribution at mature endplates. This distribution gives the mean and variance of the quantum of transmitter. According to the vesicle hypothesis, this quantum is due to exocytosis of the contents of a single synaptic vesicle. Multimodal amplitude-frequency histograms are observed in varying degrees at developing endplates and at peripheral and central synapses, each of which has a specific active zone structure. These multimodal histograms may be due to the near synchronous exocytosis of more than one vesicle. In the present work, a theoretical treatment is given of the rise of intraterminal calcium after the stochastic opening of a calcium channel within a particular active zone geometry. The stochastic interaction of this calcium with the vesicle-associated proteins involved in exocytosis is then used to calculate the probability of quantal secretions from one or several vesicles at each active zone type. It is shown that this procedure can account for multiquantal spontaneous release that may occur at varicosities and boutons, compared with that at the active zones of motor nerve terminals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Moor H., Pfenninger K. Synaptic fine structure. Adv Cytopharmacol. 1971 May;1:273–290. [PubMed] [Google Scholar]
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Richerson G. B., Stevens C. F. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Fisher C., Florin T., Quine M., Robinson J. The effect of calcium ions and temperature on the binomial parameters that control acetylcholine release by a nerve impulse at amphibian neuromuscular synapses. J Physiol. 1977 Oct;271(3):641–672. doi: 10.1113/jphysiol.1977.sp012019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
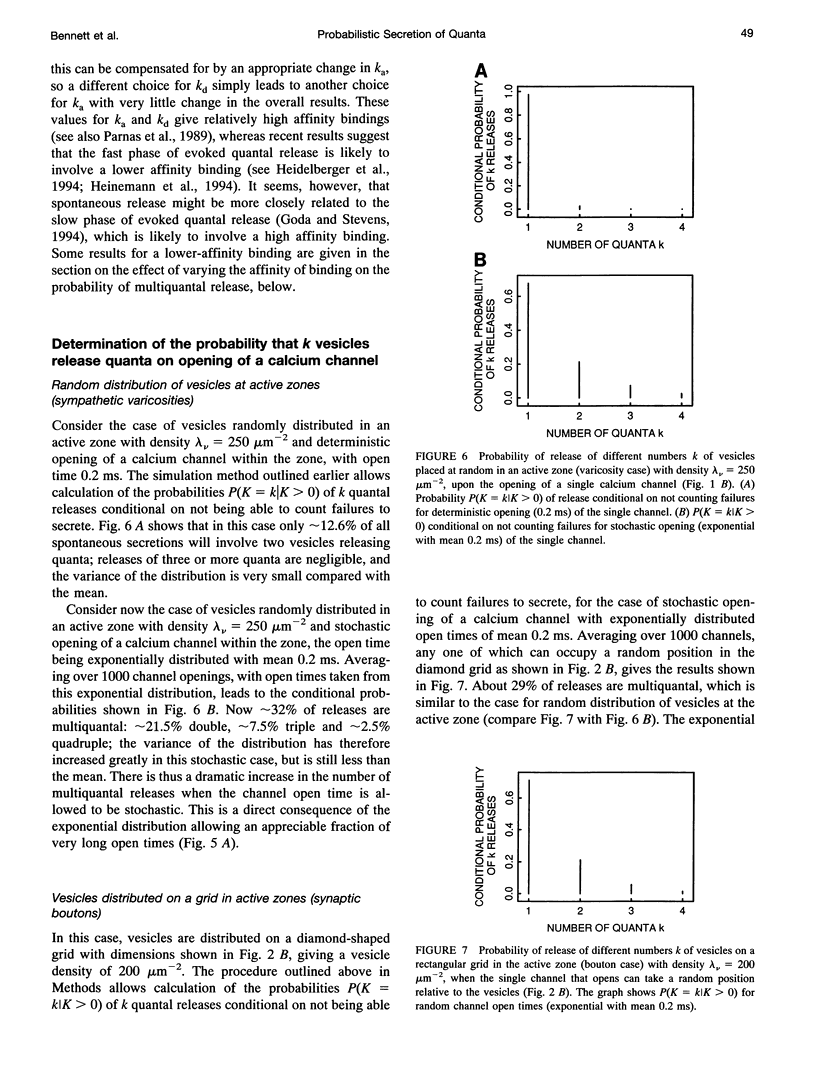
- Bennett M. R. Quantal secretion from single visualized synaptic varicosities of sympathetic nerve terminals. Adv Second Messenger Phosphoprotein Res. 1994;29:399–423. doi: 10.1016/s1040-7952(06)80028-5. [DOI] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. Effects of stimulation on the multiquantal spontaneous synaptic potentials in guinea pig hypogastric ganglia. Neurosci Lett. 1981 Feb 23;22(1):57–61. doi: 10.1016/0304-3940(81)90285-8. [DOI] [PubMed] [Google Scholar]
- Cohen I. S., van der Kloot W., Barton S. B. Bursts of miniature end-plate potentials can be released from localized regions of the frog motor nerve terminal. Brain Res. 1981 Sep 28;221(2):382–386. doi: 10.1016/0006-8993(81)90787-3. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A., Li C. G., Rand M. J., Reid J. J., Thaina P., Wong-Dusting H. K. Effects of omega-conotoxin GVIA on autonomic neuroeffector transmission in various tissues. Br J Pharmacol. 1990 Oct;101(2):437–447. doi: 10.1111/j.1476-5381.1990.tb12727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
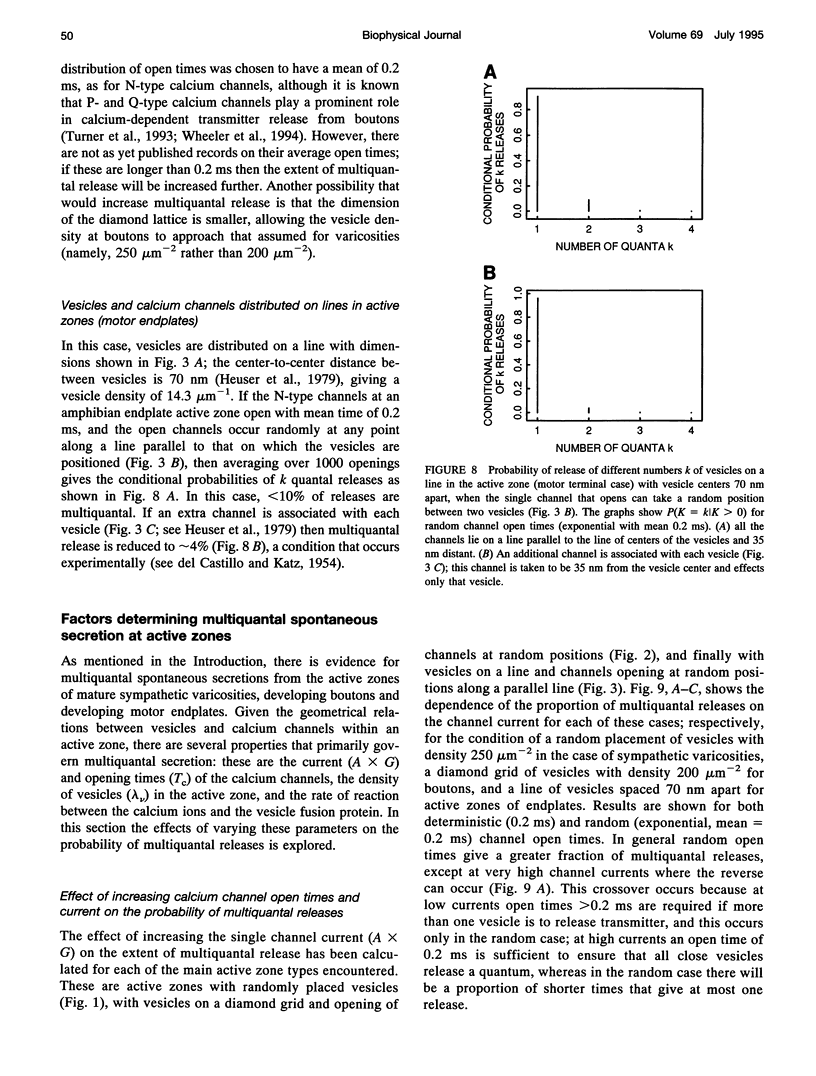
- Delcour A. H., Lipscombe D., Tsien R. W. Multiple modes of N-type calcium channel activity distinguished by differences in gating kinetics. J Neurosci. 1993 Jan;13(1):181–194. doi: 10.1523/JNEUROSCI.13-01-00181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
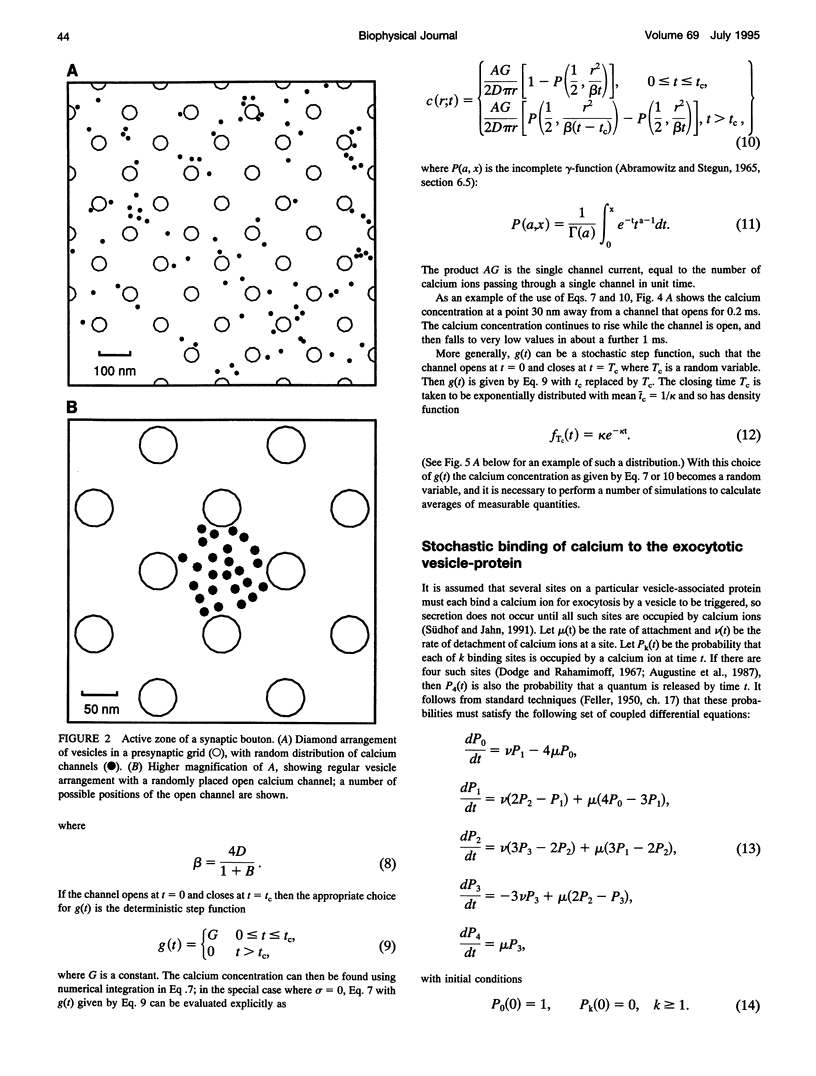
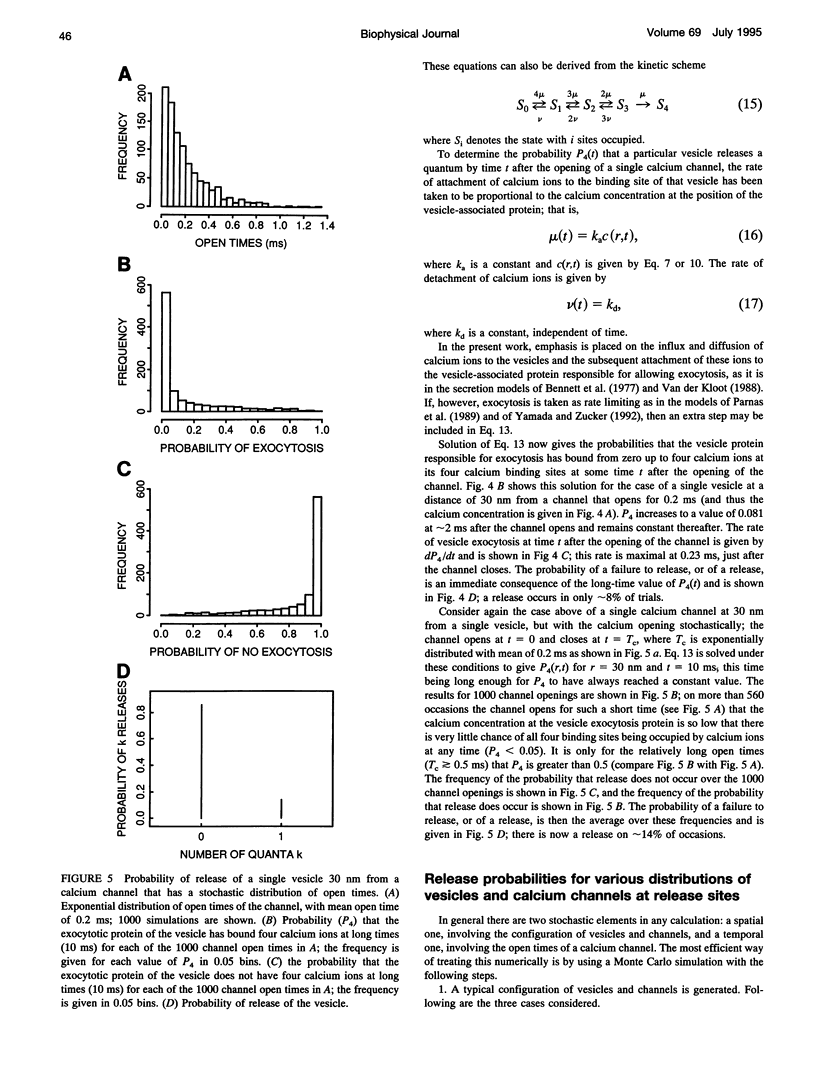
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Akert K., Sandri C., Moor H. Ultrastructure of the "active zone" in the frog neuromuscular junction. Brain Res. 1973 Nov 23;62(2):373–380. doi: 10.1016/0006-8993(73)90699-9. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
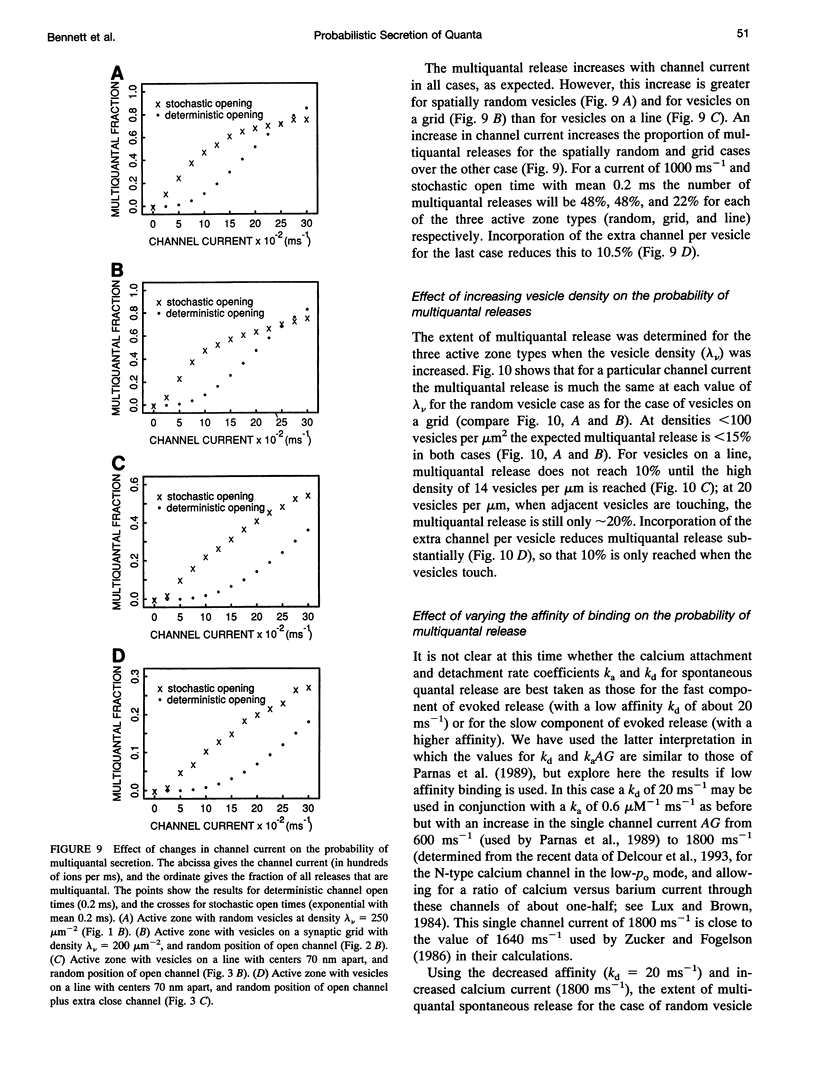
- Ellinor P. T., Zhang J. F., Randall A. D., Zhou M., Schwarz T. L., Tsien R. W., Horne W. A. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993 Jun 3;363(6428):455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Erxleben C., Kriebel M. E. Subunit composition of the spontaneous miniature end-plate currents at the mouse neuromuscular junction. J Physiol. 1988 Jun;400:659–676. doi: 10.1113/jphysiol.1988.sp017142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
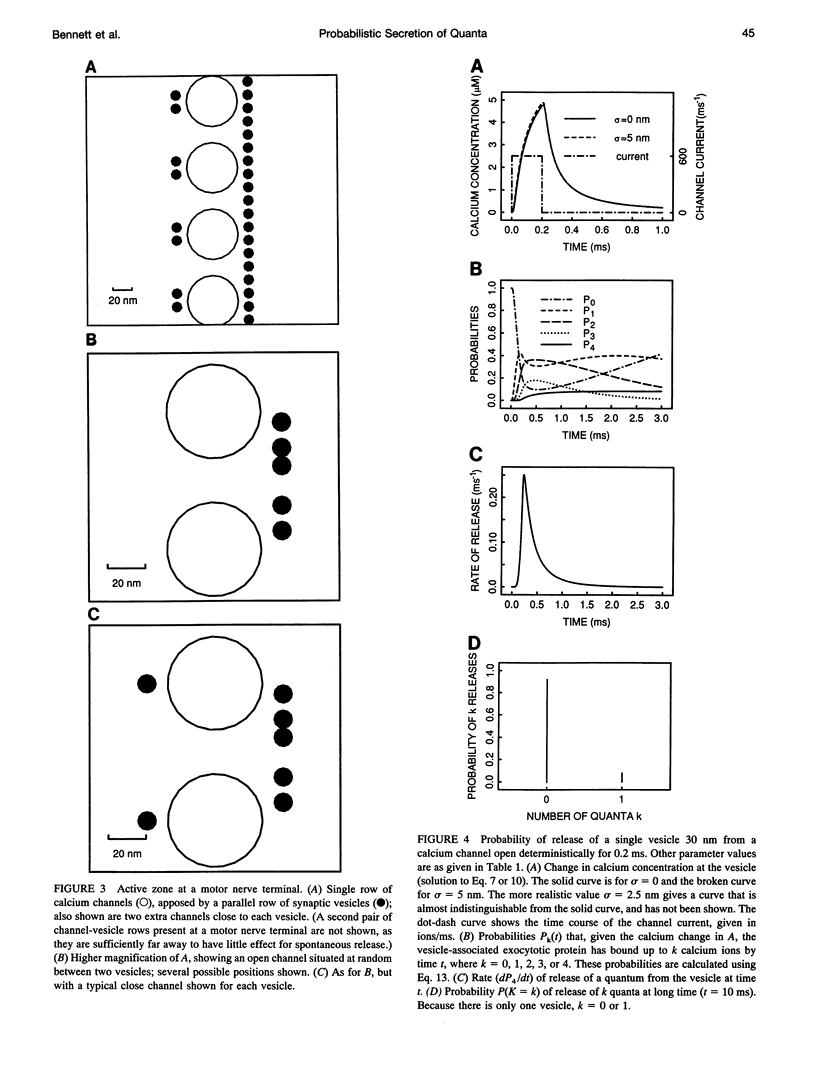
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
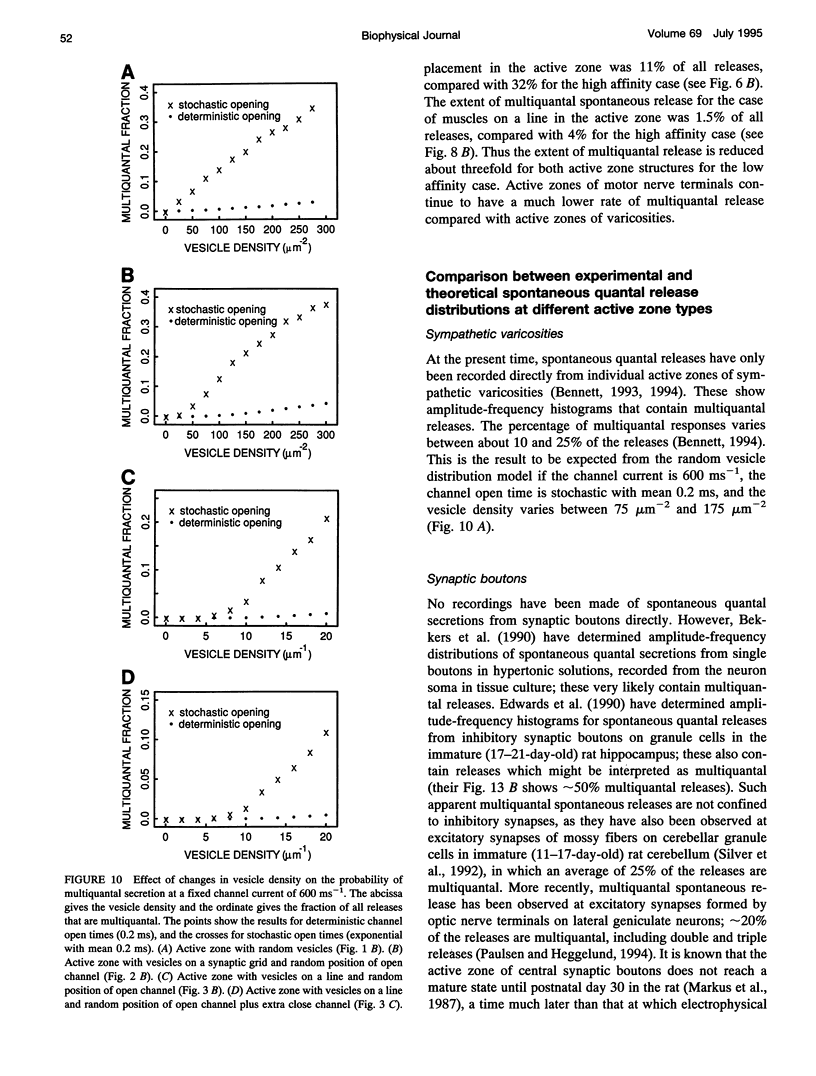
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y., Stevens C. F. Two components of transmitter release at a central synapse. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Pawson P. A. Dependence of spontaneous release at frog junctions on synaptic strength, external calcium and terminal length. J Physiol. 1989 Nov;418:397–410. doi: 10.1113/jphysiol.1989.sp017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás A. I., Miles R., Sík A., Tóth K., Tamamaki N., Freund T. F. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993 Dec 16;366(6456):683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
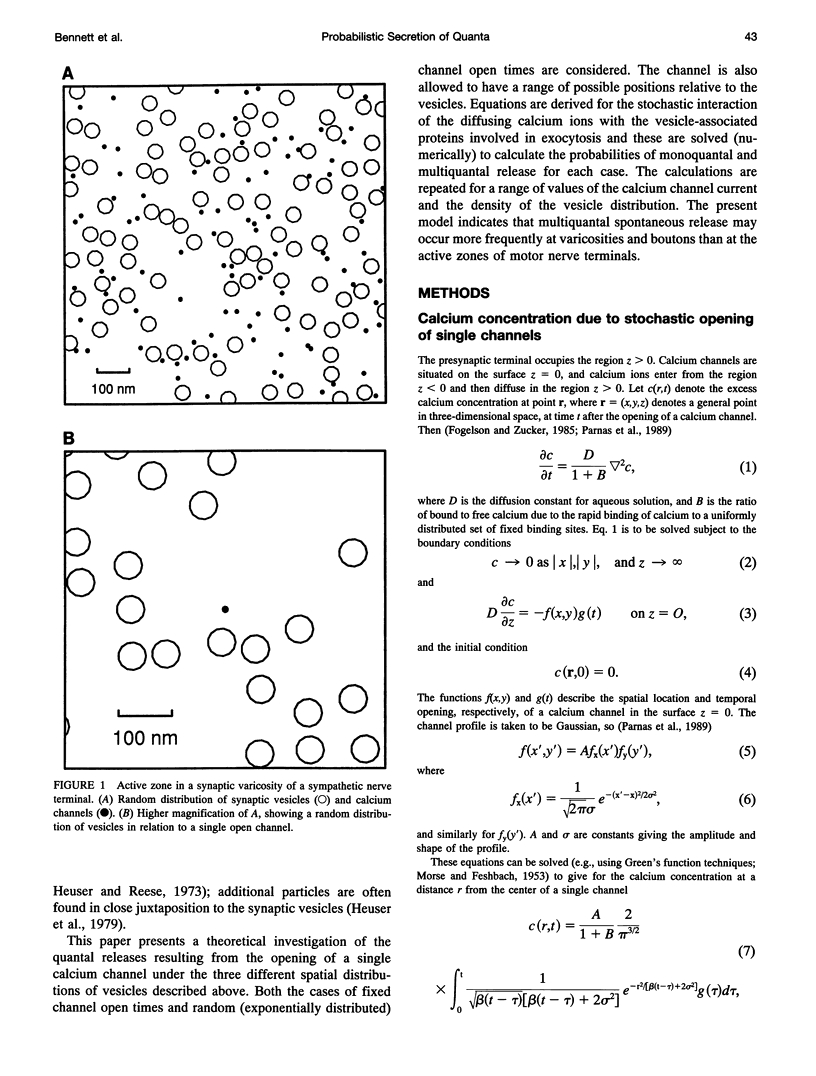
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne A. L., Kemp J. A. The effect of omega-conotoxin GVIA on synaptic transmission within the nucleus accumbens and hippocampus of the rat in vitro. Br J Pharmacol. 1991 Jul;103(3):1733–1739. doi: 10.1111/j.1476-5381.1991.tb09855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Ko C. P. Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs. J Neurocytol. 1985 Jun;14(3):487–512. doi: 10.1007/BF01217757. [DOI] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Llados F., Matteson D. R. Histograms of the unitary evoked potential of the mouse diaphragm show multiple peaks. J Physiol. 1982 Jan;322:211–222. doi: 10.1113/jphysiol.1982.sp014033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavidis N. A., Bennett M. R. Probabilistic secretion of quanta from visualized sympathetic nerve varicosities in mouse vas deferens. J Physiol. 1992 Aug;454:9–26. doi: 10.1113/jphysiol.1992.sp019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Lo Y. J., Wang T., Poo M. M. Repetitive impulse activity potentiates spontaneous acetylcholine secretion at developing neuromuscular synapses. J Physiol (Paris) 1991;85(2):71–78. [PubMed] [Google Scholar]
- Luebke J. I., Dunlap K., Turner T. J. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993 Nov;11(5):895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Brown A. M. Patch and whole cell calcium currents recorded simultaneously in snail neurons. J Gen Physiol. 1984 May;83(5):727–750. doi: 10.1085/jgp.83.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod G. T., Lavidis N. A., Bennett M. R. Calcium dependence of quantal secretion from visualized sympathetic nerve varicosities on the mouse vas deferens. J Physiol. 1994 Oct 1;480(Pt 1):61–70. doi: 10.1113/jphysiol.1994.sp020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus E. J., Petit T. L., LeBoutillier J. C. Synaptic structural changes during development and aging. Brain Res. 1987 Oct;432(2):239–248. doi: 10.1016/0165-3806(87)90048-4. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. An analysis of the release of acetylcholine from preganglionic nerve terminals. J Physiol. 1975 Feb;245(2):447–466. doi: 10.1113/jphysiol.1975.sp010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed N., Helm P. J., Rahamimoff R. Confocal microscopy reveals coordinated calcium fluctuations and oscillations in synaptic boutons. J Neurosci. 1993 Feb;13(2):632–649. doi: 10.1523/JNEUROSCI.13-02-00632.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Parnas H., Hovav G., Parnas I. Effect of Ca2+ diffusion on the time course of neurotransmitter release. Biophys J. 1989 May;55(5):859–874. doi: 10.1016/S0006-3495(89)82885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen O., Heggelund P. The quantal size at retinogeniculate synapses determined from spontaneous and evoked EPSCs in guinea-pig thalamic slices. J Physiol. 1994 Nov 1;480(Pt 3):505–511. doi: 10.1113/jphysiol.1994.sp020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Estimation of parameters for a model of transmitter release at synapses. Biometrics. 1976 Mar;32(1):61–68. [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., Rahamimoff R. Neuromuscular synapse: stochastic properties of spontaneous release of transmitter. Science. 1970 Nov 6;170(3958):648–649. doi: 10.1126/science.170.3958.648. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J., Charlton M. P. Transmission at voltage-clamped giant synapse of the squid: evidence for cooperativity of presynaptic calcium action. Proc Natl Acad Sci U S A. 1985 Jan;82(2):622–625. doi: 10.1073/pnas.82.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streichert L. C., Sargent P. B. Bouton ultrastructure and synaptic growth in a frog autonomic ganglion. J Comp Neurol. 1989 Mar 1;281(1):159–168. doi: 10.1002/cne.902810113. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991 May;6(5):665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Triller A., Korn H. Transmission at a central inhibitory synapse. III. Ultrastructure of physiologically identified and stained terminals. J Neurophysiol. 1982 Sep;48(3):708–736. doi: 10.1152/jn.1982.48.3.708. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Adams M. E., Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz M. M., Sugimori M., Cherksey B., Llinás R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992 Dec;9(6):1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The kinetics of quantal releases during end-plate currents at the frog neuromuscular junction. J Physiol. 1988 Aug;402:605–626. doi: 10.1113/jphysiol.1988.sp017225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautrin J., Kriebel M. E. Characteristics of slow-miniature endplate currents show a subunit composition. Neuroscience. 1991;41(1):71–88. doi: 10.1016/0306-4522(91)90201-x. [DOI] [PubMed] [Google Scholar]
- Wheeler D. B., Sather W. A., Randall A., Tsien R. W. Distinctive properties of a neuronal calcium channel and its contribution to excitatory synaptic transmission in the central nervous system. Adv Second Messenger Phosphoprotein Res. 1994;29:155–171. doi: 10.1016/s1040-7952(06)80014-5. [DOI] [PubMed] [Google Scholar]
- Yamada W. M., Zucker R. S. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992 Mar;61(3):671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]


