Abstract
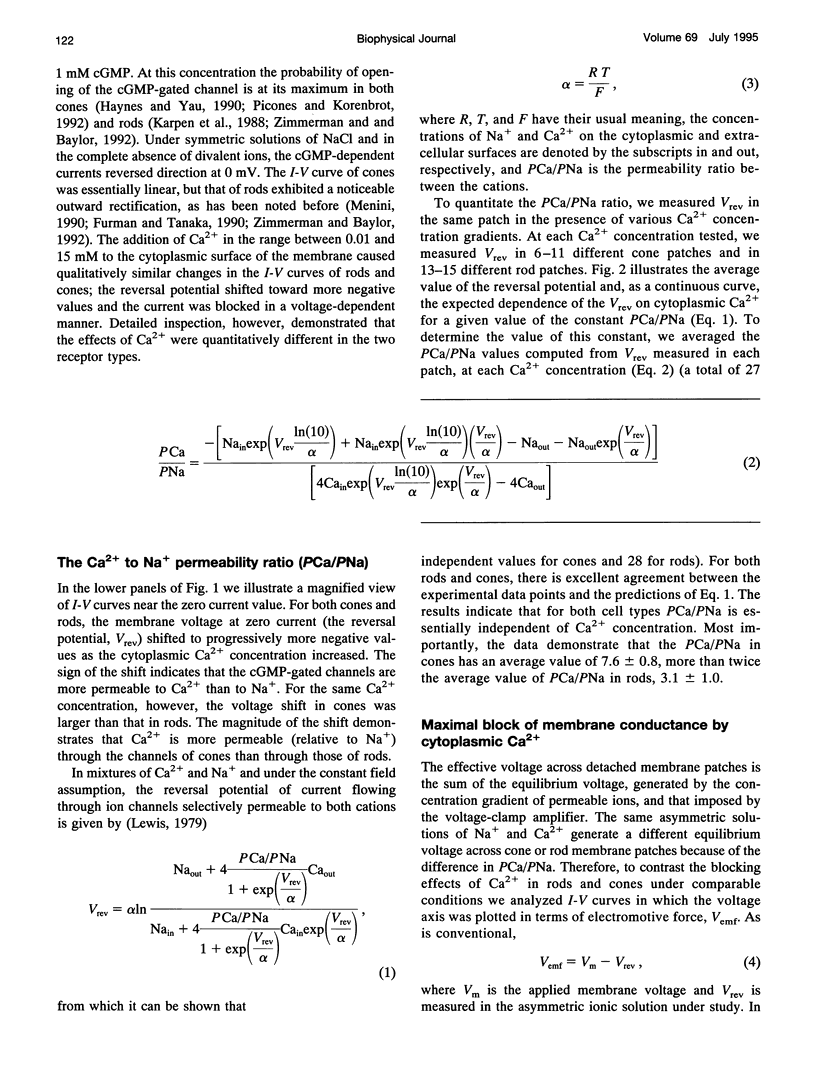
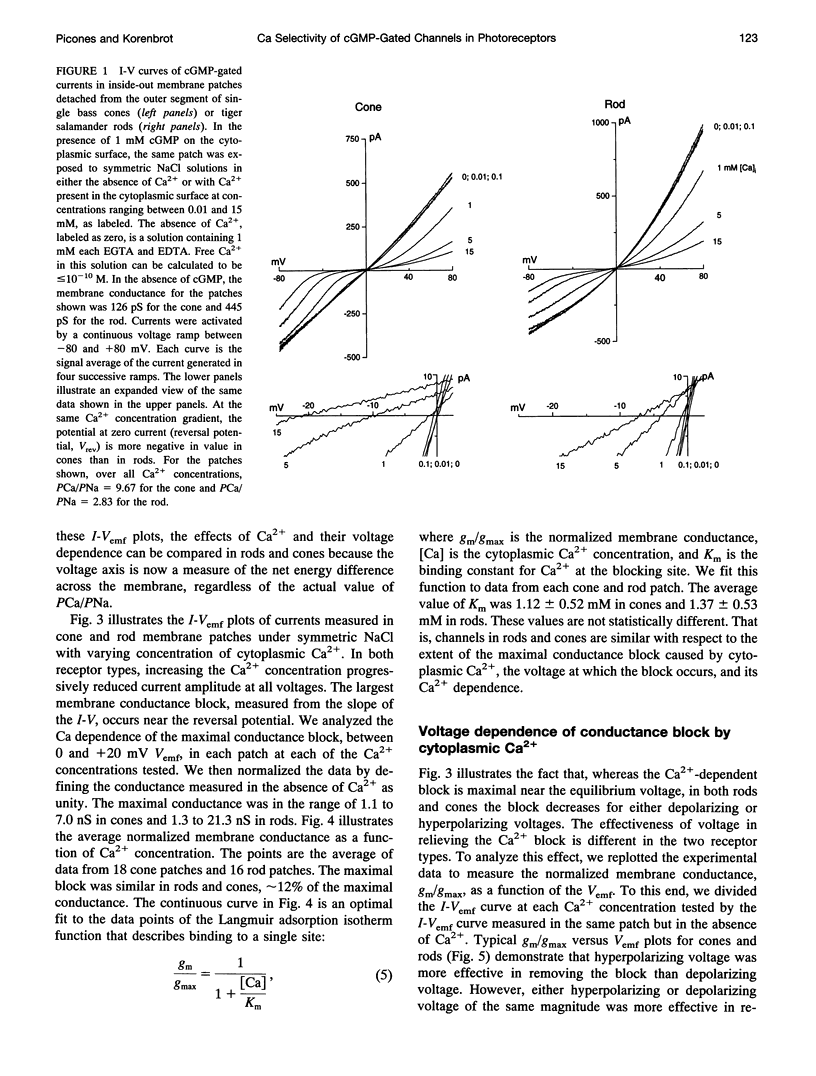
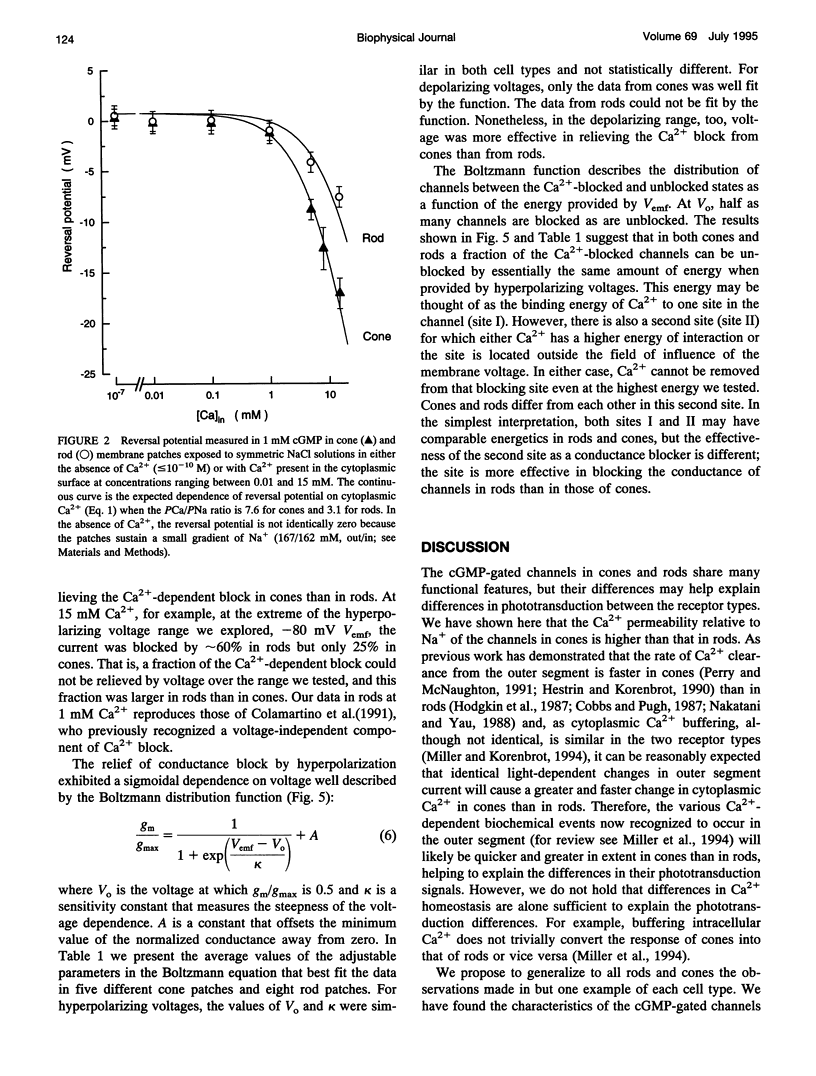
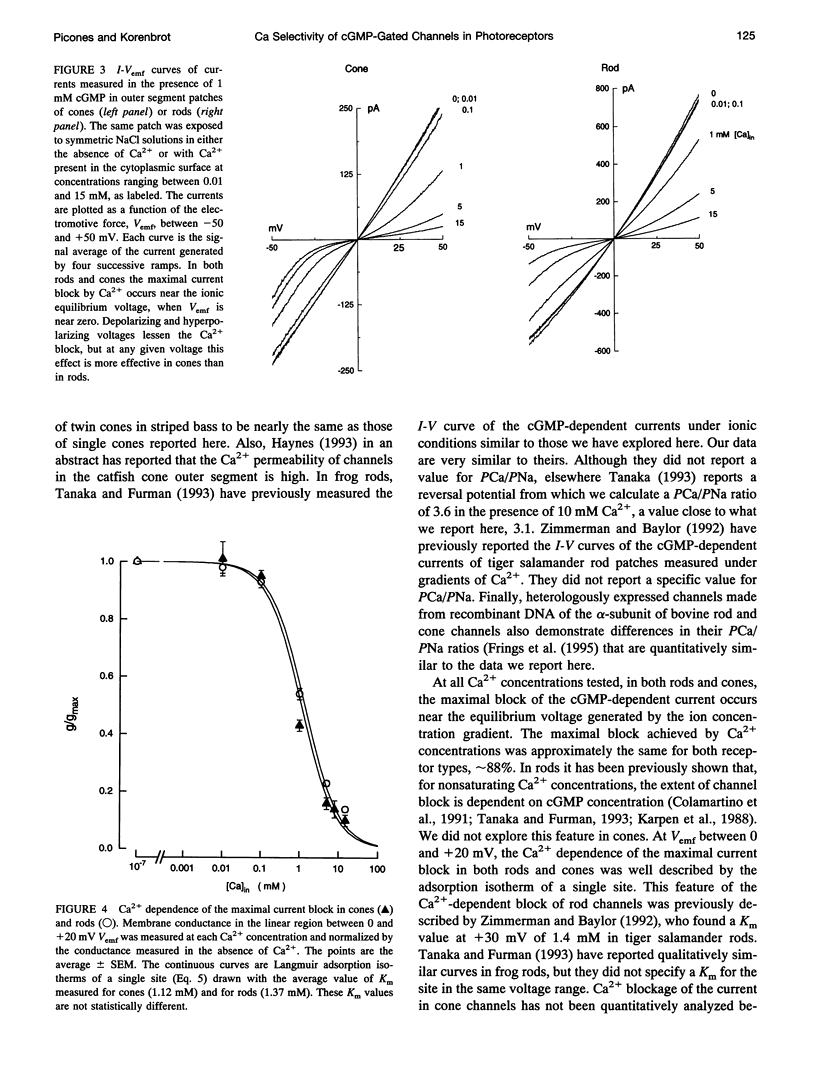
We studied the ionic permeability of cGMP-dependent currents in membrane patches detached from the outer segment of retinal cone and rod photoreceptors. Reversal potentials measured in membranes exposed to symmetric Na+ but with varying cytoplasmic Ca2+ concentrations reveal that the permeability ratio, PCa/PNa, is higher in the cGMP-gated channels of cones (7.6 +/- 0.8) than in those of rods (3.1 +/- 1.0). Ca2+ blocks both channels in a voltage-dependent manner. At any Ca2+ concentration, the channel block is maximal near the ionic reversal potential. The maximal block is essentially identical in channels of cones and rods with respect to its extent and voltage and Ca2+ dependence. The Ca2+ block is relieved by voltage, but the features of this relief differ markedly between rods and cones. Whereas the Boltzmann distribution function describes the relief of block by hyperpolarizing voltages, any given voltage is more effective in relieving the Ca2+ block in cones than in rods. Similarly, depolarizing voltages more effectively relieve Ca2+ block in cones than in rods. Our results suggest that channels contain two binding sites for Ca2+, one of which is similar in the two receptor types. The second site either interacts more strongly with Ca2+ than the first one or it is located differently in the membrane, so as to be less sensitive to membrane voltage. The channels in rods and cones differ in the features of this second site. The difference in Ca2+ permeability between the channels is likely to result in light-dependent changes in cytoplasmic Ca2+ concentration that are larger and faster in cones than in rods. The functional differences between channels, therefore, may be critically important in explaining the differences in the phototransduction signal of the two photoreceptor types.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cobbs W. H., Pugh E. N., Jr Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. J Physiol. 1987 Dec;394:529–572. doi: 10.1113/jphysiol.1987.sp016884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamartino G., Menini A., Torre V. Blockage and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J Physiol. 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eismann E., Müller F., Heinemann S. H., Kaupp U. B. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Matthews H. R. Calcium and the mechanism of light adaptation in vertebrate photoreceptors. Trends Neurosci. 1990 Sep;13(9):378–384. doi: 10.1016/0166-2236(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Furman R. E., Tanaka J. C. Monovalent selectivity of the cyclic guanosine monophosphate-activated ion channel. J Gen Physiol. 1990 Jul;96(1):57–82. doi: 10.1085/jgp.96.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Haynes L. W. Block of the cyclic GMP-gated channel of vertebrate rod and cone photoreceptors by l-cis-diltiazem. J Gen Physiol. 1992 Nov;100(5):783–801. doi: 10.1085/jgp.100.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W., Yau K. W. Single-channel measurement from the cyclic GMP-activated conductance of catfish retinal cones. J Physiol. 1990 Oct;429:451–481. doi: 10.1113/jphysiol.1990.sp018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S., Korenbrot J. I. Activation kinetics of retinal cones and rods: response to intense flashes of light. J Neurosci. 1990 Jun;10(6):1967–1973. doi: 10.1523/JNEUROSCI.10-06-01967.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Brown R. L., Stryer L., Baylor D. A. Interactions between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods. J Gen Physiol. 1993 Jan;101(1):1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Molecular mechanics of the cyclic-GMP-activated channel of retinal rods. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):325–332. doi: 10.1101/sqb.1988.053.01.039. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I., Miller D. L. Calcium ions act as modulators of intracellular information flow in retinal rod phototransduction. Neurosci Res Suppl. 1986;4:S11–S34. doi: 10.1016/0168-0102(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton P. A. Light response of vertebrate photoreceptors. Physiol Rev. 1990 Jul;70(3):847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Korenbrot J. I. Kinetics of light-dependent Ca fluxes across the plasma membrane of rod outer segments. A dynamic model of the regulation of the cytoplasmic Ca concentration. J Gen Physiol. 1987 Sep;90(3):397–425. doi: 10.1085/jgp.90.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Korenbrot J. I. Differences in calcium homeostasis between retinal rod and cone photoreceptors revealed by the effects of voltage on the cGMP-gated conductance in intact cells. J Gen Physiol. 1994 Nov;104(5):909–940. doi: 10.1085/jgp.104.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Korenbrot J. I. In retinal cones, membrane depolarization in darkness activates the cGMP-dependent conductance. A model of Ca homeostasis and the regulation of guanylate cyclase. J Gen Physiol. 1993 Jun;101(6):933–960. doi: 10.1085/jgp.101.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Korenbrot J. I. Phototransduction and adaptation in rods, single cones, and twin cones of the striped bass retina: a comparative study. Vis Neurosci. 1993 Jul-Aug;10(4):653–667. doi: 10.1017/s0952523800005356. [DOI] [PubMed] [Google Scholar]
- Miller J. L., Picones A., Korenbrot J. I. Differences in transduction between rod and cone photoreceptors: an exploration of the role of calcium homeostasis. Curr Opin Neurobiol. 1994 Aug;4(4):488–495. doi: 10.1016/0959-4388(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. J., McNaughton P. A. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991 Feb;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A., Korenbrot J. I. Permeation and interaction of monovalent cations with the cGMP-gated channel of cone photoreceptors. J Gen Physiol. 1992 Oct;100(4):647–673. doi: 10.1085/jgp.100.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A., Korenbrot J. I. Spontaneous, ligand-independent activity of the cGMP-gated ion channels in cone photoreceptors of fish. J Physiol. 1995 Jun 15;485(Pt 3):699–714. doi: 10.1113/jphysiol.1995.sp020763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M. J., MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993 Sep;11(3):459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol. 1991 Jul;98(1):95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. C., Furman R. E. Divalent effects on cGMP-activated currents in excised patches from amphibian photoreceptors. J Membr Biol. 1993 Feb;131(3):245–256. doi: 10.1007/BF02260113. [DOI] [PubMed] [Google Scholar]
- Tanaka J. C. The effects of protons on 3',5'-cGMP-activated currents in photoreceptor patches. Biophys J. 1993 Dec;65(6):2517–2523. doi: 10.1016/S0006-3495(93)81294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol. 1992 Apr;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]


