Abstract
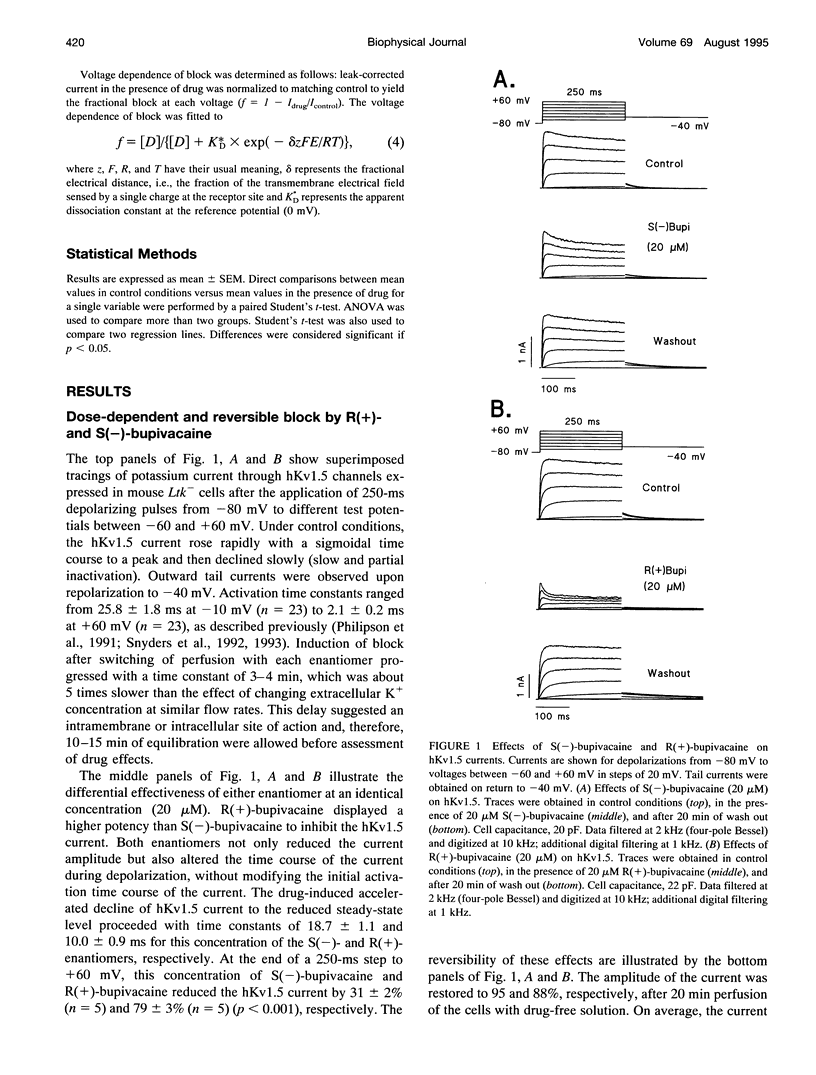
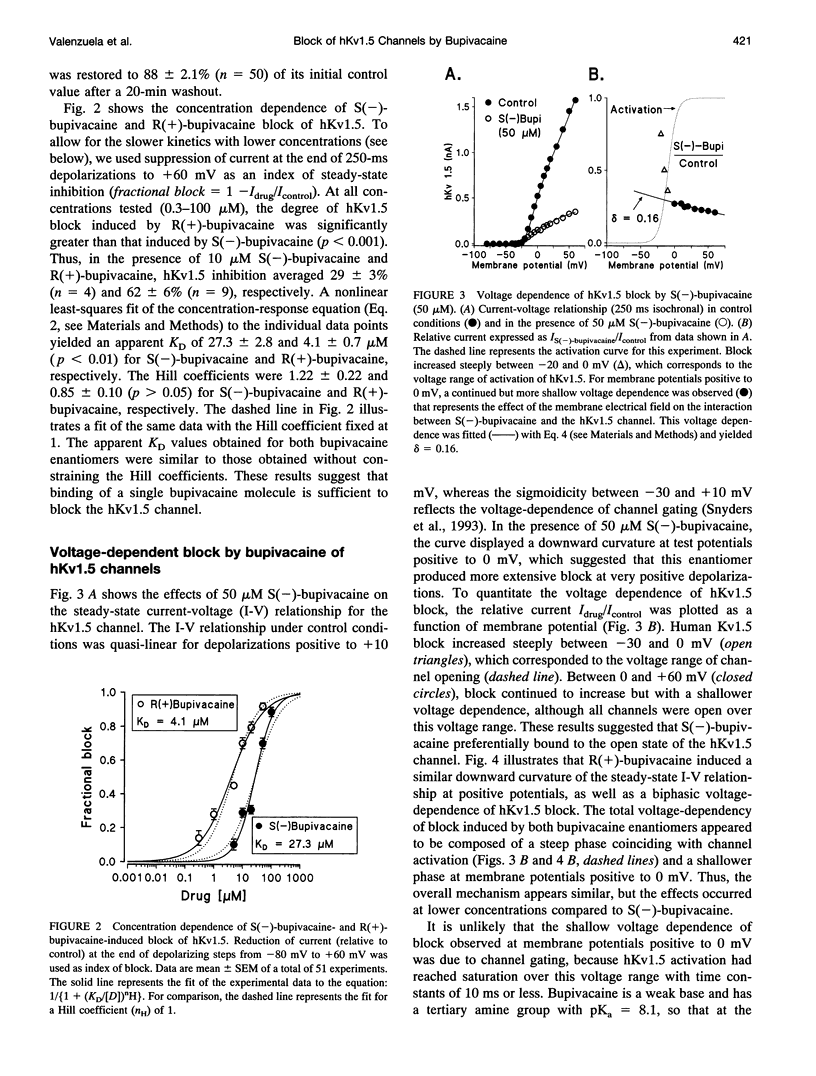
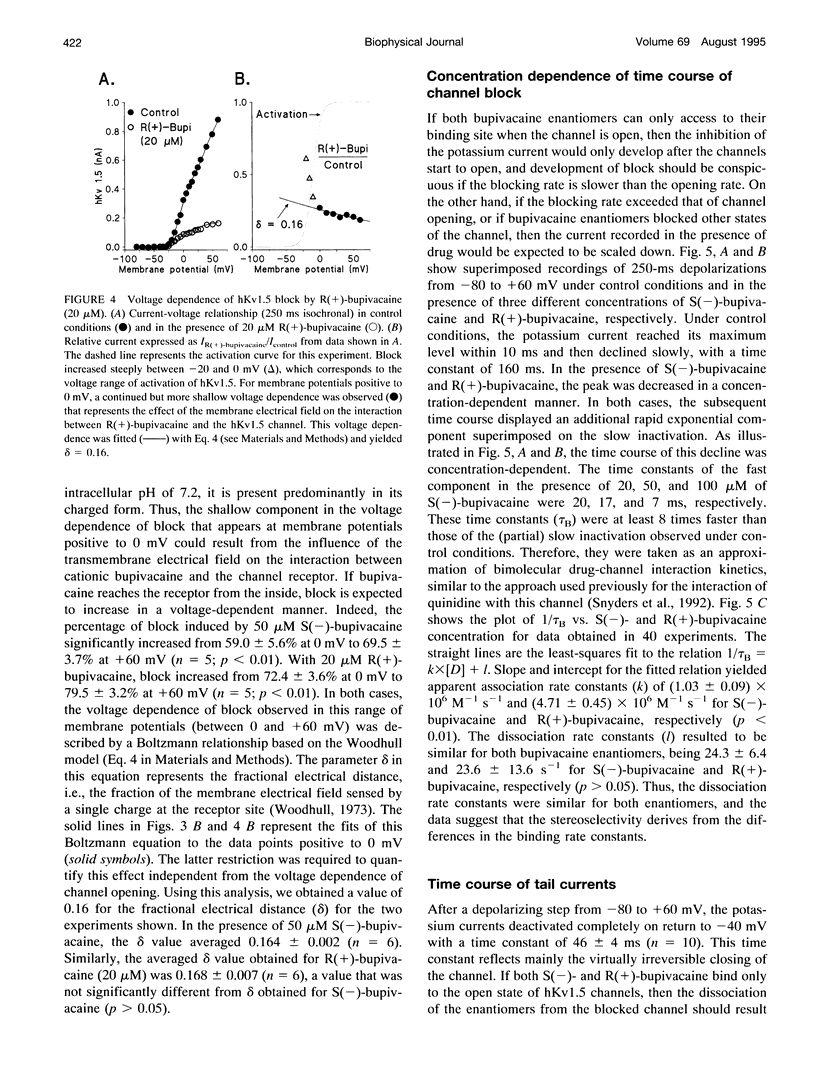
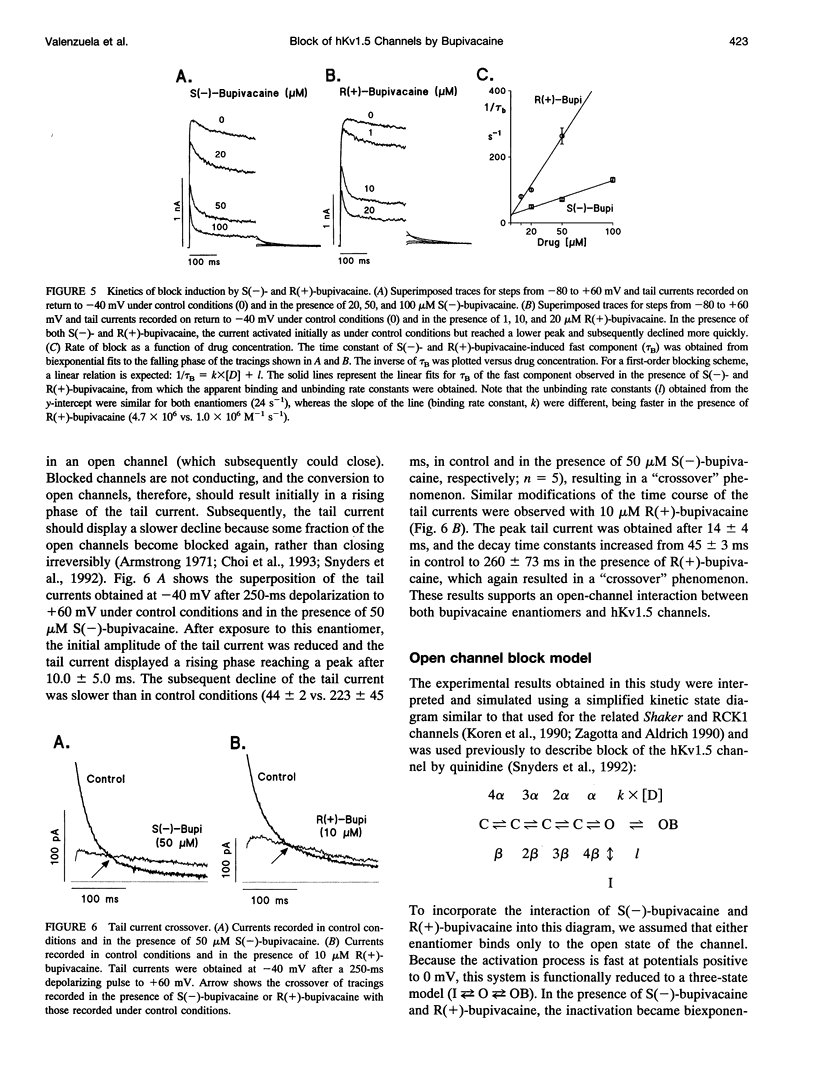
Stereoselective drug-channel interactions may help to elucidate the molecular basis of voltage-gated potassium channel block by local anesthetic drugs. We studied the effects of the enantiomers of bupivacaine on a cloned human cardiac potassium channel (hKv1.5). This channel was stably expressed in a mouse Ltk- cell line and studied using the whole-cell configuration of the patch-clamp technique. Both enantiomers modified the time course of this delayed rectifier current. Exposure to 20 microM of either S(-)-bupivacaine or R(+)-bupivacaine did not modify the activation time constant of the current, but reduced the peak outward current and induced a subsequent exponential decline of current with time constants of 18.7 +/- 1.1 and 10.0 +/- 0.9 ms, respectively. Steady-state levels of block (assessed with 250-ms depolarizing pulses to +60 mV) averaged 30.8 +/- 2.5% (n = 6) and 79.5 +/- 3.2% (n = 6) (p < 0.001), for S(-)- and R(+)-bupivacaine, respectively. The concentration dependence of hKv1.5 inhibition revealed apparent KD values of 27.3 +/- 2.8 and 4.1 +/- 0.7 microM for S(-)-bupivacaine and R(+)-bupivacaine, respectively, with Hill coefficients close to unity, suggesting that binding of one enantiomer molecule per channel was sufficient to block potassium permeation. Analysis of the rate constants of association (k) and dissociation (l) yielded similar values for l (24.9 s-1 vs. 23.6 s-1 for S(-)- and R(+)-bupivacaine, respectively) but different association rate constants (1.0 x 10(6) vs. 4.7 x 10(6) M-1 s-1 for S(-)- and R(+)-bupivacaine, respectively). Block induced by either enantiomer displayed a shallow voltage dependence in the voltage range positive to 0 mV, i.e., where the channel is fully open, consistent with an equivalent electrical distance delta of 0.16 +/- 0.01. This suggested that at the binding site, both enantiomers of bupivacaine experienced 16% of the applied transmembrane electrical field, referenced to the inner surface. Both bupivacaine enantiomers reduced the tail current amplitude recorded on return to -40 mV and slowed their time course relative to control, resulting in a "crossover" phenomenon. These data indicate 1) the charged form of both bupivacaine enantiomers block the hKv1.5 channel after it opens, 2) binding occurs within the transmembrane electrical field, 3) unbinding is required before the channel can close, 4) block of hKv1.5 channels by bupivacaine is markedly stereoselective, with the R(+)-enantiomer being the more potent one, 5) this stereoselective block was associated with a 1.11 -kcal/mol difference in binding energy between both enantiomers, and 6) the stereoselectivity derives mainly from a difference in the association rate constants, suggesting that the S(-)-enantiomer is less likely to access the binding site in an optimal configuration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberg G. Toxicological and local anaesthetic effects of optically active isomers of two local anaesthetic compounds. Acta Pharmacol Toxicol (Copenh) 1972;31(4):273–286. [PubMed] [Google Scholar]
- Albright G. A. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979 Oct;51(4):285–287. doi: 10.1097/00000542-197910000-00001. [DOI] [PubMed] [Google Scholar]
- Aps C., Reynolds F. An intradermal study of the local anaesthetic and vascular effects of the isomers of bupivacaine. Br J Clin Pharmacol. 1978 Jul;6(1):63–68. doi: 10.1111/j.1365-2125.1978.tb01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery P., Redon D., Schaenzer G., Rusy B. The influence of serum potassium on the cerebral and cardiac toxicity of bupivacaine and lidocaine. Anesthesiology. 1984 Aug;61(2):134–138. doi: 10.1097/00000542-198408000-00004. [DOI] [PubMed] [Google Scholar]
- Boyle W. A., Nerbonne J. M. A novel type of depolarization-activated K+ current in isolated adult rat atrial myocytes. Am J Physiol. 1991 Apr;260(4 Pt 2):H1236–H1247. doi: 10.1152/ajpheart.1991.260.4.H1236. [DOI] [PubMed] [Google Scholar]
- Burm A. G., van der Meer A. D., van Kleef J. W., Zeijlmans P. W., Groen K. Pharmacokinetics of the enantiomers of bupivacaine following intravenous administration of the racemate. Br J Clin Pharmacol. 1994 Aug;38(2):125–129. doi: 10.1111/j.1365-2125.1994.tb04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Use-dependent block and use-dependent unblock of the delayed rectifier K+ current by almokalant in rabbit ventricular myocytes. Circ Res. 1993 Nov;73(5):857–868. doi: 10.1161/01.res.73.5.857. [DOI] [PubMed] [Google Scholar]
- Castle N. A. Bupivacaine inhibits the transient outward K+ current but not the inward rectifier in rat ventricular myocytes. J Pharmacol Exp Ther. 1990 Dec;255(3):1038–1046. [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993 Mar;10(3):533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Hondeghem L. M. Evidence for a specific receptor site for lidocaine, quinidine, and bupivacaine associated with cardiac sodium channels in guinea pig ventricular myocardium. Circ Res. 1985 Apr;56(4):496–506. doi: 10.1161/01.res.56.4.496. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Hondeghem L. M. Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology. 1985 Apr;62(4):396–405. [PubMed] [Google Scholar]
- Courtney K. R., Kendig J. J. Bupivacaine is an effective potassium channel blocker in heart. Biochim Biophys Acta. 1988 Mar 22;939(1):163–166. doi: 10.1016/0005-2736(88)90058-2. [DOI] [PubMed] [Google Scholar]
- Fedida D., Wible B., Wang Z., Fermini B., Faust F., Nattel S., Brown A. M. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res. 1993 Jul;73(1):210–216. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- Follmer C. H., Cullinan C. A., Colatsky T. J. Differential block of cardiac delayed rectifier current by class Ic antiarrhythmic drugs: evidence for open channel block and unblock. Cardiovasc Res. 1992 Nov;26(11):1121–1130. doi: 10.1093/cvr/26.11.1121. [DOI] [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys J. 1981 May;34(2):271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck C. D., Boyden P. A. Age-related appearance of outward currents may contribute to developmental differences in ventricular repolarization. Circ Res. 1992 Dec;71(6):1390–1403. doi: 10.1161/01.res.71.6.1390. [DOI] [PubMed] [Google Scholar]
- Kasten G. W., Martin S. T. Bupivacaine cardiovascular toxicity: comparison of treatment with bretylium and lidocaine. Anesth Analg. 1985 Sep;64(9):911–916. [PubMed] [Google Scholar]
- Koren G., Liman E. R., Logothetis D. E., Nadal-Ginard B., Hess P. Gating mechanism of a cloned potassium channel expressed in frog oocytes and mammalian cells. Neuron. 1990 Jan;4(1):39–51. doi: 10.1016/0896-6273(90)90442-i. [DOI] [PubMed] [Google Scholar]
- Luduena F. P., Bogado E. F., Tullar B. F. Optical isomers of mepivacaine and bupivacaine. Arch Int Pharmacodyn Ther. 1972 Dec;200(2):359–369. [PubMed] [Google Scholar]
- Philipson L. H., Hice R. E., Schaefer K., LaMendola J., Bell G. I., Nelson D. J., Steiner D. F. Sequence and functional expression in Xenopus oocytes of a human insulinoma and islet potassium channel. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):53–57. doi: 10.1073/pnas.88.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Roberds S. L., Knoth K. M., Po S., Blair T. A., Bennett P. B., Hartshorne R. P., Snyders D. J., Tamkun M. M. Molecular biology of the voltage-gated potassium channels of the cardiovascular system. J Cardiovasc Electrophysiol. 1993 Feb;4(1):68–80. doi: 10.1111/j.1540-8167.1993.tb01214.x. [DOI] [PubMed] [Google Scholar]
- Scott D. B., Lee A., Fagan D., Bowler G. M., Bloomfield P., Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989 Nov;69(5):563–569. [PubMed] [Google Scholar]
- Snyders D. J., Tamkun M. M., Bennett P. B. A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol. 1993 Apr;101(4):513–543. doi: 10.1085/jgp.101.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon D., Bunegin L., Albin M. The effect of magnesium sulfate administration on cerebral and cardiac toxicity of bupivacaine in dogs. Anesthesiology. 1990 Feb;72(2):341–346. doi: 10.1097/00000542-199002000-00021. [DOI] [PubMed] [Google Scholar]
- Sánchez-Chapula J. Effects of bupivacaine on membrane currents of guinea-pig ventricular myocytes. Eur J Pharmacol. 1988 Nov 8;156(3):303–308. doi: 10.1016/0014-2999(88)90274-9. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Knoth K. M., Walbridge J. A., Kroemer H., Roden D. M., Glover D. M. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 1991 Mar 1;5(3):331–337. doi: 10.1096/fasebj.5.3.2001794. [DOI] [PubMed] [Google Scholar]
- Tucker G. T., Mather L. E. Pharmacology of local anaesthetic agents. Pharmacokinetics of local anaesthetic agents. Br J Anaesth. 1975 Feb;47 Suppl:213–224. [PubMed] [Google Scholar]
- Valenzuela C., Sánchez-Chapula J., Delpón E., Elizalde A., Pérez O., Tamargo J. Imipramine blocks rapidly activating and delays slowly activating K+ current activation in guinea pig ventricular myocytes. Circ Res. 1994 Apr;74(4):687–699. doi: 10.1161/01.res.74.4.687. [DOI] [PubMed] [Google Scholar]
- Wang G. K. Binding affinity and stereoselectivity of local anesthetics in single batrachotoxin-activated Na+ channels. J Gen Physiol. 1990 Nov;96(5):1105–1127. doi: 10.1085/jgp.96.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993 Dec;73(6):1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- Wheeler D. M., Bradley E. L., Woods W. T., Jr The electrophysiologic actions of lidocaine and bupivacaine in the isolated, perfused canine heart. Anesthesiology. 1988 Feb;68(2):201–212. doi: 10.1097/00000542-198802000-00005. [DOI] [PubMed] [Google Scholar]
- White M. M., Bezanilla F. Activation of squid axon K+ channels. Ionic and gating current studies. J Gen Physiol. 1985 Apr;85(4):539–554. doi: 10.1085/jgp.85.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]


