Abstract
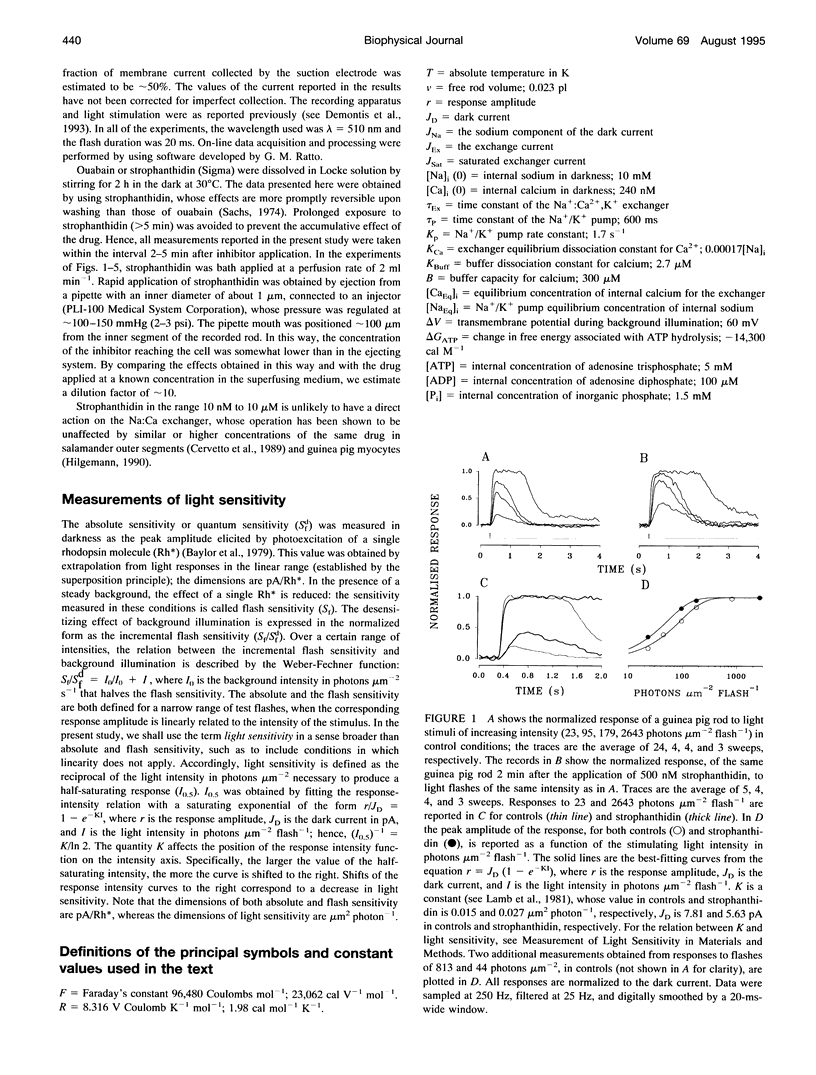
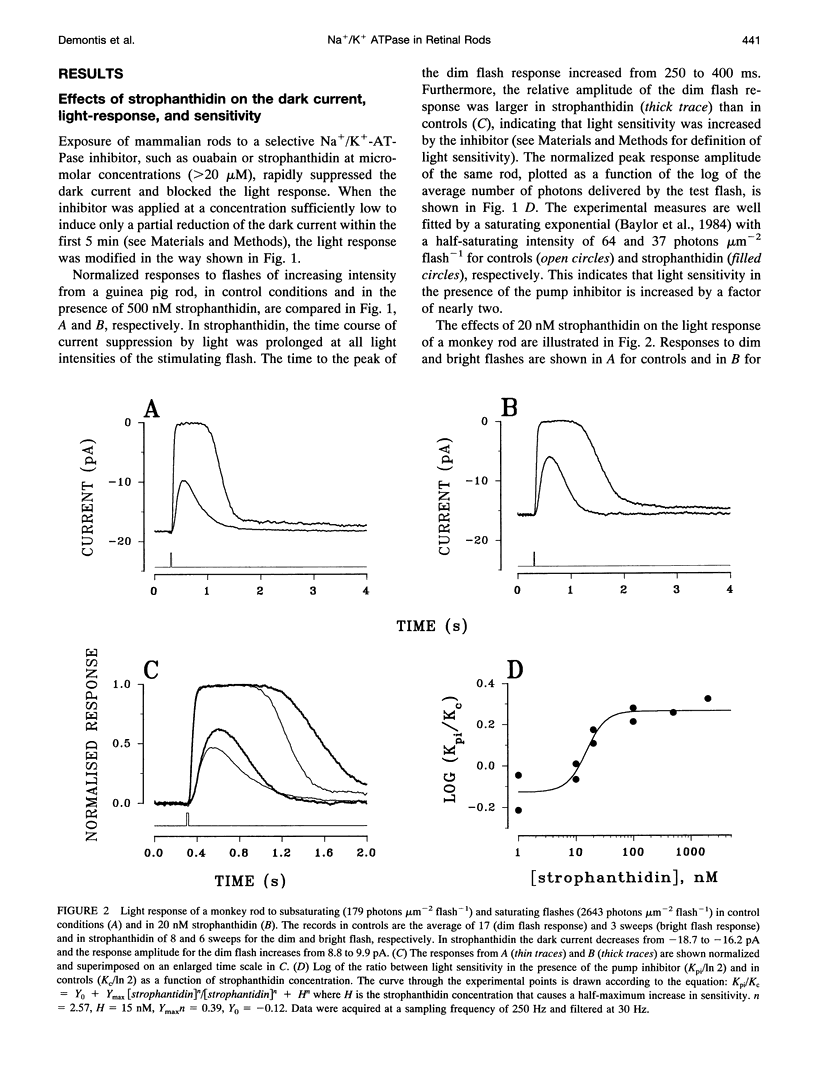
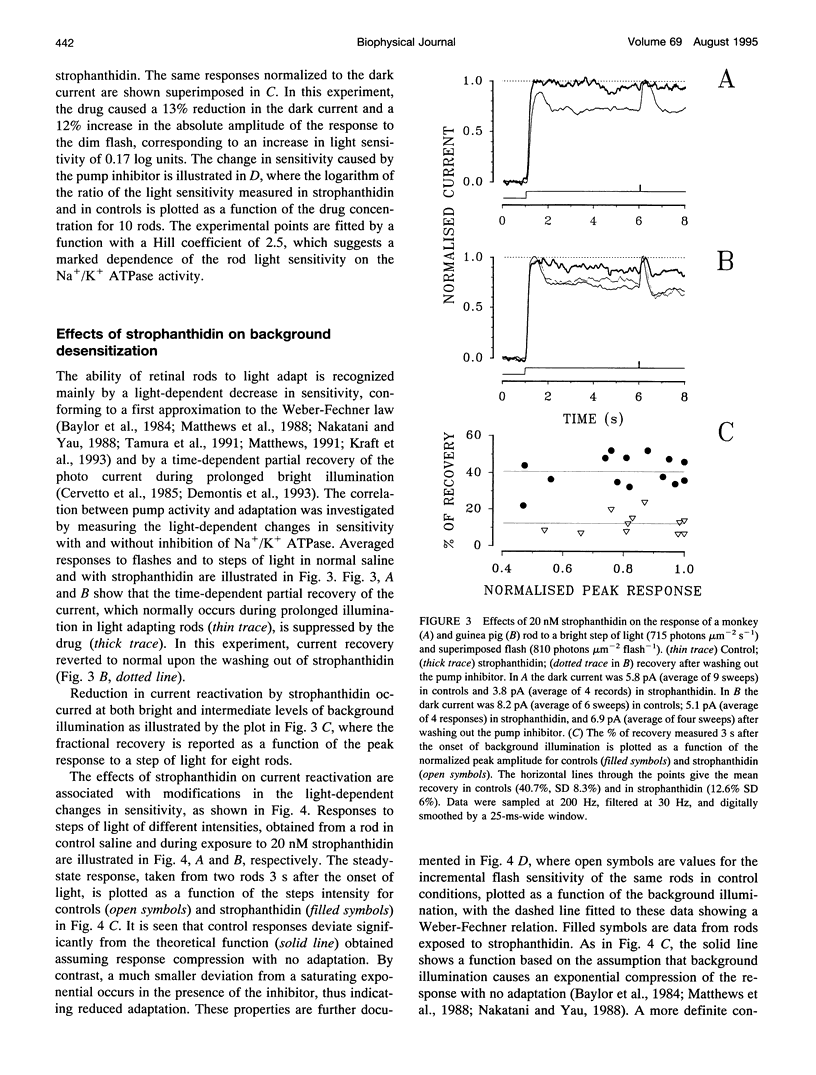
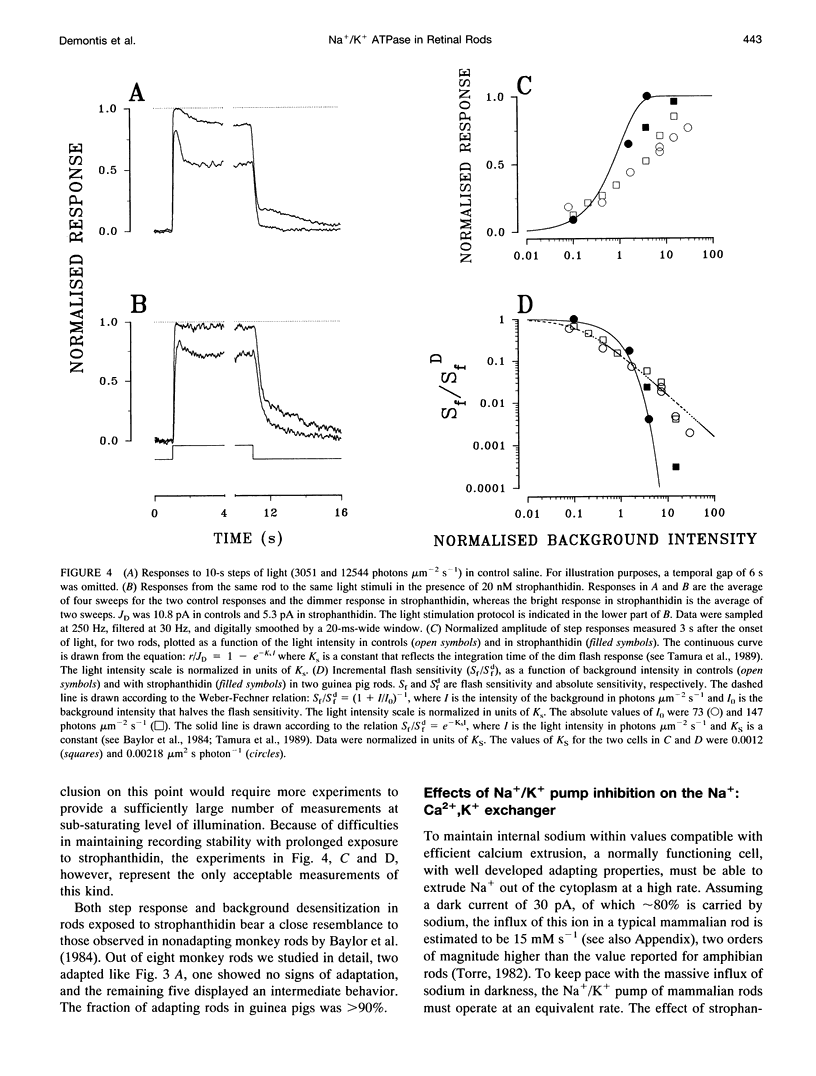
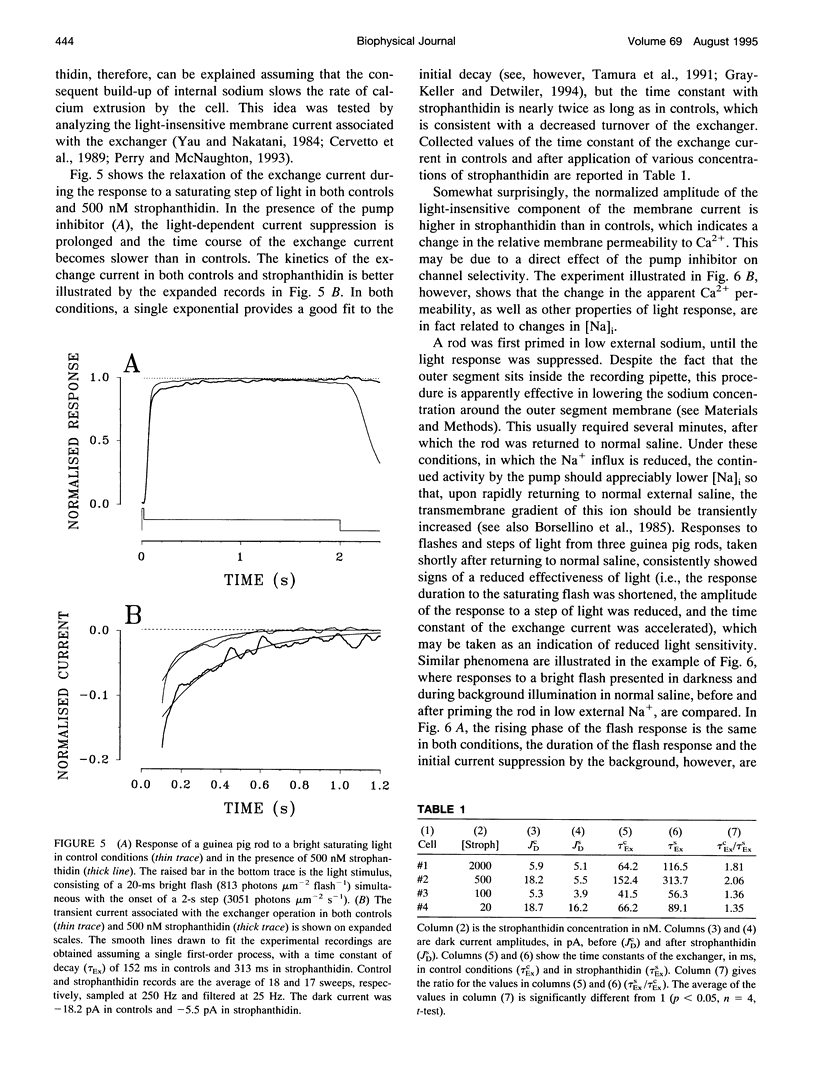
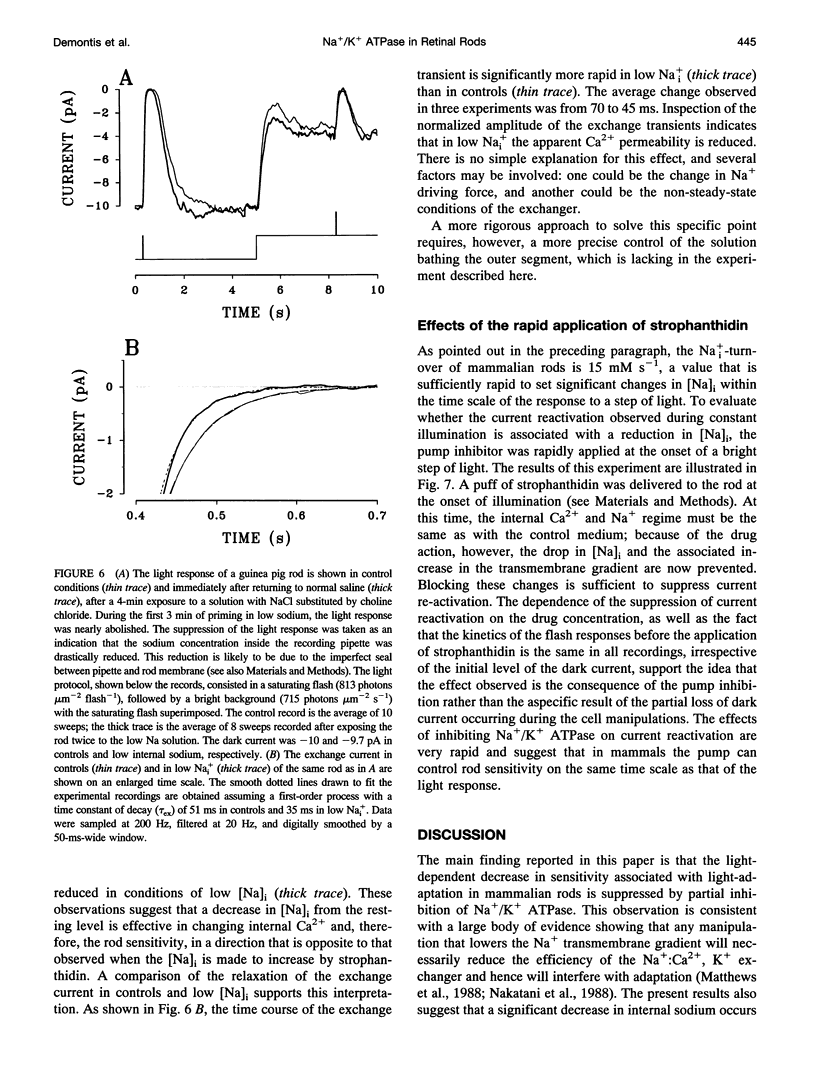
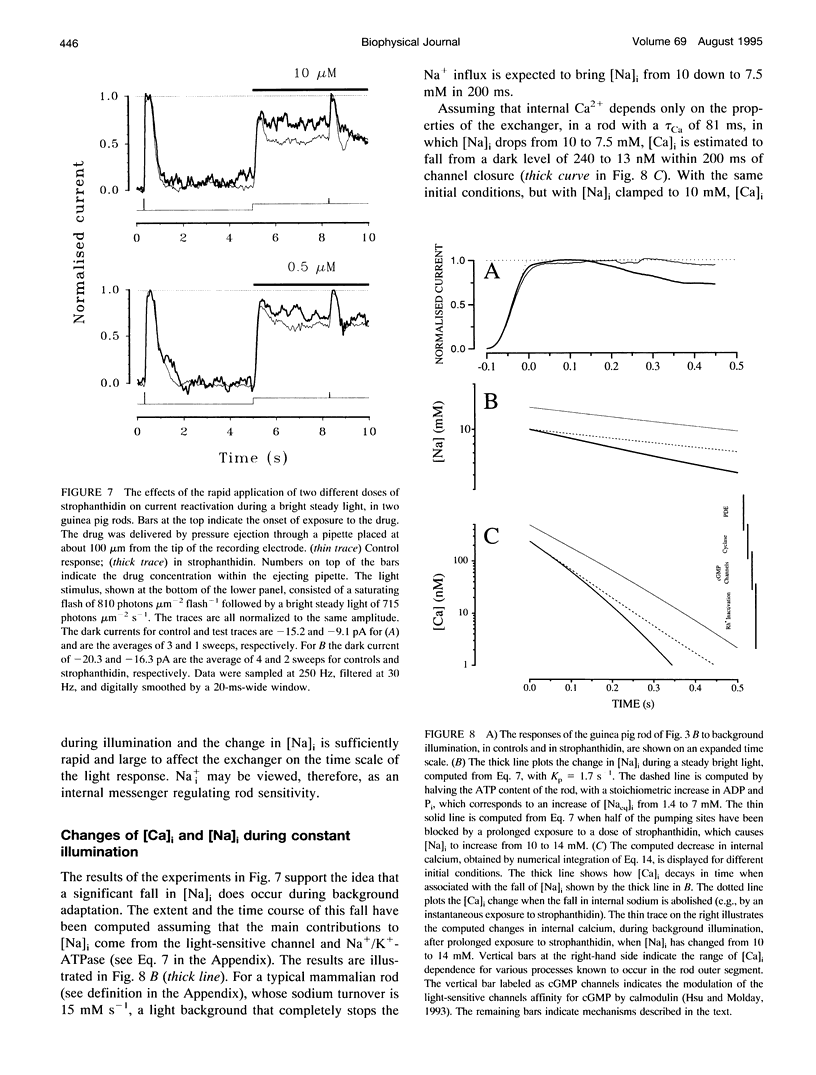
Membrane current and light response were recorded from rods of monkey and guinea pig by means of suction electrodes. The correlation between adaptation and the Na+/K+ pump was investigated by measuring light-dependent changes in sensitivity with and without inhibition of Na+/K+ ATPase by strophanthidin. Strophanthidin was found to reduce the dark current, to slow the time course of the photoresponse, and to increase light sensitivity. At concentrations between 20 and 500 nM, the pump inhibitor suppressed in a reversible way the current re-activation occurring during prolonged illumination and modified the light-dependent decrease in sensitivity, which in control conditions approximates to a Weber-Fechner function. The effects of the pump inhibitor on the adaptive properties of rods are associated with an increased time constant of the membrane current attributed to the operation of the Na+:Ca2+,K+ exchanger. The effects of rapid application of the pump inhibitor on the current re-activation are consistent with the idea that significant changes in the internal sodium occur in rods of mammals during background illumination and that they play an important role in the process of light adaptation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Torre V., Rispoli G., Marroni P. Mechanisms of light adaptation in toad rods. Exp Biol. 1985;44(3):147–157. [PubMed] [Google Scholar]
- Demontis G. C., Bisti S., Cervetto L. Light sensitivity, adaptation and saturation in mammalian rods. Prog Brain Res. 1993;95:15–24. doi: 10.1016/s0079-6123(08)60353-2. [DOI] [PubMed] [Google Scholar]
- Dizhoor A. M., Ray S., Kumar S., Niemi G., Spencer M., Brolley D., Walsh K. A., Philipov P. P., Hurley J. B., Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991 Feb 22;251(4996):915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca W. A., Gray-Keller M. P., Detwiler P. B., Palczewski K. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W. Regulation and deregulation of cardiac Na(+)-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 1990 Mar 15;344(6263):242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:371–398. doi: 10.1113/jphysiol.1987.sp016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Koch K. W. Role of cGMP and Ca2+ in vertebrate photoreceptor excitation and adaptation. Annu Rev Physiol. 1992;54:153–175. doi: 10.1146/annurev.ph.54.030192.001101. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993 Apr 29;362(6423):855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I., Miller D. L. Cytoplasmic free calcium concentration in dark-adapted retinal rod outer segments. Vision Res. 1989;29(8):939–948. doi: 10.1016/0042-6989(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Kraft T. W., Schneeweis D. M., Schnapf J. L. Visual transduction in human rod photoreceptors. J Physiol. 1993 May;464:747–765. doi: 10.1113/jphysiol.1993.sp019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R. Incorporation of chelator into guinea-pig rods shows that calcium mediates mammalian photoreceptor light adaptation. J Physiol. 1991 May;436:93–105. doi: 10.1113/jphysiol.1991.sp018541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- McCarthy S. T., Younger J. P., Owen W. G. Free calcium concentrations in bullfrog rods determined in the presence of multiple forms of Fura-2. Biophys J. 1994 Nov;67(5):2076–2089. doi: 10.1016/S0006-3495(94)80691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Perry R. J., McNaughton P. A. The mechanism of ion transport by the Na(+)-Ca2+,K+ exchange in rods isolated from the salamander retina. J Physiol. 1993 Jul;466:443–480. [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Cyclic GMP and calcium: the internal messengers of excitation and adaptation in vertebrate photoreceptors. Vision Res. 1990;30(12):1923–1948. doi: 10.1016/0042-6989(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Interaction of external K, Na, and cardioactive steroids with the Na-K pump of the human red blood cell. J Gen Physiol. 1974 Feb;63(2):123–143. doi: 10.1085/jgp.63.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp P. P., Basu D. K., Li X. B., Szerencsei R. T. Regulation of intracellular free Ca2+ concentration in the outer segments of bovine retinal rods by Na-Ca-K exchange measured with fluo-3. II. Thermodynamic competence of transmembrane Na+ and K+ gradients and inactivation of Na(+)-dependent Ca2+ extrusion. J Biol Chem. 1991 Dec 5;266(34):22983–22990. [PubMed] [Google Scholar]
- Schnetkamp P. P., Basu D. K., Szerencsei R. T. Na+-Ca2+ exchange in bovine rod outer segments requires and transports K+. Am J Physiol. 1989 Jul;257(1 Pt 1):C153–C157. doi: 10.1152/ajpcell.1989.257.1.C153. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P., Szerencsei R. T. Intracellular Ca2+ sequestration and release in intact bovine retinal rod outer segments. Role in inactivation of Na-Ca+K exchange. J Biol Chem. 1993 Jun 15;268(17):12449–12457. [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol. 1991 Jul;98(1):95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Light adaptation in cat retinal rods. Science. 1989 Aug 18;245(4919):755–758. doi: 10.1126/science.2772634. [DOI] [PubMed] [Google Scholar]
- Torre V. The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol. 1982 Dec;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]


