Abstract
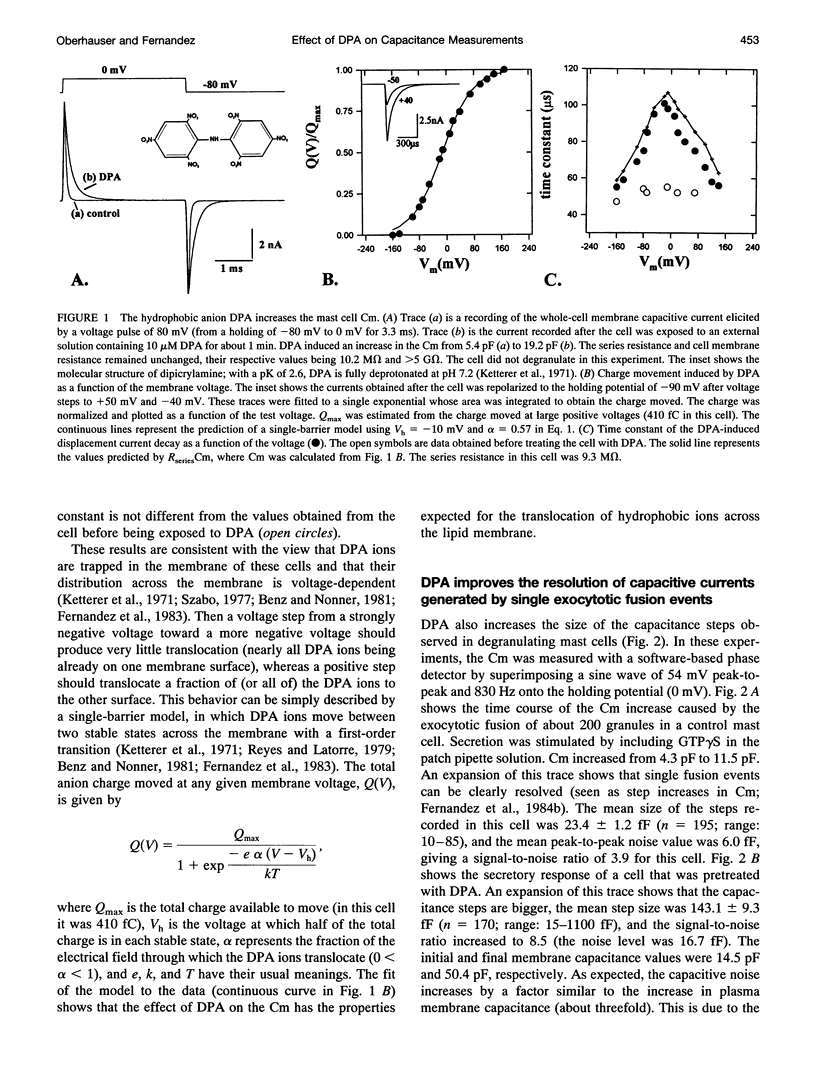
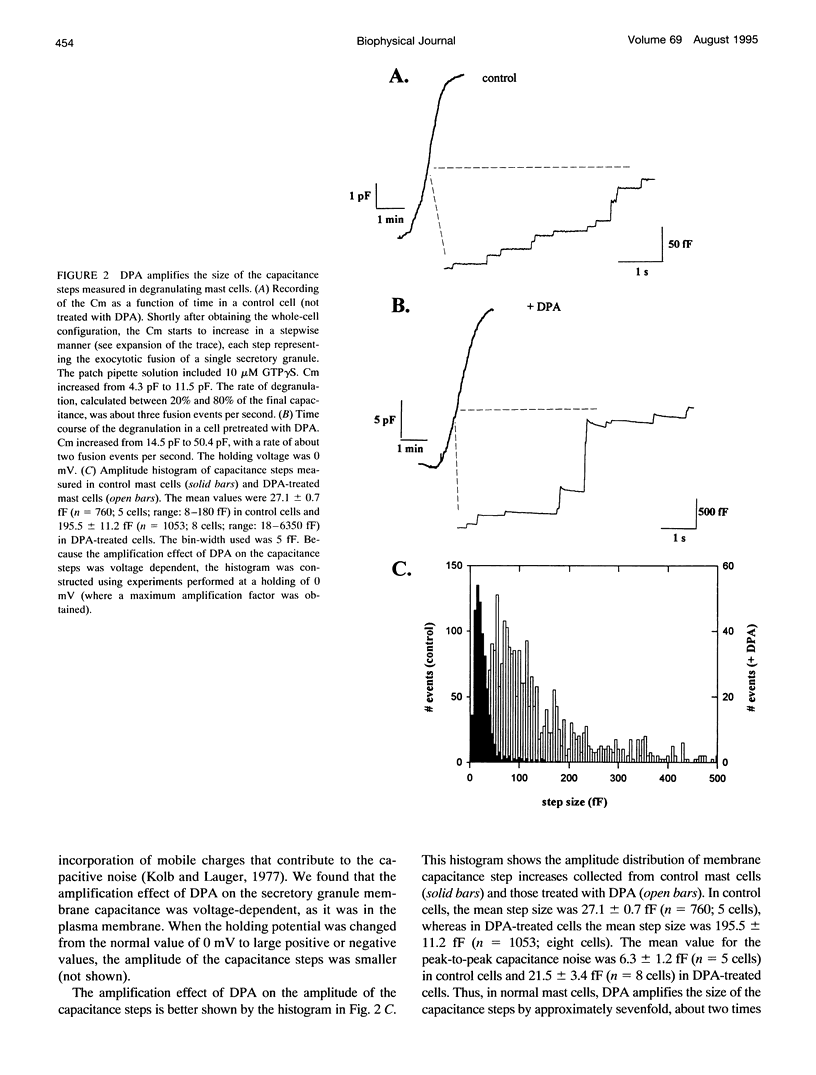
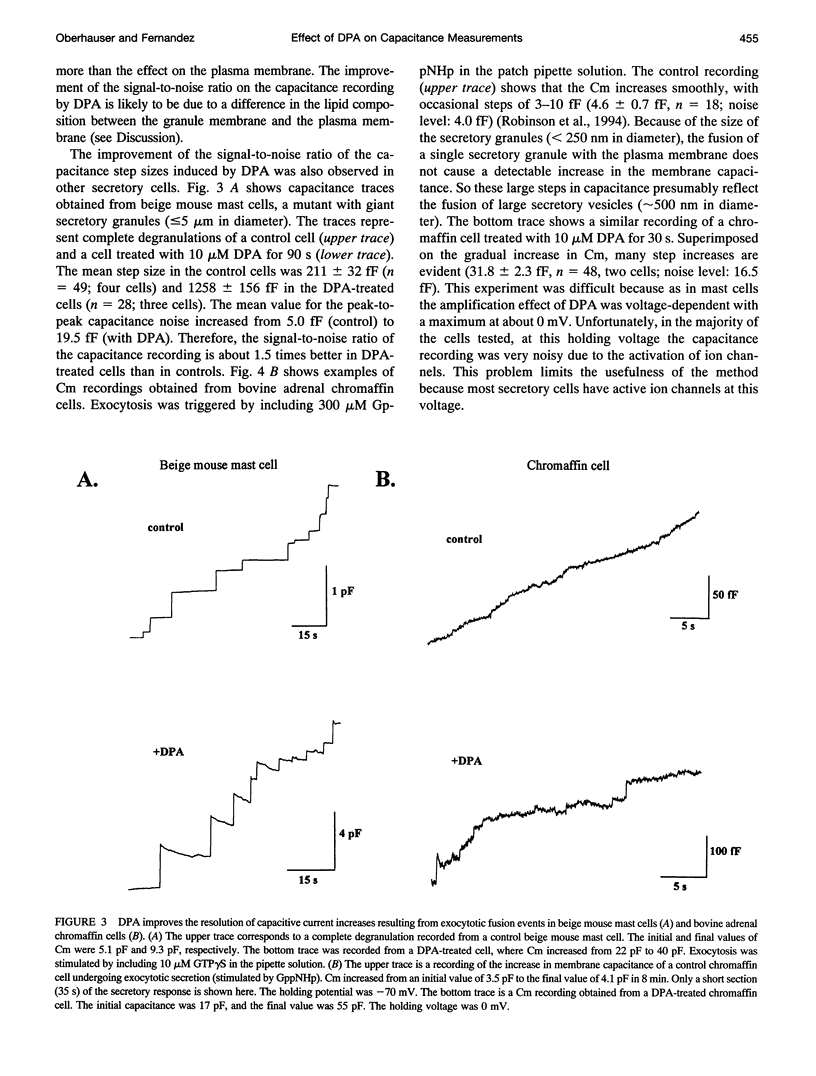
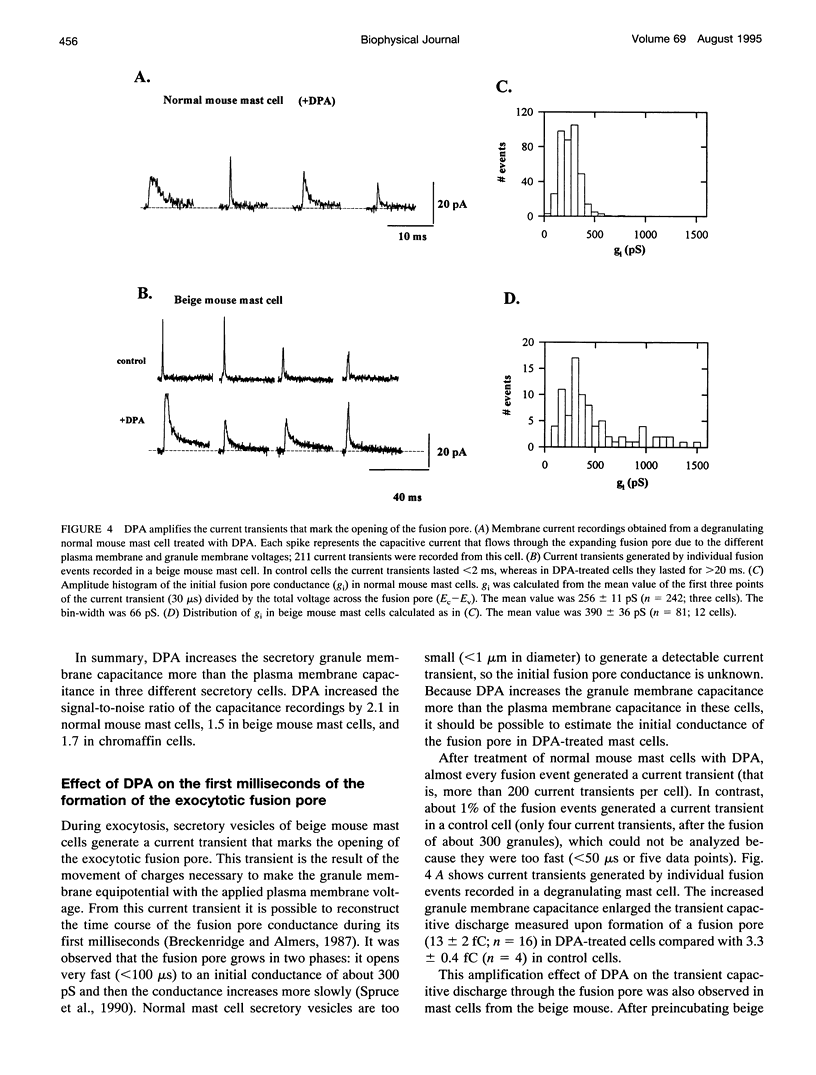
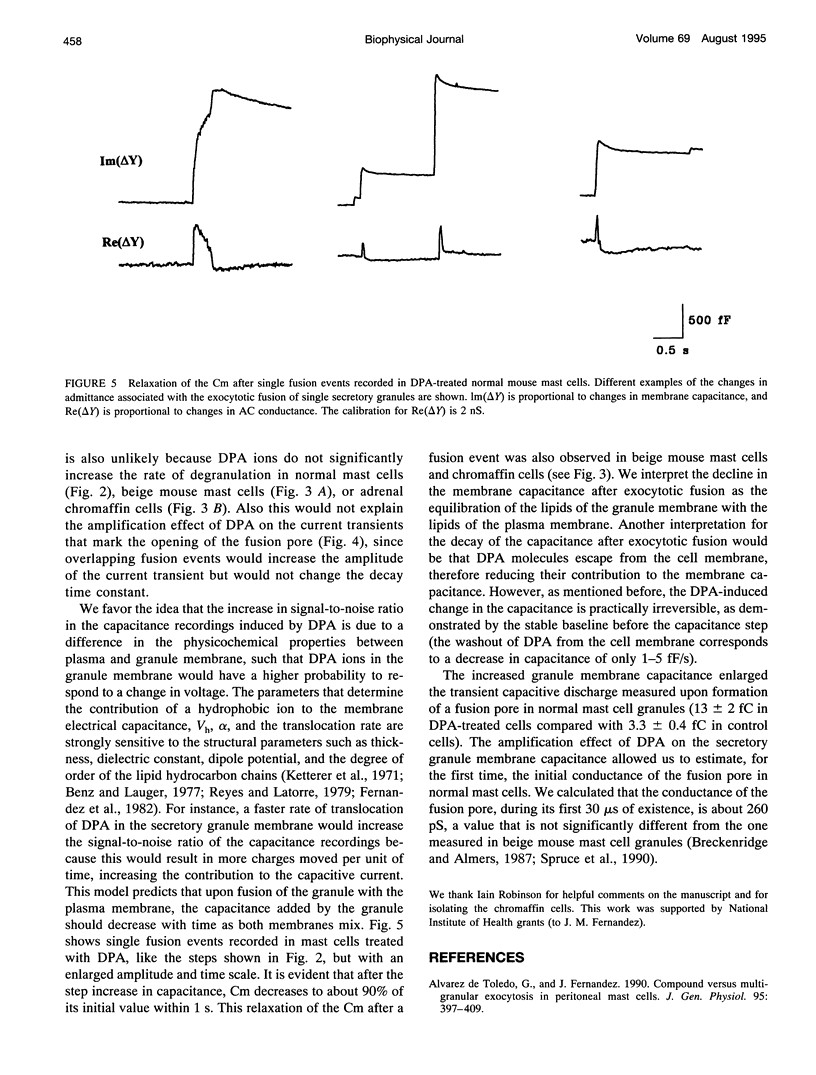
The detection of exocytotic fusion in patch-clamped secretory cells depends on measuring an increase in the cell membrane capacitance as new membrane is added to the plasma membrane. However, in the majority of secretory cells, secretory vesicles are too small (< 200 nm in diameter) to cause a detectable signal. We have found that incubations of normal mouse mast cells with the hydrophobic anion dipicrylamine (DPA), increases cell membrane capacitance by about three times. The large capacitive current induced by DPA was voltage-dependent, having a maximum value at -10 mV. The DPA-induced charge movement could be described by a single barrier model in which the DPA molecules move between two stable states in the bulk lipid matrix of the membrane. More importantly, the DPA treatment produced a sevenfold increase in the size of the capacitance steps observed upon the exocytotic fusion of single secretory granules. A similar amplification of DPA on the secretory vesicle capacitance was observed in a cell with larger (< or = 5 microns in diameter) or with smaller secretory granules (< 250 nm in diameter). Additionally, the increased granule membrane capacitance enlarged the transient capacitive discharge measured upon formation of a fusion pore in normal mast cell granules. Our results indicate that hydrophobic ions provide an important tool for high resolution studies of membrane capacitance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez de Toledo G., Fernandez J. M. Compound versus multigranular exocytosis in peritoneal mast cells. J Gen Physiol. 1990 Mar;95(3):397–409. doi: 10.1085/jgp.95.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Läuger P. Transport kinetics of dipicrylamine through lipid bilayer membranes. Effects of membrane structure. Biochim Biophys Acta. 1977 Jul 14;468(2):245–258. doi: 10.1016/0005-2736(77)90118-3. [DOI] [PubMed] [Google Scholar]
- Benz R., Nonner W. Structure of the axolemma of frog myelinated nerve: relaxation experiments with a lipophilic probe ion. J Membr Biol. 1981 Apr 15;59(2):127–134. doi: 10.1007/BF01875710. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Dilger J. P., Benz R. Optical and electrical properties of thin monoolein lipid bilayers. J Membr Biol. 1985;85(2):181–189. doi: 10.1007/BF01871270. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fernández J. M., Bezanilla F., Taylor R. E. Effect of chloroform on charge movement in the nerve membrane. Nature. 1982 May 13;297(5862):150–152. doi: 10.1038/297150a0. [DOI] [PubMed] [Google Scholar]
- Fernández J. M., Taylor R. E., Bezanilla F. Induced capacitance in the squid giant axon. Lipophilic ion displacement currents. J Gen Physiol. 1983 Sep;82(3):331–346. doi: 10.1085/jgp.82.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn W. B., Bruner L. J., Midland M. M., Wisniewski J. Hydrophobic ion probe studies of membrane dipole potentials. Biochim Biophys Acta. 1983 Jan 19;727(2):357–366. doi: 10.1016/0005-2736(83)90421-2. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Läuger P. Electrical noise from lipid bilayer membranes in the presence of hydrophobic ions. J Membr Biol. 1977 Dec 15;37(3-4):321–345. doi: 10.1007/BF01940938. [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Fernandez J. M. Patch clamp studies of single intact secretory granules. Biophys J. 1993 Nov;65(5):1844–1852. doi: 10.1016/S0006-3495(93)81246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J., Latorre R. Effect of the anesthetics benzyl alcohol and chloroform on bilayers made from monolayers. Biophys J. 1979 Nov;28(2):259–279. doi: 10.1016/S0006-3495(79)85175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejtek P., Wang S. Domains and anomalous adsorption isotherms of dipalmitoylphosphatidylcholine membranes and lipophilic ions: pentachlorophenolate, tetraphenylborate, and dipicrylamine. Biophys J. 1991 May;59(5):1064–1073. doi: 10.1016/S0006-3495(91)82321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Szabo G. Electrical characteristics of ion transport in lipid bilayer membranes. Ann N Y Acad Sci. 1977 Dec 30;303:266–280. [PubMed] [Google Scholar]
- Turin L., Béhé P., Plonsky I., Dunina-Barkovskaya A. Hydrophobic ion transfer between membranes of adjacent hepatocytes: a possible probe of tight junction structure. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9365–9369. doi: 10.1073/pnas.88.20.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]


