Abstract
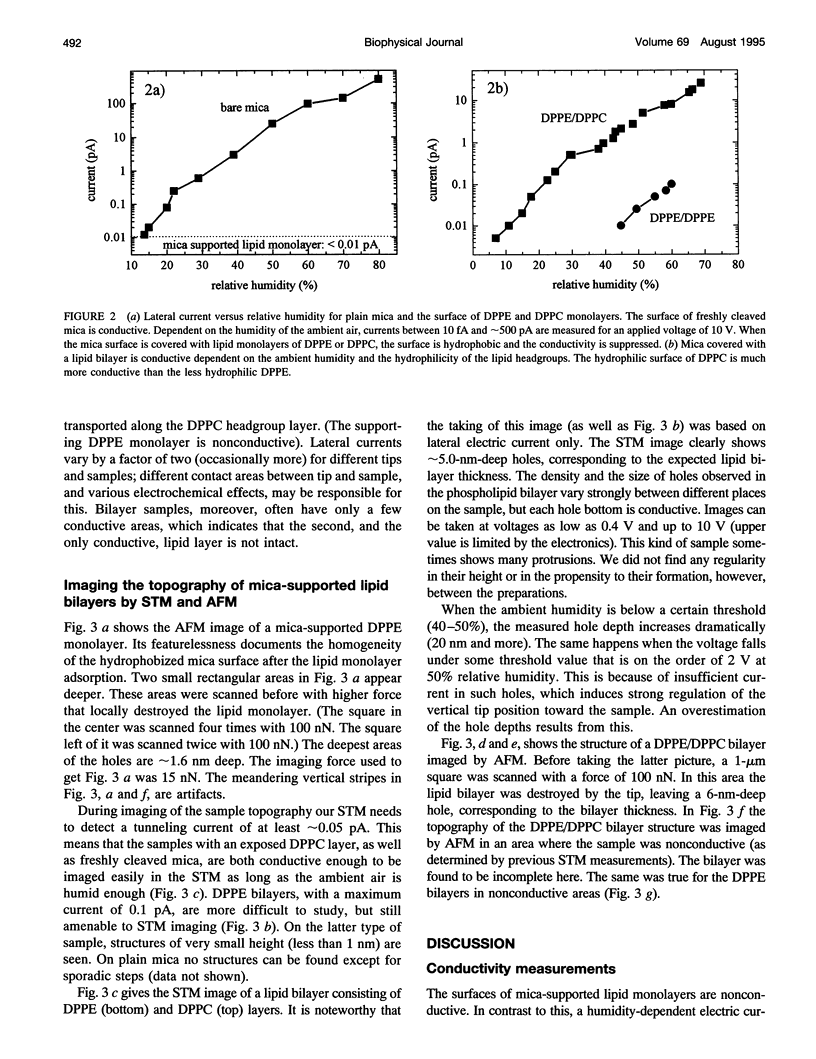
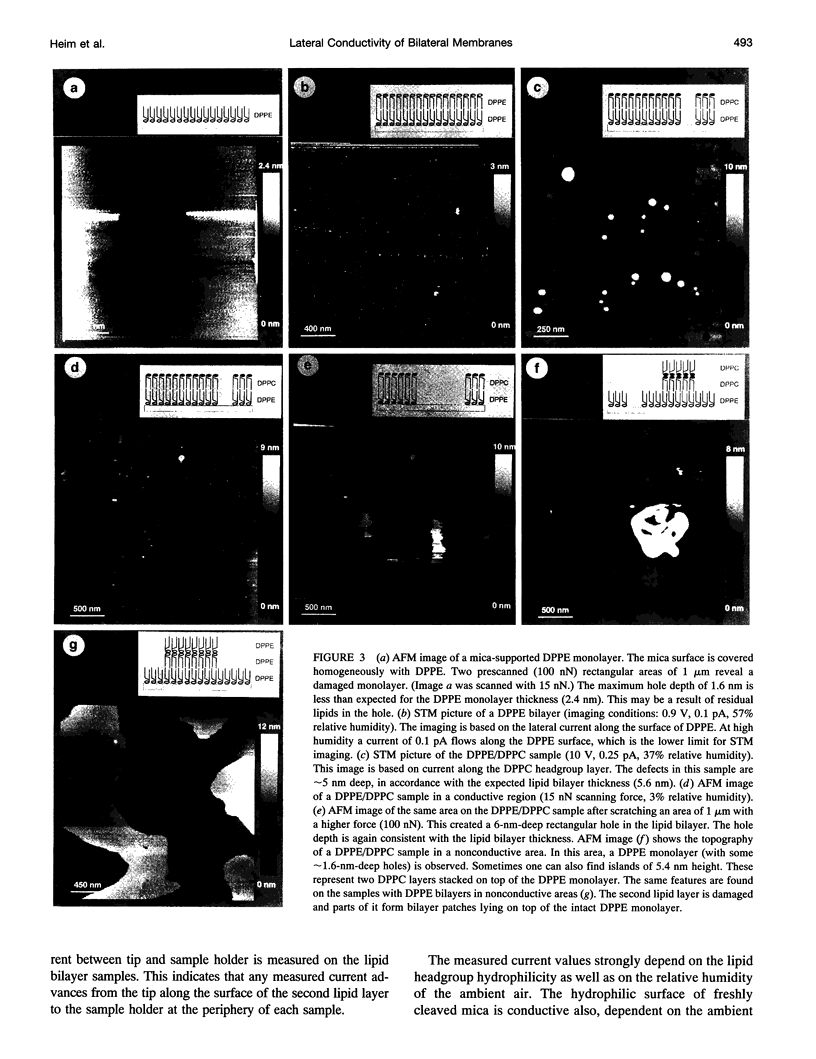
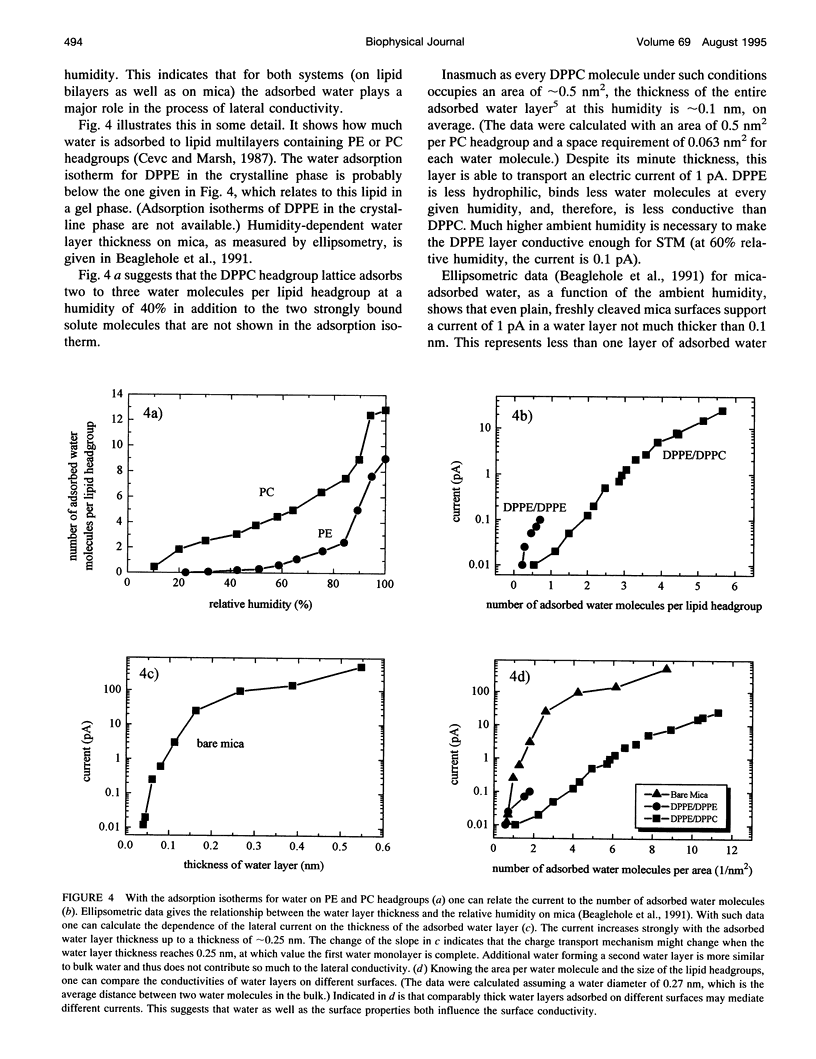
Lateral electric conductivity of mica-supported lipid monolayers and of the corresponding lipid bilayers has been studied by means of scanning tunneling microscopy (STM). The surface of freshly cleaved mica itself was found to be conductive when exposed to humid air. Lipid monolayers were transferred onto such a surface by means of the Langmuir-Blodgett technique, which makes the mica surface hydrophobic and suppresses the electric current along the surface in the experimentally accessible humidity (5-80%) and applied voltage (0-10 V) range. This is true for dipalmitoylphosphatidylethanolamine (DPPE) as well as dipalmitoylphosphatidylcholine (DPPC) monolayers. Repeated deposition of DPPC layers by means of the Langmuir-Blodgett LB technique does not lead to the formation of a stable surface-supported bilayer because of the high hydrophilicity of the phosphatidylcholine headgroups that causes DPPC/DPPC bilayers to peel off the supporting surface during the sample preparation. In contrast to this, a DPPE or a DPPC monolayer on top of a DPPE monolayer gives rise to a rather stable mica-supported bilayer that can be studied by STM. Electric currents between 10 and 100 fA, depending on the ambient humidity, flow along the DPPE bilayer surface, in the humidity range between 35 and 60%. The DPPC surface, which is more hydrophilic, is up to 100 times more conductive under comparable conditions. Anomalous high lateral conductivity thus depends on, and probably proceeds via, the surface-adsorbed water layers. The prominence of ambient humidity and surface hydrophilicity on the measured lateral currents suggests this. The combination of our STM data and previously published water adsorption isotherms as a function of the relative humidity indicate that one layer or less of adsorbed water suffices for mediating the measurable lateral currents. The fact that similar observations are also made for other hydrophilic substrates supports the conclusion that lateral conductivity via surface-adsorbed water is a rather general phenomenon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaglehole D, Radlinska EZ, Ninham BW, Christenson HK. Inadequacy of Lifshitz theory for thin liquid films. Phys Rev Lett. 1991 Apr 22;66(16):2084–2087. doi: 10.1103/PhysRevLett.66.2084. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Guckenberger R., Heim M., Cevc G., Knapp H. F., Wiegräbe W., Hillebrand A. Scanning tunneling microscopy of insulators and biological specimens based on lateral conductivity of ultrathin water films. Science. 1994 Dec 2;266(5190):1538–1540. doi: 10.1126/science.7985024. [DOI] [PubMed] [Google Scholar]
- Guckenberger R., Kösslinger C., Gatz R., Breu H., Levai N., Baumeister W. A scanning tunneling microscope (STM) for biological applications: design and performance. Ultramicroscopy. 1988;25(2):111–121. doi: 10.1016/0304-3991(88)90218-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jendrasiak G. L., Mendible J. C. The effect of the phase transition on the hydration and electrical conductivity of phospholipids. Biochim Biophys Acta. 1976 Feb 23;424(2):133–148. doi: 10.1016/0005-2760(76)90183-1. [DOI] [PubMed] [Google Scholar]
- Kasianowicz J., Benz R., McLaughlin S. How do protons cross the membrane-solution interface? Kinetic studies on bilayer membranes exposed to the protonophore S-13 (5-chloro-3-tert-butyl-2'-chloro-4' nitrosalicylanilide). J Membr Biol. 1987;95(1):73–89. doi: 10.1007/BF01869632. [DOI] [PubMed] [Google Scholar]
- Kell D. B. On the functional proton current pathway of electron transport phosphorylation. An electrodic view. Biochim Biophys Acta. 1979 Jul 3;549(1):55–99. doi: 10.1016/0304-4173(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Läuger P. Dynamics of ion transport systems in membranes. Physiol Rev. 1987 Oct;67(4):1296–1331. doi: 10.1152/physrev.1987.67.4.1296. [DOI] [PubMed] [Google Scholar]
- MUELLER P., RUDIN D. O., TIEN H. T., WESCOTT W. C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 1962 Jun 9;194:979–980. doi: 10.1038/194979a0. [DOI] [PubMed] [Google Scholar]
- Morgan H., Taylor D. M., Oliveira O. N., Jr Proton transport at the monolayer-water interface. Biochim Biophys Acta. 1991 Feb 25;1062(2):149–156. doi: 10.1016/0005-2736(91)90386-m. [DOI] [PubMed] [Google Scholar]
- Prats M., Tocanne J. F., Teissié J. Lateral diffusion of protons along phospholipid monolayers. J Membr Biol. 1987;99(3):225–227. doi: 10.1007/BF01995703. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of temperature-dependent solvation forces between DNA double helices. Biophys J. 1992 Jan;61(1):260–271. doi: 10.1016/S0006-3495(92)81832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of the intermolecular forces between counterion-condensed DNA double helices. Evidence for long range attractive hydration forces. Biophys J. 1992 Jan;61(1):246–259. doi: 10.1016/S0006-3495(92)81831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai I., Kawamura Y. Lateral electrical conduction along a phosphatidylcholine monolayer. Biochim Biophys Acta. 1987 Nov 13;904(2):405–409. doi: 10.1016/0005-2736(87)90391-9. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissie J., Tsong T. Y. Electric field induced transient pores in phospholipid bilayer vesicles. Biochemistry. 1981 Mar 17;20(6):1548–1554. doi: 10.1021/bi00509a022. [DOI] [PubMed] [Google Scholar]
- Teissié J., Prats M., Soucaille P., Tocanne J. F. Evidence for conduction of protons along the interface between water and a polar lipid monolayer. Proc Natl Acad Sci U S A. 1985 May;82(10):3217–3221. doi: 10.1073/pnas.82.10.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]





