Abstract
Purpose
Rhegmatogenous retinal detachment (RRD) is a sight-threatening condition requiring prompt surgical intervention. Various adjunctive techniques are employed to enhance subretinal fluid drainage and retinal reattachment. This study aimed to evaluate the outcomes of perfluorocarbon liquid (PFCL) versus posterior retinotomy (PR) during pars plana vitrectomy (PPV) for RRD, focusing on anatomical success, visual acuity, intraocular pressure (IOP), and complications.
Methods
This is a prospective randomized controlled trial that included 58 eyes with RRD, divided into Group A (PFCL, n = 29) and Group B (PR, n = 29). Preoperative assessments included best-corrected visual acuity (BCVA), IOP, axial length, lens status, macula status, and PVR grade. Outcomes were evaluated at 1 week, 1 month, 2 months, and 3 months postoperatively. Primary outcomes included retinal reattachment rates and the number of operations; secondary outcomes included BCVA, IOP changes, complications like cataract development, retinal redetachment, epiretinal membrane (ERM) formation, and single-surgery success.
Results
Retinal reattachment rates were comparable (76% in Group A vs. 66% in Group B, p = 0.387), as well as the number of operations (p = 0.375). Moreover, BCVA improved significantly in both groups (p < 0.05), with no intergroup differences. IOP increased postoperatively in both groups (p < 0.001), with no significant differences. No differences were observed in cataract formation or retinal redetachment. However, ERM incidence was significantly higher in the PR group (27% vs. 11%, p = 0.049).
Conclusion
PFCL and PR are effective for RRD repair, with similar anatomical and visual outcomes. However, PFCL may reduce ERM risk, making it preferable in certain cases. These findings guide surgical decision-making and highlight the need for further research.
Trial registration
The study was retrospectively registered at ClinicalTrials.gov (NCT06919211) on April 4, 2025.
Keywords: Rhegmatogenous retinal detachment, Pars plana vitrectomy, Perfluorocarbon liquid, Posterior retinotomy, Epiretinal membrane
Introduction
Rhegmatogenous retinal detachment (RRD) is a sight-threatening condition involving the separation of the neurosensory retina from the underlying retinal pigment epithelium due to the passage of fluid through a retinal tear [1]. In many instances, the detachment is preceded by vitreoretinal alterations, including vitreous liquefaction and syneresis, which exert tractional forces on the retina, in addition to degenerative lesions that predispose to retinal breaks [2]. The primary surgical goal in RRD is to achieve durable retinal reattachment by sealing retinal breaks and relieving vitreous traction [3]. Pars plana vitrectomy (PPV) is a cornerstone technique for RRD repair, and adjunctive strategies such as perfluorocarbon liquid (PFCL) use and posterior retinotomy (PR) are frequently employed to aid in subretinal fluid (SRF) drainage and retinal reattachment [4].
Drainage retinotomy involves creating a retinal incision to evacuate SRF, especially in cases where retinal rigidity or extensive proliferative vitreoretinopathy (PVR) prevents spontaneous reattachment [5]. In such scenarios, PR facilitates fluid drainage and releases traction that cannot be resolved through vitreous removal or buckling procedures alone [6]. This technique is also applied in advanced myopia, ischemic vasculopathies, and massive subretinal proliferation, which refers to dense fibrocellular membranes beneath the detached retina, often forming part of PVR grade C and impeding retinal redraping [7]. However, PR carries risks such as intraoperative bleeding, retinal incarceration, and the development of postoperative PVR [8].
Conversely, PFCLs are heavy liquid fluorinated hydrocarbons introduced intraoperatively to assist in retinal stabilization. They aid in the displacement of SRF through anterior retinal breaks without the need for retinotomy, thereby minimizing surgical trauma [9]. PFCL provides intraoperative retinal tamponade, supports posterior pole flattening, and simplifies subretinal fluid management [10]. While PFCL toxicity remains a concern, particularly with retained material or prolonged exposure, its temporary intraoperative use is generally well tolerated [11].
Despite the routine use of both PFCL and PR in vitreoretinal surgery, clear guidelines and comparative data regarding their efficacy in managing SRF during RRD surgery remain scarce [12]. Moreover, there is limited consensus on the optimal technique, and considerable variability exists among surgeons regarding the use of PFCL or PR, with decisions often based on intraoperative judgment rather than standard criteria [13, 14].
This prospective randomized controlled trial was designed to compare PFCL and PR as intraoperative techniques for SRF drainage in primary RRD managed with PPV. In this study, PFCL was used as a temporary intraoperative tool, while PR was applied as a primary, predefined strategy, not merely a rescue intervention. The aim was to evaluate anatomical success, visual acuity outcomes, intraocular pressure (IOP), and complication profiles between the two techniques under standardized surgical conditions.
The findings are expected to inform clinical practice and contribute to the development of evidence-based guidelines for selecting SRF drainage strategies in RRD repair. Additionally, this work may serve as a foundation for future investigations into tailored surgical approaches based on individual patient or anatomical risk factors.
Methods
Study design and subjects
A prospective randomized controlled clinical trial was conducted with a study sample of 58 eyes with RRD enrolled from Ebsar Eye Center, Cairo, Egypt, to compare the surgical outcomes of PFCL and PR in PPV to manage RRD. The efficacy, safety, and postoperative outcomes of the two techniques were evaluated in a controlled and systematic manner.
The study included patients who met the following criteria: a clear diagnosis of primary RRD requiring surgery, 18 years or older, and the potential to return for follow-up visits for 3 months post-surgery. These criteria were established to ensure that the study population was appropriate for assessing the results of surgical therapy and to provide consistent monitoring of healing and long-term results. Patients were excluded if they presented with tractional or exudative retinal detachment, had surgery on the affected eye in the past, or presented with severe eye disorders like advanced glaucoma or endophthalmitis. These were instituted to keep the study group homogeneous, reduce confusing factors, and have only those cases of primary RRD without any other conditions that could affect surgery outcomes or recovery.
Patients were allocated randomly using computer-generated numbers to two intervention groups with a 1:1 ratio to compare the efficacy of different surgical techniques for SRF drainage through simple randomization to avoid biased distribution and accurately determine the safety and efficacy of both surgical interventions. Group assignment was concealed to minimize allocation bias. Group A (PFCL Group) patients underwent PPV with PFCL application to facilitate SRF drainage and retinal reattachment, and was considered the experimental group. Group B (PR Group) patients underwent PPV with PR for SRF drainage directly.
The study was conducted following the ethical principles outlined in the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board (IRB)/Ethics Committee of the Faculty of Medicine, Al-Azhar University (Approval No. [Ophth._19/2024Med.Research]). Written informed consent was obtained from all participants before their inclusion in the study. Patient confidentiality was maintained throughout the study, and all data were anonymized for analysis.
Study interventions
A thorough preoperative evaluation was conducted on all patients to establish baseline ocular characteristics for postoperative comparison and ensure proper initial status documentation. This comprehensive assessment allowed for reliable attribution of changes in visual acuity, retinal condition, or other ocular parameters to the surgery and postoperative care. Additionally, the preoperative findings played a crucial role in surgical planning, enabling the surgeons to tailor the procedure to each patient's specific needs.
Key preoperative assessments included Best-Corrected Visual Acuity (BCVA) determination using a Snellen chart, intraocular pressure (IOP) measurement via Goldmann application tonometry, and axial length measurement through optical biometry. Lens status was categorized as phakic, pseudophakic, or aphakic, while macular status was documented as attached or detached using optical coherence tomography (OCT). Furthermore, the presence and severity of PVR were classified according to the revised Retina Society classification system. Further baseline characteristics were documented to account for potential confounding variables. These included history of ocular trauma, presence of choroidal detachment confirmed by B-scan ultrasonography, existence of giant retinal tears defined as tears involving more than 90 degrees of retinal circumference, and high myopia, characterized by a refractive error exceeding −6.00 diopters. Other relevant ocular comorbidities such as lattice degeneration, previous intraocular surgeries (e.g., cataract extraction, trabeculectomy, or retinal laser procedures), and clinical signs suggestive of chronicity or retinal scarring were also recorded. These detailed evaluations provided essential information for both surgical decision-making and postoperative analysis.
Beyond these fundamental ocular assessments, a meticulous examination of the retinal detachment was performed. This included determining the extent and location of the detachment, identifying the number and position of retinal breaks, and assessing for any associated vitreous hemorrhage or other pathological findings. The fundus was carefully examined using indirect ophthalmoscopy and non-contact slit-lamp biomicroscopy to gain a comprehensive understanding of the retinal and vitreous condition.
In addition to ocular assessments, a thorough systemic evaluation was conducted to identify underlying health conditions that could influence surgical outcomes or postoperative recovery. Patients were screened for systemic diseases such as diabetes and hypertension, which are known to affect ocular health and healing processes.
Following surgery, the same parameters were reassessed at regular intervals to monitor recovery, detect potential complications, and evaluate the overall success of the intervention. This structured follow-up ensured a comprehensive assessment of surgical outcomes and facilitated the timely management of postoperative issues.
Surgical protocol
All surgeries were conducted by two experienced vitreoretinal surgeons using a uniform 23-gauge pars plana vitrectomy (PPV) technique under local or general anesthesia. After core vitrectomy and induction of posterior vitreous detachment when necessary, peripheral vitreous shaving was performed with scleral indentation.
In Group A (PFCL group), intraoperative injection of perfluorocarbon liquid (PFCL) was used to mechanically flatten the detached retina and displace subretinal fluid (SRF) toward the pre-existing retinal breaks. As shown in Fig. 1A, diathermy was applied to the edges of the primary retinal tear to stabilize the margins prior to PFCL injection. Once reattachment was confirmed, a PFCL–air exchange was carried out, followed by internal tamponade with either silicone oil (5000 centistokes) or expansile gas (C3F8 or SF6), selected based on case-specific parameters. Figure 1B illustrates the same case after PFCL injection, during endolaser photocoagulation of the retinal breaks.
Fig. 1.
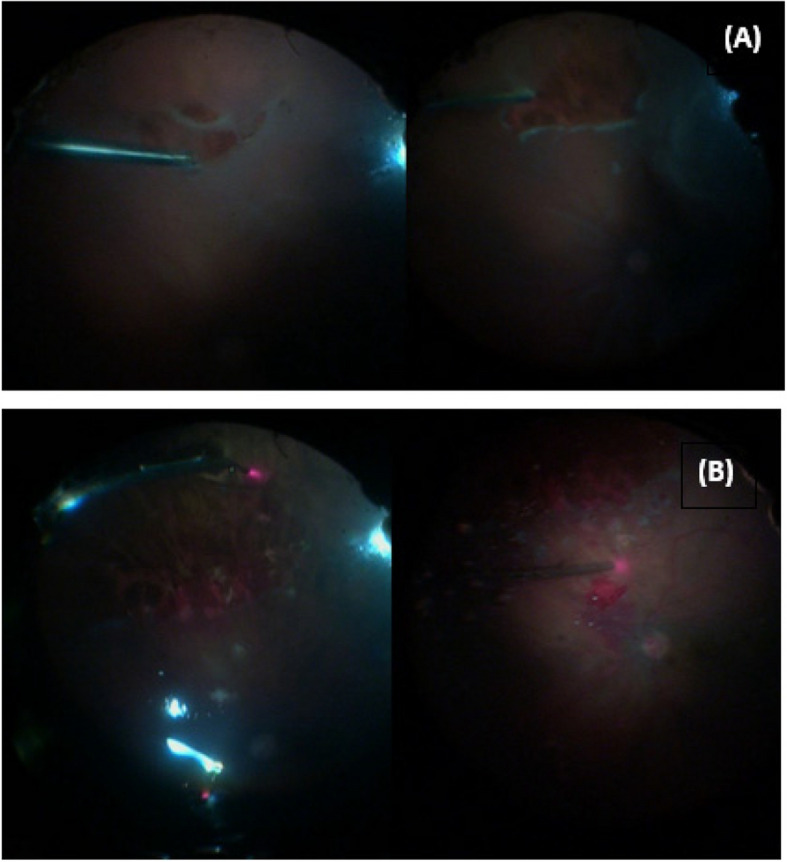
A Intraoperative photograph of a rhegmatogenous retinal detachment (RRD) case during diathermy application to the primary retinal tear. B Intraoperative image of the same case following perfluorocarbon liquid (PFCL) injection, with endolaser photocoagulation being applied around the retinal break
In Group B (PR group), for cases where SRF could not be adequately drained through peripheral breaks due to retinal stiffness or posterior fluid pocketing, a controlled posterior retinotomy (PR) was created using an endodiathermy probe. As illustrated in Fig. 2, SRF was actively aspirated through the newly created retinotomy site, which was subsequently surrounded by laser photocoagulation to prevent postoperative complications. The procedure concluded with fluid–air exchange and internal tamponade using silicone oil or gas, following the same criteria as Group A.
Fig. 2.

Intraoperative photo showing posterior retinotomy (PR) created using diathermy for SRF drainage in a case of RRD (Group B)
In both groups, endolaser photocoagulation was applied to all identified retinal breaks. No patients received scleral buckling. Postoperative management included standard topical corticosteroids, antibiotics, and cycloplegics. The standardized operative technique and postoperative care minimized inter-surgeon variability and ensured procedural consistency across the study arms.
Outcome measures
Primary outcome
Retinal reattachment was confirmed postoperatively through detailed fundus evaluation using indirect ophthalmoscopy and spectral-domain OCT. In cases where media opacity precluded direct visualization, B-scan ultrasonography was used to verify retinal apposition.
Primary retinal reattachment was defined as successful reattachment following the initial surgery and tamponade removal without need for additional surgery. Final retinal reattachment referred to the reattachment status at the last follow-up visit (after up to 6 months), regardless of additional procedures required.
Secondary outcome
Secondary outcome measures included Visual Acuity Outcomes, with BCVA measured using a Snellen chart at each follow-up visit (1 week, 1 month and 3 months postoperatively). This allowed for tracking visual recovery over time and identifying any trends or delays in improvement. The Incidence of Intraoperative Complications, such as iatrogenic retinal breaks, was documented during the surgical procedure to evaluate the safety and technical challenges associated with each technique. IOP was also monitored at each follow-up visit using Goldmann applanation tonometry to detect any postoperative elevation or abnormalities that could indicate complications like ocular hypertension or glaucoma. Furthermore, the study assessed Postoperative Complications, including Cataract development assessed using the Lens Opacities Classification System III (LOCS-III) score, the recurrence of retinal detachment, the development of epiretinal membranes, and single-surgery success. These complications were carefully documented to evaluate the long-term stability of the surgical outcomes and to identify any factors that might contribute to suboptimal results.
Sample size calculation
A priori power analysis was performed using G*Power software (version 3.1.9.7) to determine the required sample size. Based on previous studies that reported ERM incidence rates ranging from 10 to 35% following PR or PFCL use [6], we aimed to detect a clinically significant absolute difference of 25% in ERM rates between the two groups, with a power (1–β) of 80% and a two-sided alpha level of 0.05. This analysis indicated that a minimum of 25 patients per group would be necessary to detect this difference. Our study enrolled 58 eyes (29 in each group), which exceeds the calculated sample size and provides adequate power to assess clinically meaningful differences in primary outcomes.
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 26. Continuous variables were tested for normality using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD) and compared using the independent samples t-test. Non-normally distributed data were expressed as median (interquartile range, IQR) and compared using the Mann–Whitney U test. Categorical variables were presented as counts and percentages, and compared using the chi-square test or Fisher’s exact test when expected frequencies were small.
Univariate analysis was conducted to explore associations between baseline variables (e.g., age, axial length, PVR grade, lens status) and postoperative outcomes including epiretinal membrane (ERM) formation and retinal redetachment. Multivariate logistic regression was not feasible due to convergence failure caused by limited sample size and low event frequency. A p-value < 0.05 was considered statistically significant.
Results
The results revealed no significant differences between the two groups in baseline demographic or preoperative characteristics (Table 1). Similarly, there were no statistically significant differences in relevant baseline ocular risk factors or comorbidities (Table 2).
Table 1.
Demographic characteristics and preoperative ocular status
| Characteristic | Group A (n = 29) | Group B (n = 29) | Statistical Test | P-Value |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 40.72 ± 19.28 | 47.9 ± 15.83 | U = 338 | 0.202 |
| Range | 11–70 | 6–67 | ||
| Sex, n (%) | ||||
| Male | 23 (79%) | 16 (55%) | χ2 = 2.81 | 0.093 |
| Female | 6 (21%) | 13 (45%) | ||
| Axial Length, n (%) | ||||
| < 26 mm | 22 (75.9%) | 24 (82.8%) | χ2 = 0.11 | 0.746 |
| > 26 mm | 7 (24.1%) | 5 (17.2%) | ||
| Lens Status, n (%) | ||||
| Phakic | 20 (69%) | 21 (72%) | χ2 = 0.08 | 0.773 |
| Pseudophakic | 9 (31%) | 8 (28%) | ||
| Macula Status, n (%) | ||||
| On | 13 (45%) | 14 (48%) | χ2 = 0.07 | 0.792 |
| Off | 16 (55%) | 15 (52%) | ||
| Grade of Proliferative Vitreoretinopathy (PVR), n (%) | ||||
| A | 2 (7%) | 2 (7%) | χ2 = 1.89 | 0.388 |
| B | 9 (31%) | 14 (48%) | ||
| C | 18 (62%) | 13 (45%) | ||
A P-value < 0.05 was considered statistically significant
χ2 chi-square test, U Mann–Whitney U test
Table 2.
Baseline ocular comorbidities and anatomical risk factors
| Variable | Group A (n = 29) | Group B (n = 29) | p-value |
|---|---|---|---|
| History of ocular trauma | 3 (10.0%) | 2 (7.1%) | 0.712 |
| Choroidal detachment | 2 (6.7%) | 1 (3.6%) | 0.621 |
| Giant retinal tears | 2 (6.7%) | 2 (7.1%) | 0.956 |
| High myopia (> −6.00 D) | 4 (13.3%) | 3 (10.7%) | 0.765 |
| Lattice degeneration | 5 (16.7%) | 6 (21.4%) | 0.641 |
| Previous intraocular surgery | 4 (13.3%) | 4 (14.3%) | 0.912 |
A P-value < 0.05 was considered statistically significant
D diopters, U Mann–Whitney U test
Primary outcome measures
At 3 months postoperatively, retinal reattachment was achieved in 76% (22/29) of eyes in Group A vs. 66% (19/29) in Group B (χ2 = 0.75, p = 0.387, 95% CI for difference: −12.4% to 32.4%), indicating no significant difference (Table 3). Most cases in both groups required only one surgery (76% vs. 66%, p = 0.375).
Table 3.
Primary outcome measures of the studied groups
| Outcome | Group A (n = 29) | Group B (n = 29) | Statistical Test | P-Value |
|---|---|---|---|---|
| Retinal Reattachment at 3 Months After Intraocular Tamponade Removal, n (%) | ||||
| Yes | 22 (76%) | 19 (66%) | χ2 = 0.75 | 0.387 |
| No | 7 (24%) | 10 (34%) | ||
| Number of Operations Required to Achieve Retinal Reattachment, n (%) | ||||
| 1 | 22 (76%) | 19 (66%) | χ2 = 1.96 | 0.375 |
| 2 | 3 (10%) | 7 (24%) | ||
| 3 | 4 (14%) | 3 (10%) | ||
A P-value < 0.05 was considered statistically significant
χ2 chi-square test
In Group A, the three reoperations were due to persistent subretinal fluid (n = 1) and new peripheral breaks (n = 2). In Group B, five eyes required reoperations due to recurrent PVR (n = 3), unresolved subretinal fluid (n = 1), and new breaks (n = 1).
Secondary outcome measures
BCVA (LogMAR) improved progressively in both groups postoperatively with no significant intergroup differences at any time point (Table 4, Fig. 3). The preoperative BCVA was similar between the groups (U = 411, p = 0.885), and improvements at 3 months were observed in both: Group A (1.13 ± 0.59) vs. Group B (1.02 ± 0.44), U = 0.82, p = 0.416.
Table 4.
BCVA and IOP changes in the studied groups
| Parameter | Group A (n = 29) | Group B (n = 29) | Statistical Test | P-Value |
|---|---|---|---|---|
| Best-Corrected Visual Acuity (BCVA) (LogMAR), Mean ± SD (Range) | ||||
| Preoperative | 1.88 ± 0.64 (0.4—2.8) | 1.86 ± 0.62 (0.4—2.8) | U = 411 | 0.885 |
| After 1 week | 1.66 ± 0.54 (0.5—2.3) | 1.65 ± 0.57 (0.4—2.8) | U = 398 | 0.727 |
| After 1 month | 1.42 ± 0.51 (0.4—2.3) | 1.32 ± 0.51 (0.4—2.8) | U = 359 | 0.337 |
| After 2 months | 1.18 ± 0.58 (0.1—2.3) | 1.17 ± 0.45 (0.4—2.3) | t = 0.114 | 0.909 |
| After 3 months | 1.13 ± 0.59 (0.2—2.3) | 1.02 ± 0.44 (0.3—2.3) | U = 0.82 | 0.416 |
| Intraocular Pressure (IOP) (mmHg), Mean ± SD (Range) | ||||
| Preoperative | 14.17 ± 4.24 (6—26) | 13.24 ± 3.72 (6—22) | t = 0.88 | 0.378 |
| 3-Month Postoperative | 20.00 ± 4.72 (12—28) | 20.14 ± 4.00 (12—28) | t = 0.12 | 0.905 |
A P-value < 0.05 was considered statistically significant
U Mann–Whitney test, t Student's t-test
Fig. 3.
Changes in BCVA in PFCL Group and PR Group at preoperative, 1 week, 1 month, 2 months, and 3 months postoperative time points
IOP increased postoperatively in both groups (from 14.17 ± 4.24 to 20.00 ± 4.72 in Group A and from 13.24 ± 3.72 to 20.14 ± 4.00 in Group B), but the differences between groups were not statistically significant (p = 0.905 at 3 months).
Cataract formation (as graded by LOCS-III) was comparable in both groups: 38% in Group A and 41% in Group B (p = 0.95). Retinal redetachment occurred in 24% vs. 34% of eyes in Groups A and B, respectively (p = 0.387). Importantly, the rate of ERM development was significantly higher in the PR group (27%) than in the PFCL group (11%), with a χ2 = 2.08, p = 0.049 (95% CI for difference: 0.2% to 31.6%). Single-surgery success rates were similar (76% Group A vs. 66% Group B, p = 0.865) (Table 5, Fig. 4).
Table 5.
Postoperative complications in the studied groups
| Complication | Group A (n = 29) | Group B (n = 29) | Statistical Test | P-Value |
|---|---|---|---|---|
| Cataract (LOCS-III Score), n (%) | ||||
| Yes | 11 (38%) | 12 (41%) | χ2 = 0.10 | 0.95 |
| No | 9 (31%) | 9 (31%) | ||
| Pseudophakic | 9 (31%) | 8 (28%) | ||
| Retinal Redetachment, n (%) | ||||
| Yes | 7 (24%) | 10 (34%) | χ2 = 0.75 | 0.387 |
| No | 22 (76%) | 19 (66%) | ||
| Development of Epiretinal Membrane, n (%) | ||||
| Yes | 3 (11%) | 8 (27%) | χ2 = 2.08 | 0.049 |
| No | 26 (89%) | 21 (73%) | ||
| Single-Surgery Success, n (%) | ||||
| Yes | 22 (76%) | 19 (66%) | χ2 = 0.75 | 0.865 |
| No | 7 (24%) | 10 (34%) | ||
A P-value < 0.05 was considered statistically significant
Abbreviations: LOCS-III Lens Opacities Classification System III, χ2 chi-square test
Fig. 4.

Incidence of Epiretinal Membrane Development in the Studied Groups
Univariate analysis of postoperative outcomes
To assess potential predictors of postoperative complications, univariate analysis was performed. ERM development was significantly more frequent in the PR group compared to the PFCL group (27% vs. 11%, p = 0.049). No significant associations were found between ERM formation and patient age (p = 0.532), axial length (p = 0.638), lens status (p = 0.819), macular status (p = 0.771), or PVR grade (p = 0.605).
Similarly, retinal redetachment did not significantly correlate with treatment group (p = 0.387), PVR grade (p = 0.412), axial length (p = 0.623), or macular status (p = 0.479). These findings suggest that the choice of surgical technique may influence ERM formation but that other demographic or anatomical risk factors did not significantly affect postoperative outcomes in this sample (Table 6).
Table 6.
Univariate logistic regression analyses of factors associated with ERM formation
| Variable | Univariate OR (95% CI) | p-value |
|---|---|---|
| Age (per year increase) | 1.02 (0.98–1.06) | 0.302 |
| Sex (Male vs. Female) | 0.52 (0.14–1.90) | 0.325 |
| High myopia | 1.20 (0.22–6.42) | 0.830 |
| Macula-off detachment | 1.34 (0.35–5.14) | 0.671 |
| PVR grade C | 1.75 (0.47–6.53) | 0.403 |
| Use of PR (vs. PFCL) | 3.00 (1.02–8.87) | 0.046 |
Abbreviations: OR odds ratio, CI confidence interval, ERM Epiretinal membrane, RRD Rhegmatogenous retinal detachment, PVR Proliferative vitreoretinopathy, PFCL Perfluorocarbon liquid, PR Posterior retinotomy, BCVA Best-corrected visual acuity
Discussion
This study confirms that both PFCL and PR are effective adjunctive techniques for retinal reattachment in RRD surgery, with no statistically significant difference in success rates at three months. Most eyes achieved reattachment after a single procedure, and the slightly higher reattachment rate in the PFCL group—though not significant—may reflect its intraoperative utility in cases with severe PVR or complex SRF [15]. PFCL facilitates retinal stabilization and SRF drainage during surgery, which may enhance precision and reduce reoperation [15]. Nonetheless, PR remains a viable alternative, particularly when PFCL is contraindicated or unavailable. The slightly lower reattachment in PR may relate to iatrogenic breaks and their association with ERM or redetachment [16, 17].
A subset of PR cases (24%) required two surgeries, compared to 10% in PFCL, indicating that PR may predispose to persistent SRF or secondary complications [18]. Still, PR is useful in cases with fibrotic or incarcerated retina where other methods may fail [19]. These findings align with previous studies showing similar anatomical outcomes between PFCL and PR/subretinal fluid drainage (SRFD) groups [20, 21].
BCVA improved significantly in both groups, with no differences at any follow-up. Preoperative BCVA in LogMAR was comparable, and both groups showed progressive visual recovery from week one to month three, consistent with findings that anatomical reattachment is key to functional restoration [22]. Despite different mechanisms—PFCL offering tamponade and PR aiding in SRF drainage—visual outcomes remained comparable [23, 24]. This suggests neither technique offers superior early visual rehabilitation. Factors such as PVR severity, macular detachment duration, and photoreceptor status likely influenced outcomes [25, 26]. Longer follow-up may clarify differences in late visual recovery related to neuroadaptation and remodeling.
IOP was comparable pre- and postoperatively. The postoperative rise in IOP seen in both groups is consistent with known effects of tamponade agents, inflammation, and steroid response [27]. Elevated IOP is well documented after PPV, particularly with gas or silicone oil tamponade [28, 29]. The lack of intergroup differences suggests that neither PFCL nor PR increases the risk of early IOP complications. PFCL may also help reduce retinal displacement [30], as reported by Cheng et al. [31], reaffirming the need for IOP monitoring to prevent complications like glaucoma [32]. Prolonged follow-up is necessary to evaluate sustained IOP elevation risk [33].
Postoperative complications were largely comparable. Cataract formation and redetachment occurred at similar rates, consistent with literature showing cataracts are a near-universal outcome of PPV, regardless of technique [34, 35]. Oxidative stress and altered intraocular dynamics from tamponades may contribute [36]. Redetachment was slightly higher in the PR group, likely due to SRF persistence and increased risk of secondary PVR [37–39], though this difference lacked statistical significance.
Notably, ERM incidence was significantly higher in the PR group. This may stem from retinotomy-related trauma, inflammation, or disruption of the blood-retinal barrier [17, 40–42]. PFCL may reduce ERM risk by avoiding additional retinal breaks [43]. While our ERM findings differ from some reports, they suggest a possible association between PR and reactive gliosis [42]. Despite the higher incidence of ERM in the PR group, no significant differences in BCVA were observed between groups throughout the follow-up period. However, qualitative functional impacts such as metamorphopsia were not assessed and may be underestimated. Despite higher complication rates in the PR group, the comparable single-operation success supports that complications likely emerged after initial reattachment.
To further explore predictors of retinal reattachment following surgery, a univariate logistic regression analysis was conducted. Among the variables examined, preoperative macula-on status and absence of PVR grade C were significantly associated with successful reattachment at 3 months. These findings are consistent with previously published systematic reviews and meta-analyses that emphasize the prognostic importance of macular status and PVR severity in determining anatomical outcomes after RRD repair [44, 45]. Although the surgical method (PFCL vs. PR) did not emerge as a statistically significant predictor in univariate analysis, the trend favoring PFCL in reducing ERM risk reinforces its clinical utility in select cases.
These findings emphasize the need for individualized surgical planning. PFCL is particularly useful in complex detachments requiring precise intraoperative retinal control, while PR is an acceptable alternative when PFCL is impractical. Although PFCL involves higher material costs, its potential to reduce complications like ERM may offer long-term benefit.
Conclusion
This prospective randomized controlled trial provides evidence that both perfluorocarbon liquid (PFCL) and posterior retinotomy (PR) are effective adjunct techniques for subretinal fluid drainage during pars plana vitrectomy in rhegmatogenous retinal detachment (RRD). Both groups demonstrated comparable anatomical reattachment rates, visual acuity improvement, and intraocular pressure changes. However, a significantly higher incidence of epiretinal membrane (ERM) formation in the PR group suggests that PFCL may offer a lower risk of this specific postoperative complication, supporting its preferential use in selected clinical scenarios.
The study's strengths include its randomized design, standardized surgical protocols, and comprehensive assessment of anatomical and functional outcomes. However, limitations must be acknowledged. These include the modest sample size (58 eyes), the relatively short follow-up period (three months), and its single-center nature, all of which may affect the external validity and limit the detection of late-onset complications. Additionally, the exclusion of highly complex RRD cases and the absence of a subgroup analysis, constrained by sample size, may have limited the exploration of differential treatment responses among patient subsets.
Future research should prioritize multicenter collaborations, extended follow-up durations to better capture long-term outcomes such as ERM progression and visual recovery, and cost-effectiveness evaluations. Incorporating patient-reported outcomes and stratified subgroup analyses may further refine surgical decision-making. Ultimately, larger trials are warranted to identify patient characteristics that predict optimal benefit from PFCL or PR and to improve the personalization of RRD surgical care.
Acknowledgements
We sincerely appreciate EdigenomiX Scientific Co., Ltd. for their expert editing and proofreading, which greatly improved the clarity and quality of our manuscript. Their meticulous attention to detail and support in refining the document for publication is gratefully acknowledged.
Clinical trial number
Not applicable.
Abbreviations
- BCVA
Best-corrected Visual Acuity
- ERM
Epiretinal Membrane
- IOP
Intraocular Pressure
- PFCL
Perfluorocarbon Liquid
- PPV
Pars Plana Vitrectomy
- PR
Posterior Retinotomy
- RRD
Rhegmatogenous Retinal Detachment
- LOCS-III
Lens Opacities Classification System III
- SRFD
Subretinal fluid drainage
- LogMAR
Logarithm of the minimum angle of resolution
Authors’ contributions
E. M.: Conceptualization, study design, data interpretation, manuscript drafting, and final approval. H. E.: Data collection, statistical analysis, manuscript revision, and approval of the final version. M. M.: Literature review, data validation, and manuscript writing. O. H.: Patient recruitment, data acquisition, and manuscript editing. S. M.: Methodology development, statistical analysis, and manuscript review. A. M.: Surgical procedures, postoperative evaluation, and manuscript revision. F. M.: Data analysis, results interpretation, and critical manuscript review. A. A.: Study coordination, technical support, and manuscript proofreading. E. A.: Literature review, data validation, and manuscript editing. E. T.: Methodology development, statistical analysis, and critical manuscript review. T. G.: Supervision, critical manuscript review, and final approval for publication. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted following the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Al-Azhar University (Approval No. [Ophth._19/2024Med.Research]), Cairo, Egypt. Written informed consent was obtained from all participants before enrollment.
Consent for publication
All authors have reviewed and approved the final manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laíns I, Wang JC, Cui Y, Katz R, Vingopoulos F, Staurenghi G, et al. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog Retin Eye Res. 2021;84: 100951. 10.1016/j.preteyeres.2021.100951. [DOI] [PubMed]
- 2.Sen P, Shaikh S, Shetty S, Goswami A, Deshmukh K. Retinal Detachment. Retina: Medical & Surgical Management. 2 ed.: Jaypee Brother Medical Publisher; 2022. p. 449.
- 3.Sultan ZN, Agorogiannis EI, Iannetta D, Steel D, Sandinha T. Rhegmatogenous retinal detachment: a review of current practice in diagnosis and management. BMJ Open Ophthalmol. 2020;5(1):e000474. 10.1136/bmjophth-2020-000474. [DOI] [PMC free article] [PubMed]
- 4.Vo LV, Ryan EH, Ryan CM, Shah GK, Gupta OP, Capone A Jr., et al. Posterior retinotomy vs perfluorocarbon liquid to aid drainage of subretinal fluid during primary rhegmatogenous retinal detachment repair (PRO study report no. 10). J VitreoRetin Dis. 2020;4(6):494–8. 10.1177/2474126420941372. [DOI] [PMC free article] [PubMed]
- 5.Han G, Huang W, He L, Wei H, Wei L, Huang H. The efficacious combined treatment of rhegmatogenous retinal detachment (PVR ≤ C2) with inferior breaks using 25-gauge pars plana vitrectomy and air tamponade. Medicine (Baltimore). 2024;103(39): e39555. 10.1097/md.0000000000039555. [DOI] [PMC free article] [PubMed]
- 6.Ramamurthy SR, Dave VP, Chou H-D, Ozdek S, Parolini B, Dhawahir-Scala F, et al. Retinotomies and retinectomies: a review of indications, techniques, results, and complications. Surv Ophthalmol. 2023;68(6):1038–49. 10.1016/j.survophthal.2023.06.012. [DOI] [PubMed]
- 7.Fukuyama H, Ishikawa H, Gomi F. Impact of drainage retinotomy on surgical outcomes of retinal detachment: insights from the Japan-retinal detachment registry. Sci Rep. 2024;14(1):7795. 10.1038/s41598-024-58453-5. [DOI] [PMC free article] [PubMed]
- 8.Rizzo S, Tartaro R, Finocchio L, Cinelli L, Biagini I, Barca F, et al. Perfluorodecalin as medium-term tamponade in the case of retinal detachment recurrence with an inferior retinal break, which lies posteriorly to an encircling band. Retina. 2022;42(6):1203–10. 10.1097/IAE.0000000000002381. [DOI] [PubMed] [Google Scholar]
- 9.Trabelsi O, Bouladi M, Ouertani A, Trabelsi A. Short-term total tamponade with perfluorocarbon liquid and silicone oil in complex rhegmatogenous retinal detachment with severe proliferative vitreoretinopathy. Clin Ophthalmol. 2023;17:515–25. 10.2147/opth.S400156. [DOI] [PMC free article] [PubMed]
- 10.McKay BR, Bansal A, Kryshtalskyj M, Wong DT, Berger AR, Muni RH. Two-year outcomes of different subretinal fluid drainage techniques during vitrectomy for fovea-off rhegmatogenous retinal detachments: ELLIPSOID-2 study. Br J Ophthalmol. 2024. 10.1136/bjo-2023-323879. [DOI] [PubMed]
- 11.Davidović S, Babović S, Miljković A, Pavin S, Bolesnikov-Tošić A, Barišić S. Updates on treatment modalities for primary rhegmatogenous retinal detachment repair. Diagnostics. 2024;14(14):1493. 10.3390/diagnostics14141493. [DOI] [PMC free article] [PubMed]
- 12.Iovino C, Rosolia A, Damiano L, Iodice CM, Di Iorio V, Testa F, et al. Pars plana vitrectomy in inherited retinal diseases: a comprehensive review of the literature. Life. 2023;13(6):1241. 10.3390/life13061241. [DOI] [PMC free article] [PubMed]
- 13.Muni RH, Lee WW, Bansal A, Ramachandran A, Hillier RJ. A paradigm shift in retinal detachment repair: the concept of integrity. Prog Retin Eye Res. 2022;91: 101079. 10.1016/j.preteyeres.2022.101079. [DOI] [PubMed]
- 14.Machiele R, Motlagh M, Zeppieri M, Patel BC. Intraocular pressure. StatPearls: StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 15.Shettigar MP, Dave VP, Chou H-D, Fung A, Iguban E, de Ribot FM, et al. Vitreous substitutes and tamponades–A review of types, applications, and future directions. Indian J Ophthalmol. 2024;72(8):1102–11. 10.4103/IJO.IJO_2417_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasetty VM, Aye J, Patel N, Tripathi N, Hessburg T, Kumar N, et al. Outcomes and complications of primary rhegmatogenous retinal detachment repair with pars plana vitrectomy in young adults. Int J Retina Vitreous. 2023;9(1):11. 10.1186/s40942-023-00448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa K, Akiyama M, Mori K, Nakama T, Notomi S, Nakao S, et al. Drainage retinotomy confers risk of epiretinal membrane formation after vitrectomy for rhegmatogenous retinal detachment repair. Am J Ophthalmol. 2022;234:20–7. 10.1016/j.ajo.2021.07.028. [DOI] [PubMed]
- 18.Schwartz SG, Flynn HW Jr, Wang X, Kuriyan AE, Abariga SA, Lee WH. Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy. Cochrane Database Syst Rev. 2020;5(5):Cd006126. 10.1002/14651858.CD006126.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpineto P, Licata AM, Ciancaglini M. Proliferative vitreoretinopathy: a reappraisal. J Clin Med. 2023. 10.3390/jcm12165287. [DOI] [PMC free article] [PubMed]
- 20.Wurtz M, Dormegny L, Muller C, Bourcier T, Ballonzoli L, Gaucher D, et al. Perfluorocarbon liquid use during vitrectomy for macula-off retinal detachment has no impact on macular folds and metamorphopsia. Retina. 2024;44(11):1891–8. 10.1097/iae.0000000000004220. [DOI] [PubMed]
- 21.Vidne O, Blum Meirovitch S, Rabina G, AbdEelkader A, Prat D, Barequet D, et al. Perfluorocarbon liquid vs. subretinal fluid drainage during vitrectomy for the primary repair of rhegmatogenous retinal detachment: a comparative study. Curr Eye Res. 2018;43(11):1389–94. 10.1080/02713683.2018.1490436. [DOI] [PubMed]
- 22.Baumann C, Kaye SB, Steel DH. Reversing the paradigm on the urgency of acute retinal detachments defined by their foveal status: when off may be more urgent than on. BMJ Open Ophthalmol. 2024. 10.1136/bmjophth-2024-001668. [DOI] [PMC free article] [PubMed]
- 23.Bhurayanontachai P, Seepongphun U. Outcomes of a postoperative perfluorocarbon liquid tamponade for complex retinal detachments: 12 years of experience in southern Thailand. BMC Ophthalmol. 2020;20(1):358. 10.1186/s12886-020-01600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sood G, Mahajan S. Scleral imbrication with vitreoretinal surgery as a primary procedure for retinal detachment in pathological myopia. Indian J Ophthalmol. 2025;73(Suppl 1):S172–4. 10.4103/ijo.ijo_211_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AlGhazal F, Semidey VA, Rubio-Caso MJ, AlSulaiman SM, Sesma G. Surgical parameters and prognostic factors in persistent fetal vasculature: insights from a retrospective cohort study. Ophthalmol Ther. 2025. 10.1007/s40123-024-01088-6. [DOI] [PMC free article] [PubMed]
- 26.Dell’Omo R, Carosielli M, Rapino G, Affatato M, Cucciniello P, Virgili G, et al. Biomarkers of vitreous cortex remnants in eyes with primary rhegmatogenous retinal detachment. Biomarkers of Vitreous Cortex Remnants in Eyes With Primary Rhegmatogenous Retinal Detachment. 2024;8(10):1002–12. 10.1167/tvst.12.6.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer M, Ie A, Eibenberger K, Auffarth GU, Xue K. An analysis of heavy silicone oil treatment for inferior proliferative vitreoretinopathy. BMC Ophthalmol. 2025;25(1):38. 10.1186/s12886-024-03834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panda A, Panigrahi PK, Pattnaik L. Raised intraocular pressure secondary to vitreoretinal procedures: a literature review. J Ophthal Sci Res. 2024;62(1):12–9. 10.4103/tjosr.tjosr_84_23.
- 29.Pisig AU, Gizicki R, Hanout M. Vitreous Substitutes. Practical Manual of Vitreoretinal Surgery. Springer; 2024. p. 51–70.
- 30.Siwik P, Chudoba T, Cisiecki S. Retinal displacement following vitrectomy for rhegmatogenous retinal detachment: a systematic review of surgical techniques, tamponade agents, and outcomes. J Clin Med. 2025;14(1): 250. 10.3390/jcm14010250. [DOI] [PMC free article] [PubMed]
- 31.Cheng T, Bastion M. Case series and review article: perfluorocarbon heavy liquid as a short-term tamponade after vitrectomy for inferior rhegmatogenous retinal detachment. J Surg Acad. 2024;14:01–9. 10.17576/JSA.2024.1401.01.
- 32.Aldossari SH, Schargel K, Aljadaan I, Ahmad K, Gorinees R, Alzendi N, et al. Prognostic Significance of Early Postoperative Choroidal Detachment in Patients with Congenital Glaucoma Operated with Nonpenetrating Deep Sclerectomy. J Ophthalmol. 2024;2024(1):7127996. 10.1155/2024/7127996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dada T, Verma S, Gagrani M, Bhartiya S, Chauhan N, Satpute K, et al. Ocular and Systemic Factors Associated with Glaucoma. J Curr Glaucoma Pract. 2022;16(3):179–91. 10.5005/jp-journals-10078-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BJ, Jun JH, Afshari NA. Challenges and outcomes of cataract surgery after vitrectomy. Curr Opin Ophthalmol. 2025. 10.1097/icu.0000000000001096. [DOI] [PubMed]
- 35.Karakosta C, Samiotaki M, Panayotou G, Papaconstantinou DS, Moschos MM. Lens Cytoskeleton: An Update on the Etiopathogenesis of Human Cataracts. Cureus. 2024;16(3). 10.7759/cureus.56793. [DOI] [PMC free article] [PubMed]
- 36.Ge L, Su N, Fan W, Yuan S. Risk factors and management of intraocular pressure elevation after vitrectomy combined with silicone oil tamponade. Int J Gen Med. 2024. 10.2147/IJGM.S446617. [DOI] [PMC free article] [PubMed]
- 37.Quiroz-Reyes MA, Quiroz-Gonzalez EA, Quiroz-Gonzalez MA, Lima-Gomez V. Systematic review of surgical techniques for treating giant retinal tears in adults: a current assessment of approaches and interventions. Lat Am J Ophthalmol. 2024. 10.25259/LAJO_15_2024.
- 38.Guber J, Bentivoglio M, Valmaggia C, Lang C, Guber I. Predictive risk factors for retinal redetachment following uncomplicated pars plana vitrectomy for primary rhegmatogenous retinal detachment. J Clin Med. 2020. 10.3390/jcm9124037. [DOI] [PMC free article] [PubMed]
- 39.Nagpal M, Chaudhary P, Wachasundar S, Eltayib A, Raihan A. Management of recurrent rhegmatogenous retinal detachment. Indian J Ophthalmol. 2018;66:1763. 10.4103/ijo.IJO_1212_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalogeropoulos D, Lotery AJ, Gupta B, Lash S, Antonakis S. Epiretinal membranes in patients with uveitis: an update on the current state of management. Int Ophthalmol. 2024;44(1):291. 10.1007/s10792-024-03199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ożóg MK, Nowak-Wąs M, Rokicki W. Pathophysiology and clinical aspects of epiretinal membrane - review. Front Med (Lausanne). 2023;10:1121270. 10.3389/fmed.2023.1121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szigiato AA, Antaki F, Javidi S, Touma S, Duval R, Cordahi G, et al. Risk factors for epiretinal membrane formation and peeling following pars plana vitrectomy for primary rhegmatogenous retinal detachment, an OCT guided analysis. Int J Retina Vitreous. 2022;8(1): 70. 10.1186/s40942-022-00418-9. [DOI] [PMC free article] [PubMed]
- 43.Lyu J, Xia F, Zhao P. Intraoperative perfluorocarbon liquid tamponade technique for treatment of extensive retinal detachment secondary to a myopic macular hole. Retina. 2023;43(4):698–704. 10.1097/iae.0000000000003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazelwood JE, Mitry D, Singh J, Bennett HGB, Khan AA, Goudie CR. The scottish retinal detachment study: 10-year outcomes after retinal detachment repair. Eye (Lond). 2025;39(7):1318–21. 10.1038/s41433-025-03613-8. [DOI] [PMC free article] [PubMed]
- 45.Ferro Desideri L, Artemiev D, Zandi S, Zinkernagel MS, Anguita R. Proliferative vitreoretinopathy: an update on the current and emerging treatment options. Graefes Arch Clin Exp Ophthalmol. 2024;262(3):679–87. 10.1007/s00417-023-06264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.



