Abstract
beta-amyloid peptide (A beta) is the primary protein component of senile plaques in Alzheimer's disease patients. Synthetic A beta spontaneously assembles into amyloid fibrils and is neurotoxic to cortical cultures. Neurotoxicity has been associated with the degree of peptide aggregation, yet the mechanism of assembly of A beta into amyloid fibrils is poorly understood. In this work, A beta was dissolved in several different solvents commonly used in neurotoxicity assays. In pure dimethylsulfoxide (DMSO), A beta had no detectable beta-sheet content; in 0.1% trifluoroacetate, the peptide contained one-third beta-sheet; and in 35% acetonitrile/0.1% trifluoroacetate, A beta was two-thirds beta-sheet, equivalent to the fibrillar peptide in physiological buffer. Stock solutions of peptide were diluted into phosphate-buffered saline, and fibril growth was followed by static and dynamic light scattering. The growth rate was substantially faster when the peptide was predissolved in 35% acetonitrile/0.1% trifluoroacetate than in 0.1% trifluoroacetate, 10% DMSO, or 100% DMSO. Differences in growth rate were attributed to changes in the secondary structure of the peptide in the stock solvent. These results suggest that formation of an intermediate with a high beta-sheet content is a controlling step in A beta self-assembly.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Casamenti F., Giovannelli L., Scali C., Pepeu G. Administration of amyloid beta-peptides into the medial septum of rats decreases acetylcholine release from hippocampus in vivo. Brain Res. 1994 Feb 4;636(1):162–164. doi: 10.1016/0006-8993(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Barrow C. J., Yasuda A., Kenny P. T., Zagorski M. G. Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J Mol Biol. 1992 Jun 20;225(4):1075–1093. doi: 10.1016/0022-2836(92)90106-t. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Lorenzo A., Yankner B. A. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol Aging. 1992 Sep-Oct;13(5):609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Yeh J., Yankner B. A. beta-Amyloid neurotoxicity in human cortical culture is not mediated by excitotoxins. J Neurochem. 1993 Oct;61(4):1565–1568. doi: 10.1111/j.1471-4159.1993.tb13658.x. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Duffy L. K., O'Malley M. B., Nguyen J., Inouye H., Kirschner D. A. Morphology and antibody recognition of synthetic beta-amyloid peptides. J Neurosci Res. 1991 Apr;28(4):474–485. doi: 10.1002/jnr.490280404. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., McLachlan D. R., Surewicz W. K., Mizzen C. A., Snow A. D., Nguyen J. T., Kirschner D. A. Conformation and fibrillogenesis of Alzheimer A beta peptides with selected substitution of charged residues. J Mol Biol. 1994 Nov 18;244(1):64–73. doi: 10.1006/jmbi.1994.1704. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Inouye H., Surewicz W. K., Selkoe D. J., Podlisny M. B., Kirschner D. A. Fibril formation by primate, rodent, and Dutch-hemorrhagic analogues of Alzheimer amyloid beta-protein. Biochemistry. 1992 Nov 10;31(44):10716–10723. doi: 10.1021/bi00159a011. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Surewicz W. K., Kirschner D. A. pH-dependent structural transitions of Alzheimer amyloid peptides. Biophys J. 1991 Nov;60(5):1190–1201. doi: 10.1016/S0006-3495(91)82154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gamini A., Mandel M. Physicochemical properties of aqueous xanthan solutions: static light scattering. Biopolymers. 1994 Jun;34(6):783–797. doi: 10.1002/bip.360340610. [DOI] [PubMed] [Google Scholar]
- Good T. A., Murphy R. M. Aggregation state-dependent binding of beta-amyloid peptide to protein and lipid components of rat cortical homogenates. Biochem Biophys Res Commun. 1995 Feb 6;207(1):209–215. doi: 10.1006/bbrc.1995.1174. [DOI] [PubMed] [Google Scholar]
- Hilbich C., Kisters-Woike B., Reed J., Masters C. L., Beyreuther K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer's disease. J Mol Biol. 1991 Mar 5;218(1):149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- Inouye H., Fraser P. E., Kirschner D. A. Structure of beta-crystallite assemblies formed by Alzheimer beta-amyloid protein analogues: analysis by x-ray diffraction. Biophys J. 1993 Feb;64(2):502–519. doi: 10.1016/S0006-3495(93)81393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M., Mantsch H. H. Beware of proteins in DMSO. Biochim Biophys Acta. 1991 Jun 24;1078(2):231–235. doi: 10.1016/0167-4838(91)90563-f. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Berger E. P., Lansbury P. T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993 May 11;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kirschner D. A., Inouye H., Duffy L. K., Sinclair A., Lind M., Selkoe D. J. Synthetic peptide homologous to beta protein from Alzheimer disease forms amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6953–6957. doi: 10.1073/pnas.84.19.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993 Mar;2(3):404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo D. T., Copani A., Pike C. J., Whittemore E. R., Walencewicz A. J., Cotman C. W. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Tomaselli K. J., Rydel R. E. Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res. 1993 Sep 3;621(1):35–49. doi: 10.1016/0006-8993(93)90295-x. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Wisniewski H. M., Somerville R. A., Bobin S. A., Masters C. L., Iqbal K. Ultrastructural morphology of amyloid fibrils from neuritic and amyloid plaques. Acta Neuropathol. 1983;60(1-2):113–124. doi: 10.1007/BF00685355. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991 Aug 14;207(4):367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Prelli F., Castaño E., Glenner G. G., Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem. 1988 Aug;51(2):648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
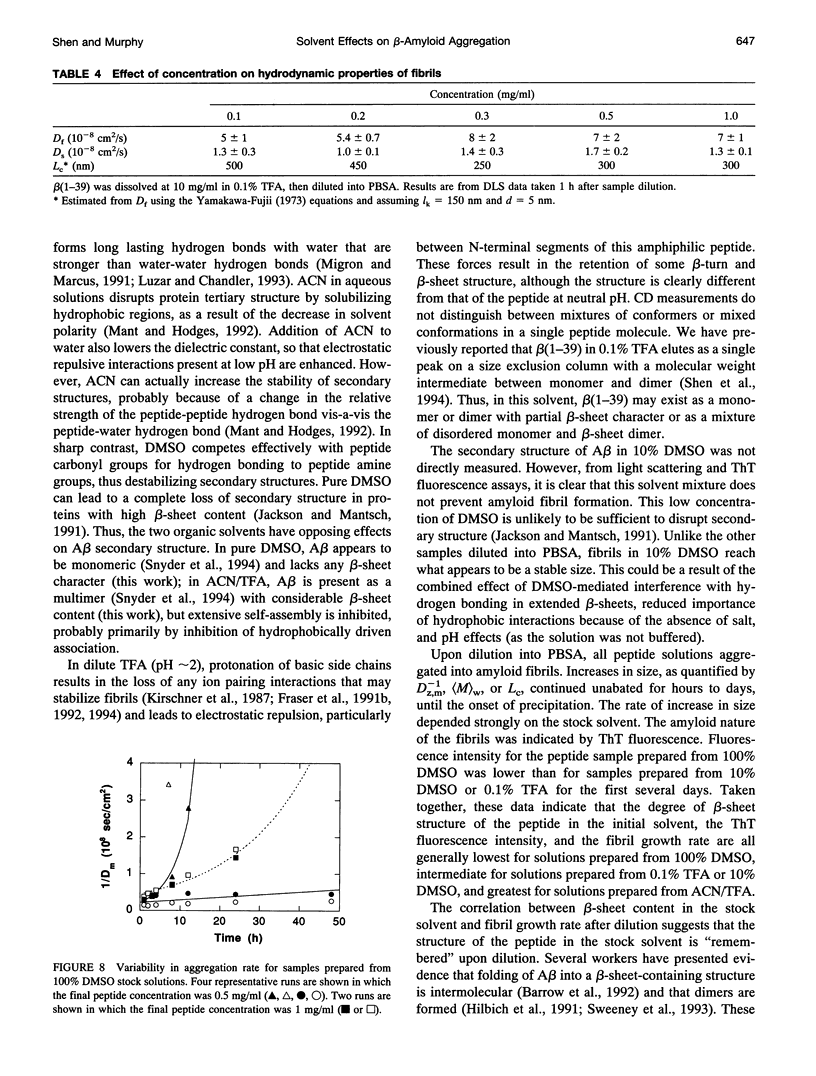
- Shen C. L., Fitzgerald M. C., Murphy R. M. Effect of acid predissolution on fibril size and fibril flexibility of synthetic beta-amyloid peptide. Biophys J. 1994 Sep;67(3):1238–1246. doi: 10.1016/S0006-3495(94)80593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. L., Scott G. L., Merchant F., Murphy R. M. Light scattering analysis of fibril growth from the amino-terminal fragment beta(1-28) of beta-amyloid peptide. Biophys J. 1993 Dec;65(6):2383–2395. doi: 10.1016/S0006-3495(93)81312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. W., Ladror U. S., Wade W. S., Wang G. T., Barrett L. W., Matayoshi E. D., Huffaker H. J., Krafft G. A., Holzman T. F. Amyloid-beta aggregation: selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys J. 1994 Sep;67(3):1216–1228. doi: 10.1016/S0006-3495(94)80591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
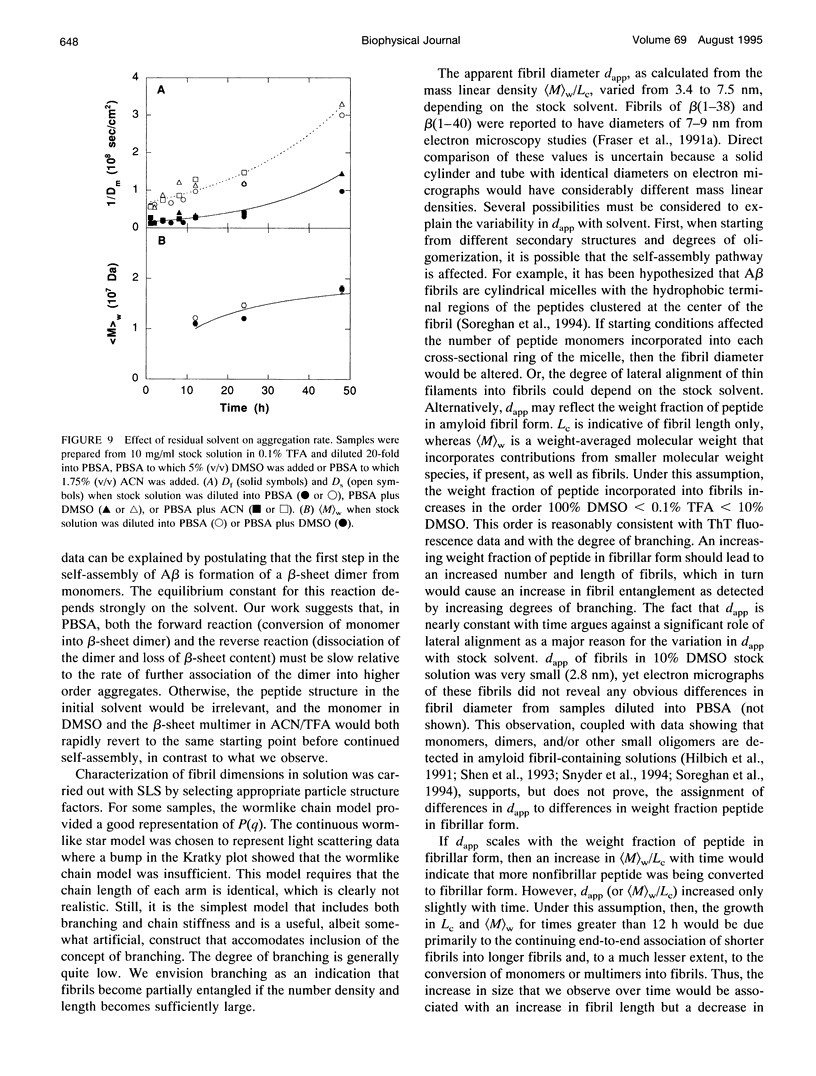
- Soreghan B., Kosmoski J., Glabe C. Surfactant properties of Alzheimer's A beta peptides and the mechanism of amyloid aggregation. J Biol Chem. 1994 Nov 18;269(46):28551–28554. [PubMed] [Google Scholar]
- Sorimachi K., Craik D. J. Structure determination of extracellular fragments of amyloid proteins involved in Alzheimer's disease and Dutch-type hereditary cerebral haemorrhage with amyloidosis. Eur J Biochem. 1994 Jan 15;219(1-2):237–251. doi: 10.1111/j.1432-1033.1994.tb19935.x. [DOI] [PubMed] [Google Scholar]
- Sweeney P. J., Darker J. G., Neville W. A., Humphries J., Camilleri P. Electrophoretic techniques for the analysis of synthetic amyloid beta-A4-related peptides. Anal Biochem. 1993 Jul;212(1):179–184. doi: 10.1006/abio.1993.1310. [DOI] [PubMed] [Google Scholar]
- Ueda K., Fukui Y., Kageyama H. Amyloid beta protein-induced neuronal cell death: neurotoxic properties of aggregated amyloid beta protein. Brain Res. 1994 Mar 14;639(2):240–244. doi: 10.1016/0006-8993(94)91736-1. [DOI] [PubMed] [Google Scholar]
- Whitson J. S., Mims M. P., Strittmatter W. J., Yamaki T., Morrisett J. D., Appel S. H. Attenuation of the neurotoxic effect of A beta amyloid peptide by apolipoprotein E. Biochem Biophys Res Commun. 1994 Feb 28;199(1):163–170. doi: 10.1006/bbrc.1994.1209. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]


