Abstract
Polarized fluorescence recovery after photobleaching (PFRAP) is a technique for measuring the rate of rotational motion of biomolecules on living, nondeoxygenated cells with characteristic times previously ranging from milliseconds to many seconds. Although very broad, that time range excludes the possibility of quantitatively observing freely rotating membrane protein monomers that typically should have a characteristic decay time of only several microseconds. This report describes an extension of the PFRAP technique to a much shorter time scale. With this new system, PFRAP experiments can be conducted with sample time as short as 0.4 microseconds and detection of possible characteristic times of less than 2 microseconds. The system is tested on rhodamine-alpha-bungarotoxin-labeled acetylcholine receptors (AChRs) on myotubes grown in primary cultures of embryonic rat muscle, in both endogenously clustered and nonclustered regions of AChR distribution. It is found that approximately 40% of the AChRs in nonclustered regions undergoes rotational diffusion fast enough to possibly arise from unrestricted monomer Brownian motion. The AChRs in clusters, on the other hand, are almost immobile. The effects of rat embryonic brain extract (which contains AChR aggregating factors) on the myotube AChR were also examined by the fast PFRAP system. Brain extract is known to abolish the presence of endogenous clusters and to induce the formation of new clusters. It is found here that rotational diffusion of AChR in the extract-induced clusters is as slow as that in endogenous clusters on untreated cells but that rotational diffusion in the nonclustered regions of extract-treated myotubes remains rapid.
Full text
PDF

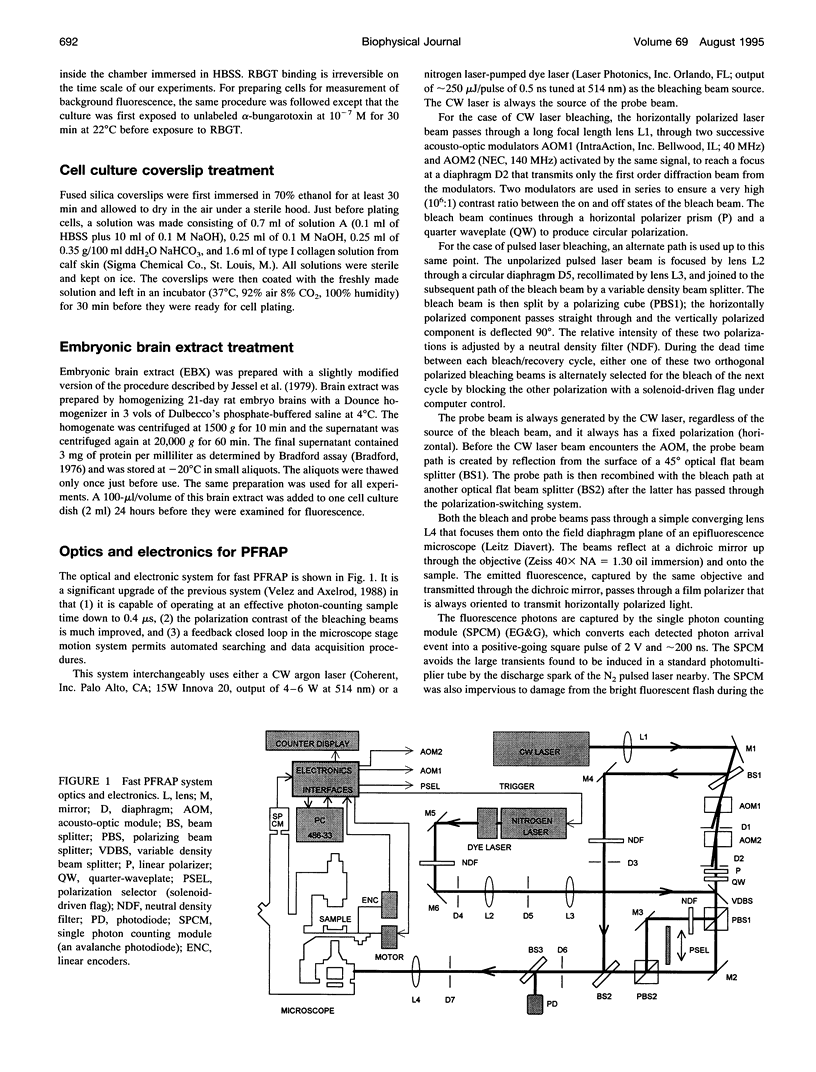


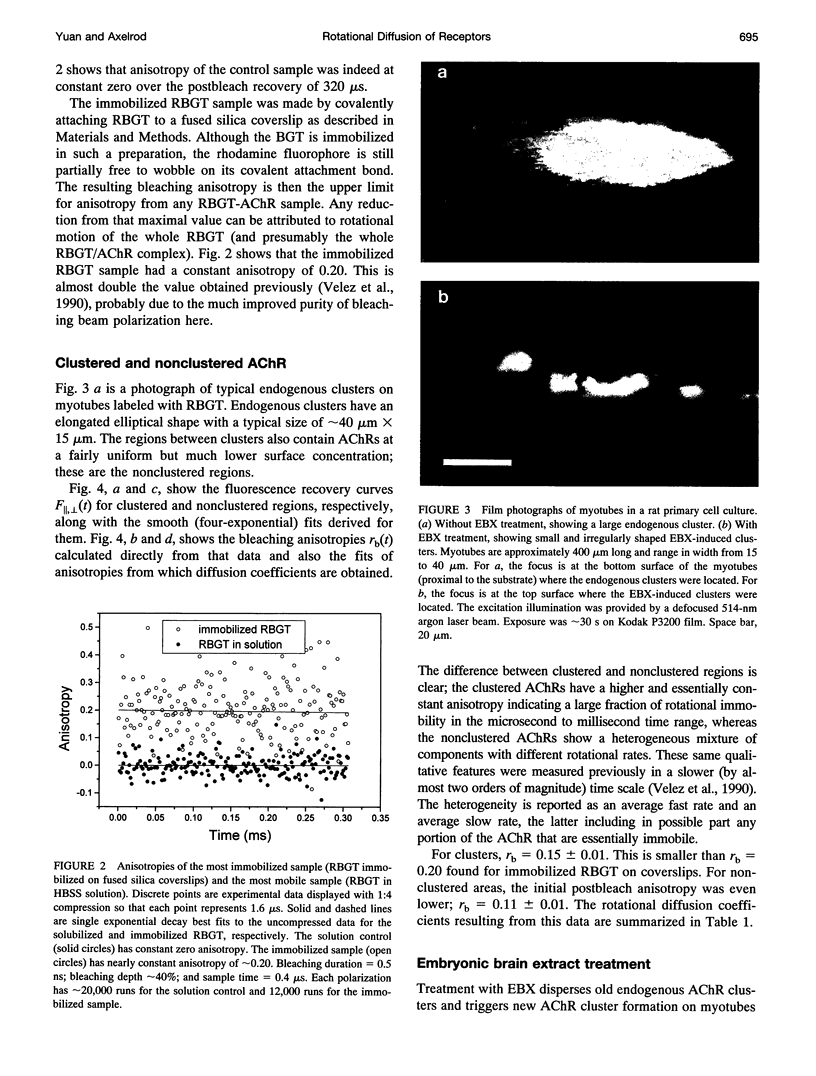
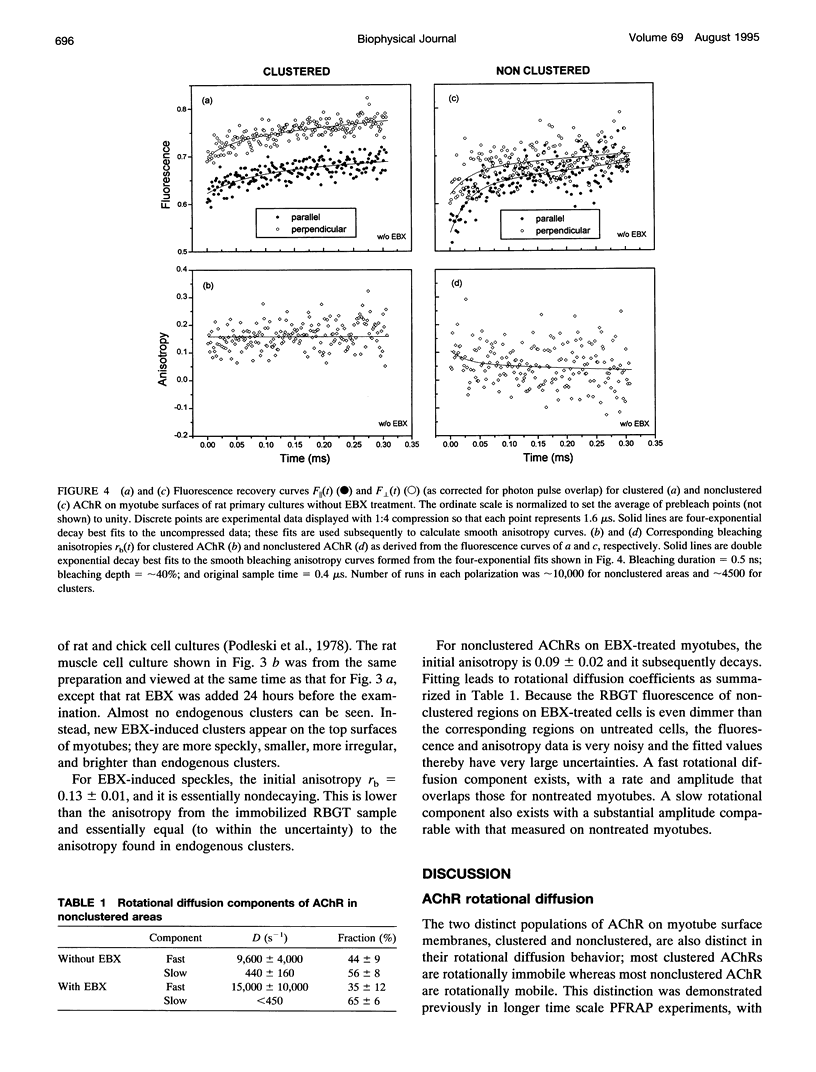




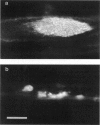
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Fluorescence polarization microscopy. Methods Cell Biol. 1989;30:333–352. [PubMed] [Google Scholar]
- Axelrod D., Ravdin P. M., Podleski T. R. Control of acetylcholine receptor mobility and distribution in cultured muscle membranes. A fluorescence study. Biochim Biophys Acta. 1978 Jul 20;511(1):23–38. doi: 10.1016/0005-2736(78)90062-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barald K. F., Phillips G. D., Jay J. C., Mizukami I. F. A component in mammalian muscle synaptic basal lamina induces clustering of acetylcholine receptors. Prog Brain Res. 1987;71:397–408. doi: 10.1016/s0079-6123(08)61841-5. [DOI] [PubMed] [Google Scholar]
- Bartholdi M., Barrantes F. J., Jovin T. M. Rotational molecular dynamics of the membrane-bound acetylcholine receptor revealed by phosphorescence spectroscopy. Eur J Biochem. 1981 Nov;120(2):389–397. doi: 10.1111/j.1432-1033.1981.tb05716.x. [DOI] [PubMed] [Google Scholar]
- Bloch R. J., Velez M., Krikorian J. G., Axelrod D. Microfilaments and actin-associated proteins at sites of membrane-substrate attachment within acetylcholine receptor clusters. Exp Cell Res. 1989 Jun;182(2):583–596. doi: 10.1016/0014-4827(89)90261-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brisson A., Unwin P. N. Quaternary structure of the acetylcholine receptor. Nature. 1985 Jun 6;315(6019):474–477. doi: 10.1038/315474a0. [DOI] [PubMed] [Google Scholar]
- Campanelli J. T., Hoch W., Rupp F., Kreiner T., Scheller R. H. Agrin mediates cell contact-induced acetylcholine receptor clustering. Cell. 1991 Nov 29;67(5):909–916. doi: 10.1016/0092-8674(91)90364-5. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Daniels M. P., Krikorian J. G., Olek A. J., Bloch R. J. Association of cytoskeletal proteins with newly formed acetylcholine receptor aggregates induced by embryonic brain extract. Exp Cell Res. 1990 Jan;186(1):99–108. doi: 10.1016/0014-4827(90)90215-v. [DOI] [PubMed] [Google Scholar]
- Daniels M. P. Localization of actin, beta-spectrin, 43 x 10(3) Mr and 58 x 10(3) Mr proteins to receptor-enriched domains of newly formed acetylcholine receptor aggregates in isolated myotube membranes. J Cell Sci. 1990 Dec;97(Pt 4):615–626. doi: 10.1242/jcs.97.4.615. [DOI] [PubMed] [Google Scholar]
- Falls D. L., Harris D. A., Johnson F. A., Morgan M. M., Corfas G., Fischbach G. D. Mr 42,000 ARIA: a protein that may regulate the accumulation of acetylcholine receptors at developing chick neuromuscular junctions. Cold Spring Harb Symp Quant Biol. 1990;55:397–406. doi: 10.1101/sqb.1990.055.01.040. [DOI] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey E. W., Nitkin R. M., Wallace B. G., Rubin L. L., McMahan U. J. Components of Torpedo electric organ and muscle that cause aggregation of acetylcholine receptors on cultured muscle cells. J Cell Biol. 1984 Aug;99(2):615–627. doi: 10.1083/jcb.99.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Cronin J., Branton D. Coupling polylysine to glass beads for plasma membrane isolation. Biochim Biophys Acta. 1978 Jan 4;506(1):81–96. doi: 10.1016/0005-2736(78)90436-4. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Siegel R. E., Fischbach G. D. Induction of acetylcholine receptors on cultured skeletal muscle by a factor extracted from brain and spinal cord. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaack D., Podleski T. R., Salpeter M. M. Ascorbic acid and acetylcholine receptor expression. Ann N Y Acad Sci. 1987;498:77–89. doi: 10.1111/j.1749-6632.1987.tb23752.x. [DOI] [PubMed] [Google Scholar]
- Lipkind G. M., Fozzard H. A. A structural model of the tetrodotoxin and saxitoxin binding site of the Na+ channel. Biophys J. 1994 Jan;66(1):1–13. doi: 10.1016/S0006-3495(94)80746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M. M., Garland P. B., Lamprecht J., Barnard E. A. Rotational mobility of the membrane-bound acetylcholine receptor of Torpedo electric organ measured by phosphorescence depolarisation. FEBS Lett. 1980 Mar 10;111(2):407–412. doi: 10.1016/0014-5793(80)80838-6. [DOI] [PubMed] [Google Scholar]
- Lupa M. T., Caldwell J. H. Effect of agrin on the distribution of acetylcholine receptors and sodium channels on adult skeletal muscle fibers in culture. J Cell Biol. 1991 Nov;115(3):765–778. doi: 10.1083/jcb.115.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastuk M. A., Fallon J. R. Agrin and the molecular choreography of synapse formation. Trends Neurosci. 1993 Feb;16(2):72–76. doi: 10.1016/0166-2236(93)90020-m. [DOI] [PubMed] [Google Scholar]
- Neugebauer K., Salpeter M. M., Podleski T. R. Differential responses of L5 and rat primary muscle cells to factors in rat brain extract. Brain Res. 1985 Oct 28;346(1):58–69. doi: 10.1016/0006-8993(85)91094-7. [DOI] [PubMed] [Google Scholar]
- Olek A. J., Pudimat P. A., Daniels M. P. Direct observation of the rapid aggregation of acetylcholine receptors on identified cultured myotubes after exposure to embryonic brain extract. Cell. 1983 Aug;34(1):255–264. doi: 10.1016/0092-8674(83)90156-3. [DOI] [PubMed] [Google Scholar]
- Peng H. B., Cheng P. C., Luther P. W. Formation of ACh receptor clusters induced by positively charged latex beads. Nature. 1981 Aug 27;292(5826):831–834. doi: 10.1038/292831a0. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Axelrod D., Ravdin P., Greenberg I., Johnson M. M., Salpeter M. M. Nerve extract induces increase and redistribution of acetylcholine receptors on cloned muscle cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2035–2039. doi: 10.1073/pnas.75.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. M., Young S. H. Diffusional and electrokinetic redistribution at the synapse: a physicochemical basis of synaptic competition. J Neurobiol. 1990 Jan;21(1):157–168. doi: 10.1002/neu.480210111. [DOI] [PubMed] [Google Scholar]
- Rahman N. A., Pecht I., Roess D. A., Barisas B. G. Rotational dynamics of type I Fc epsilon receptors on individually-selected rat mast cells studied by polarized fluorescence depletion. Biophys J. 1992 Feb;61(2):334–346. doi: 10.1016/S0006-3495(92)81840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist N. E., Werle M. J., McMahan U. J. Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron. 1992 May;8(5):865–868. doi: 10.1016/0896-6273(92)90200-w. [DOI] [PubMed] [Google Scholar]
- Rochlin M. W., Peng H. B. Localization of intracellular proteins at acetylcholine receptor clusters induced by electric fields in Xenopus muscle cells. J Cell Sci. 1989 Sep;94(Pt 1):73–83. doi: 10.1242/jcs.94.1.73. [DOI] [PubMed] [Google Scholar]
- Rousselet A., Cartaud J., Devaux P. F., Changeux J. P. The rotational diffusion of the acetylcholine receptor in Torpeda marmorata membrane fragments studied with a spin-labelled alpha-toxin: importance of the 43 000 protein(s). EMBO J. 1982;1(4):439–445. doi: 10.1002/j.1460-2075.1982.tb01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Spanton S., Holley K., Podleski T. R. Brain extract causes acetylcholine receptor redistribution which mimics some early events at developing neuromuscular junctions. J Cell Biol. 1982 May;93(2):417–425. doi: 10.1083/jcb.93.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalettar B. A., Selvin P. R., Axelrod D., Klein M. P., Hearst J. E. A polarized photobleaching study of DNA reorientation in agarose gels. Biochemistry. 1990 May 22;29(20):4790–4798. doi: 10.1021/bi00472a007. [DOI] [PubMed] [Google Scholar]
- Selvin P. R., Scalettar B. A., Langmore J. P., Axelrod D., Klein M. P., Hearst J. E. A polarized photobleaching study of chromatin reorientation in intact nuclei. J Mol Biol. 1990 Aug 20;214(4):911–922. doi: 10.1016/0022-2836(90)90345-M. [DOI] [PubMed] [Google Scholar]
- Shadiack A. M., Nitkin R. M. Agrin induces alpha-actinin, filamin, and vinculin to co-localize with AChR clusters on cultured chick myotubes. J Neurobiol. 1991 Sep;22(6):617–628. doi: 10.1002/neu.480220607. [DOI] [PubMed] [Google Scholar]
- Stollberg J., Fraser S. E. Acetylcholine receptor clustering is triggered by a change in the density of a nonreceptor molecule. J Cell Biol. 1990 Nov;111(5 Pt 1):2029–2039. doi: 10.1083/jcb.111.5.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollberg J., Fraser S. E. Acetylcholine receptors and concanavalin A-binding sites on cultured Xenopus muscle cells: electrophoresis, diffusion, and aggregation. J Cell Biol. 1988 Oct;107(4):1397–1408. doi: 10.1083/jcb.107.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollberg J., Fraser S. E. Local accumulation of acetylcholine receptors is neither necessary nor sufficient to induce cluster formation. J Neurosci. 1990 Jan;10(1):247–255. doi: 10.1523/JNEUROSCI.10-01-00247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stya M., Axelrod D. Diffusely distributed acetylcholine receptors can participate in cluster formation on cultured rat myotubes. Proc Natl Acad Sci U S A. 1983 Jan;80(2):449–453. doi: 10.1073/pnas.80.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbs M. M., Thompson N. L. Slow rotational mobilities of antibodies and lipids associated with substrate-supported phospholipid monolayers as measured by polarized fluorescence photobleaching recovery. Biophys J. 1990 Aug;58(2):413–428. doi: 10.1016/S0006-3495(90)82387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez M., Axelrod D. Polarized fluorescence photobleaching recovery for measuring rotational diffusion in solutions and membranes. Biophys J. 1988 Apr;53(4):575–591. doi: 10.1016/S0006-3495(88)83137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez M., Barald K. F., Axelrod D. Rotational diffusion of acetylcholine receptors on cultured rat myotubes. J Cell Biol. 1990 Jun;110(6):2049–2059. doi: 10.1083/jcb.110.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. G. Agrin-induced specializations contain cytoplasmic, membrane, and extracellular matrix-associated components of the postsynaptic apparatus. J Neurosci. 1989 Apr;9(4):1294–1302. doi: 10.1523/JNEUROSCI.09-04-01294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. M., Barisas B. G. Protein rotational motion in solution measured by polarized fluorescence depletion. Biophys J. 1986 Jul;50(1):41–53. doi: 10.1016/S0006-3495(86)83437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]