Abstract
Background
Metabolic dysregulation plays a crucial role in the development of diabetic vascular complications. Current models for diabetic vascular complications predominantly rely on three conventional parameter classes: demographic characteristics, clinical measures, and standard laboratory indices. In contrast, the potential prognostic value of the plasma metabolome remains substantially under characterized in this context. This study aims to systemically reframe the value of circulating metabolites, providing new insights into both assessment and pathophysiology of diabetic complications.
Methods
This study included 333,870 participants from the UK Biobank (n = 115,078) and FinnGen Biobank (n = 218,792). The initial analysis utilizing longitudinal data from 7,711 patients with diabetes was used to screen 249 plasma metabolites associated with diabetic vascular complications. These metabolites were carefully quantified using nuclear magnetic resonance (NMR) to profile the metabolites of these participants. A total of 1,457 and 1,635 people were found to have developed macrovascular (including heart failure, stroke and coronary heart disease [CHD]) and microvascular complications (including diabetic neuropathy [DN], kidney disease and retinopathy) at follow-ups, respectively. A Least Absolute Shrinkage and Selection Operator-Cox (LASSO-Cox) regression was conducted to define the potential biomarkers, adjusting for conventional factors including age, sex, race, smoking status, diet intake, Townsend deprivation index, systolic and diastolic blood pressure, body mass index, plasma triglycerides, low-density lipoprotein (LDL) cholesterol, plasma creatinine and estimated glomerular filtration rate. Subsequently, a multivariate Cox proportional hazards regression model was used to estimate the hazard ratios (HRs). Finally, a bidirectional two-sample Mendelian randomization (MR) analysis was employed to evaluate the relationships between the selected metabolomics and diabetic complications to analyze causal associations.
Results
Over a 13.06 ± 3.59 years of follow-up, 15 out of 249 plasma metabolites demonstrated significant associations with incident macrovascular complications in LASSO-Cox regression, while 33 metabolites were linked to microvascular complications after 12.77 ± 3.90 years of follow-up (all P < 0.05). In the multivariate Cox proportional hazards regression, 6 metabolites including creatinine (HR = 1.32, 95% confidence interval [CI] 1.17–1.50, P < 0.001), albumin (HR = 0.87, 95% CI 0.81–0.94, P < 0.001), tyrosine (HR = 0.91, 95% CI 0.85–0.96, P = 0.001), glutamine (HR = 1.08, 95% CI 1.01–1.15, P = 0.020), lactate (HR = 1.07, 95% CI 1.01–1.14, P = 0.023), and the ratio of phospholipids to total lipids in small LDL (HR = 1.10, 95% CI 1.01–1.19, P = 0.023) were correlated with macrovascular complications, while 8 metabolites including glucose (HR = 1.25, 95% CI 1.18–1.33, P < 0.001), tyrosine (HR = 0.86, 95% CI 0.80–0.92, P < 0.001), concentration of very large high-density lipoprotein particles (HR = 0.78, 95% CI 0.68–0.90, P = 0.001), valine (HR = 1.21, 95% CI 1.08–1.36, P = 0.001), free cholesterol to total lipids in very small very low-density lipoprotein (VLDL, HR = 1.28, 95% CI 1.10–1.49, P = 0.001), alanine (HR = 1.08, 95% CI 1.01–1.15, P = 0.022), albumin (HR = 0.92, 95% CI 0.86–0.99, P = 0.027), and isoleucine (HR = 0.89, 95% CI 0.80–1.00, P = 0.041) were associated with microvascular complications. MR analysis suggested that genetic predisposition to several screened metabolites was linked to diabetic complications. For CHD, the ratio of phospholipids to total lipids in small LDL was associated with increased risk (odds ratio [OR] = 1.96, 95% CI 1.33–2.88, P = 0.015). As for reverse MR, DN was relevant to decreased level of serum ratio of docosahexaenoic acid to total fatty acids (OR = 0.97, 95% CI 0.95–0.99, P = 0.019), increased level of the ratio of triglycerides to total lipids in very large VLDL (OR = 1.03, 95% CI 1.01–1.05, P = 0.019), and pyruvate (OR = 1.03, 95% CI 1.01–1.05, P = 0.046).
Conclusions
These findings may serve as potential biomarkers for predicting the development of vascular complications in patients with diabetes, thereby improving clinical management strategies for affected patients.
Trial registration
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02899-y.
Keywords: Diabetes complications, Microvascular complications, Macrovascular complications, Metabolomics
Background
Diabetes and associated vascular disorders are noteworthy global public health challenges. Recent epidemiological data indicate that diabetes affected approximately 1 in 11 adults worldwide in 2015 [1]. Projections suggest this prevalence will rise dramatically, potentially affecting 693 million adults globally by 2045 [2]. Diabetic vascular complications exhibit substantial pathophysiological overlap at the vascular level, collectively contributing to worse clinical outcomes compared to isolated diabetic conditions [3]. Recent research on these complications has greatly advanced the understanding of the pathogenesis of the disease. The systematic integration of shared determinants and underlying pathophysiological mechanisms across disease entities facilitates the understanding and management of diabetic vascular complications. Therefore, it is of great importance to simultaneously explore the impact of factors at risk on multiple systems and various angiopathies when investigating diabetic vascular complications.
Diabetic complications, including macrovascular complications such as heart failure (HF), coronary heart disease (CHD), and stroke, as well as microvascular disorders such as diabetic kidney disease (DKD), diabetic retinopathy (DR), and diabetic neuropathy (DN), can lead to serious cardiovascular damage, renal failure, blindness, and other consequences, posing a noteworthy decline in life quality and a significant increase in the mortality risk [4]. The likelihood of HF in patients with diabetes is more than twice that of individuals without diabetes [5]. At least half of patients with diabetes will develop DN in their lifetime [6]. Therefore, identifying biomarkers or key contributors to diabetes vascular complications is of great clinical importance for the management of diabetes.
Diabetes is fundamentally characterized by persistent metabolic dysregulation, which serves as a pivotal driver in both the development and progression of diabetic vascular complications through underlying pathophysiological mechanisms [7–12]. Increasing studies highlights the diagnostic and predictive potential of metabolomics in diabetic complications, reinforcing the critical role of metabolites in vascular pathogenesis [13, 14]. Notably, the study related to DKD revealed extensive metabolic dysregulation, where urea and creatinine serve as renal impairment biomarkers, while elevated circulating galactose, d-fructose, and lactate induce blood pH alterations that may initiate DKD progression [14].
However, though some studies have proved the significance of metabolomics in the progression of certain diabetic complications, including DR and DKD, there is currently still no research systemically analyzing metabolomics in all diabetic complications, which is crucial for investigating the common pathophysiological mechanisms and metabolic pathways of these complications [15, 16].
Consequently, there is an urgent need for a comprehensive assessment of plasma metabolomics at the onset of diverse diabetic complications [17]. This current research strives to comprehensively evaluate the relationships of plasma metabolites and the future risk of diabetic vascular complications using longitudinal data. Additionally, employing Mendelian randomization (MR) to elucidate the potential genetic causal impact of selected metabolites on diabetic complications, we ultimately delineated the role of metabolomics in the initiation and progression of diabetic vascular complication.
Methods
Study participants
The UK Biobank is a prospective cohort study initiated in 2006–2010, enrolling over 500,000 participants from the United Kingdom. It has comprehensive datasets encompassing disease diagnoses, circulating metabolites, plasma proteins, genome sequencing data, and so on.
The FinnGen study, a genomic and personalized medicine initiative supported by nearly 500,000 Finnish individuals, aims to advance precision medicine by elucidating genetic determinants of diseases. This project establishes a unique resource for investigating genetic variations and their clinical implications in disease prevention, diagnosis, and therapeutic development.
A total of 333,870 participants from UK Biobank (n = 115,078) and FinnGen Biobank round 5 (n = 218,792) were included in this study, consisting of two specific subpopulations. The workflow of this study was described in Fig. 1.
Fig. 1.
The overall workflow of the study. The diagram represents the inclusion criteria and analytical procedures of the study. ROC=receiver operating characteristics curve; NRI=net reclassification index; GWAS=genome-wide association studies; MR=Mendelian randomization; LASSO=least absolute shrinkage and selection operator; MR-PRESSO=Mendelian randomization pleiotropy residual sum and outlier; IDI=integrated discrimination index.
Firstly, metabolite data were available among 118,002 participants in the UK Biobank. Among these, 7,713 participants had diabetes mellitus at baseline. Participants with specific diabetic vascular complications at baseline identified through hospital admissions records, primary care records, or self-report history during or before the baseline period were pre-excluded for specific analysis. The exclusion criteria comprised participants with baseline diabetes complications: macrovascular complications (N = 1,957), CHD (N = 1,607), HF (N = 542), stroke (N = 226), microvascular complications (N = 1,955), DKD (N = 432), DN (N = 300), and DR (N = 1,626). Two participants were excluded from all study cohorts due to pre-existing diagnoses of all target complications at baseline. The final analytical sample comprised 7,711 participants with longitudinal follow-up data. To be specific, eight distinct cohorts with longitudinal data were included in observational study, including: 5,756 participants for macrovascular complications, 6,106 participants for CHD, 7,171 participants for HF, 7,487 participants for stroke, 5,758 participants for microvascular complications, 7,281 participants for DKD, 7,413 participants for DN, and 6,087 participants for DR, all with available longitudinal data for cohort study.
In phase II analysis, 115,078 participants from the UK Biobank and 218,792 participants from the FinnGen Biobank (Round 5) with genome-wide association studies (GWAS) data were included for MR analyses to establish the causal relationship between selected plasma metabolomics and macrovascular complications or microvascular complications.
Ascertainment of endpoints
Diabetic macrovascular complications were defined as CHD, HF, and stroke, while microvascular complications encompassed DR, DN, and DKD. Endpoints were determined by the earliest recorded occurrence across four validated sources: self-report history, hospital admissions (ICD-9/ICD-10), death registries, or primary care records, with death registration dates superseding other records for deceased cases (Supplementary Materials 1 Table S1-2). To enhance diagnostic precision, diabetes mellitus confirmation requires at least one criterion: self-reported history, fasting plasma glucose ≥ 11.1 mmol/L, HbA1c ≥ 48 mmol/mol, or active glucose-lowering medication use, self-reported history, hospital admission records, or primary care records.
Conventional risk factors
Age, sex, race, smoking status, diet intake, Townsend deprivation index, systolic and diastolic blood pressure, body mass index (BMI), plasma triglycerides, low-density lipoprotein (LDL) cholesterol, plasma creatinine and estimated glomerular filtration rate (eGFR, calculated by plasma creatinine, sex, age) were included as conventional risk factors. Detailed conventional factors and field IDs were provided in Supplementary Materials 1 Table S3-4 [18].
Quantification and quality control of metabolites
Plasma specimens were prepared in 96-well plates with plasma mimic to monitor the consistency of quantification by the UK Biobank. A mixture of two small molecules was also added to serve as a technical reference. These samples were further analyzed at Nightingale Health's laboratories in Finland between June 2019 and April 2020. A total of 249 metabolites were analyzed using nuclear magnetic resonance (NMR) to profile the metabolites of these participants.
Quality control was applied to eliminate technical and systemic errors. Pre-specified agreement on protocol was made between UK Biobank and Nightingale Health Centre to ensure the biomarker consistency throughout the project. Two internalized control samples were plated on 96-well plates with plasma samples of participants to track the consistency and eliminate batch effects. Thus, NMR quantification differs from other approaches like spectrometry, by its absence of batch effect, offering the optimal statistical value [19]. Also, four sets of internal control samples were used across 1,352 96-well plates measured [20].
A total of 168 metabolites were quantified in absolute level, referring to mmol/L, encompassing amino acids, fatty acids, glycosylated molecules, lipids, and lipoproteins. The rest 81 was quantified as a ratio. Detailed metabolites and field IDs were provided in Supplementary Materials 1 Table S5. Among all participants enrolled in the study, a total of 725 (9.4%) exhibited missing values across all metabolites, with a mean of 0.3 ± 1.50 metabolites missing per individual. These missing values were addressed through multiple imputation implemented via the R package mice (v3.16.0).
Summary statistics for GWAS datasets of FinnGen Biobanks and UK Biobank
The metabolites were further included in the ascertainment of the causal relationship with specific diabetic complications. The cohort of the UK Biobank was included as exposure data to determine the circulating plasma metabolites [21]. The cohort of FinnGen Biobanks was applied as outcome data to determine the presence of diabetic complications (https://finngen.gitbook.io/documentation/v/r5). Both cohorts comprised European participants.
Summary statistics for genetic instruments associated with the outcomes of certain diabetic complications were obtained from the FinnGen Biobank participants. Detailed characteristics were shown in Supplementary Materials 1 Table S6. The full GWAS results of these metabolites were published by IEU OpenGWAS datasets with GWAS ID provided in Supplementary Materials 1 Table S7-13.
Statistical analyses
Two steps of analytical approach were conducted to determine the potential relationship of metabolite biomarkers with diabetic vascular complications. Before analysis, all metabolites were first transformed by natural logarithmic (ln[x + 1]) and scaled by Z transformation. Also, data of metabolites exceeding the 2.5th to 97.5th percentile range were identified as outliers and replaced with the corresponding boundary values (the 2.5th percentile for lower extremes or the 97.5th percentile for upper extremes) to minimize their impact on subsequent analyses.
In phase I analysis, we selected several metabolites that were significantly related to specific diabetic complications. Here, we included individuals with longitudinal data to select significant plasma metabolites. Least absolute shrinkage and selection operator-Cox (LASSO-Cox) regression, adjusted for conventional covariates, was performed to assess metabolite effects on complications while accounting for intercorrelations. Outcomes represented complication status (presence/absence) at follow-up visits. Significant metabolites associated with each complication were identified as non-zero coefficient biomarkers in LASSO-Cox regression and then were advanced to phase II. A multivariate Cox proportional hazards regression was conducted to estimate the hazard ratio (HR). Two kinds of Cox models were constructed: Model 1 (traditional covariates: age, sex, race, smoking status, diet, Townsend deprivation index, systolic and diastolic blood pressure, BMI, plasma triglycerides, LDL cholesterol, plasma creatinine and eGFR) and Model 2 (traditional covariates combined with Phase I-derived metabolites). The discriminative performance of predictive algorithms was evaluated using the area under the receiver operating characteristic curve (AUC), Concordance index (C-index), net reclassification index (NRI), and integrated discrimination index (IDI). NRI could quantify how the newly developed model reclassified participants compared to the previous model, and IDI could assess how the model was improved. To ensure the robustness of the statistical analysis, we systematically evaluated the statistical power of the overall study. Our power analyses demonstrated excellent statistical power for detecting associations, with observed values of 1.000 for macrovascular complications and 0.998 for microvascular complications.
In phase II analysis, causal relationships were estimated by applying bidirectional MR between metabolites selected by LASSO-Cox regression and diabetic vascular complications. MR was conducted under three assumptions: (1) genetic instruments (IVs) are related to the exposures; (2) there are no other mediators between the genetic instruments and the outcome; (3) there are no correlations between the genetic instruments and the outcome. Any single-nucleotide polymorphisms (SNPs) that didn’t conform to the above assumptions were excluded from the study. The fulfillment of these assumptions was further tested by the heterogeneity test and the pleiotropy test. A heterogeneity test was conducted by applying Cochran’ s Q test, and horizontal pleiotropy was tested and excluded by the MR-Egger intercept test and Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO). Also, MR Steiger filtering analysis was conducted to ensure the robustness of the causal relationship detected.
The following principles were applied to choose the instruments: (1) SNPs associated with metabolites at the threshold of P < 5*10–8 were included; (2) SNPs were filtered using linkage disequilibrium (LD) tests, excluding those with R2 < 0.01 within a 5000 kb clumping window size. Heterogeneity and horizontal pleiotropy were tested to ensure the effectiveness at the threshold of P < 0.05; (3) MR-PRESSO tests were conducted to exclude outlier SNPs to ensure the robustness of the analysis; (4) MR Steiger filtering was conducted to exclude SNPs with false causal correlation. Heterogeneity and horizontal pleiotropy were tested to ensure the effectiveness at the threshold of P < 0.05. Five methods of MR were applied, including simple mode, MR Egger, Inverse weighted median (IVW), weighted median, and weighted mode. IVW applies Wald estimates to each SNP, deriving aggregate genetic associations between metabolite biomarkers and diabetic complications. IVW was thought to be unbiased and of most statistical value if no horizontal pleiotropy appears [22]. To ensure the robustness of the results, sensitivity analyses including leave-one-out analysis, forest plots, funnel plots, and scatter plots were conducted. False discovery rate (FDR) correction was also applied to eliminate the bias induced by multiple testing. Detailed SNPs selected were listed in Supplementary Materials 2 S1-2.
A two-sided P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using R software, version 4.4.2 (R Foundation for Statistical Computing).
Ethics
The data collection and utilization for this research were conducted under ethical guidelines. Before participation, all individuals involved have given written informed consent. Given the publicly accessible and anonymized nature of the datasets, institutional review board approval was waived for this particular analysis.
Role of funders
This work only represented the viewpoint of the authors. The funding sources had no involvement in the study design or implementation.
Results
Baseline characteristics
In phase I analysis, 7,711 participants with an average age of 59.3 ± 7.3 years (range, 40.0–70.0 years) were included. A total of 3,041 (39.4%) participants were female, 6,735 (87.3%) were White, and 925 (12%) were current smokers. Mean BMI was 31.3 ± 5.79 kg/m2 (range, 15.8–61.7 kg/m2) systolic blood pressure was 139 ± 20.3 mmHg (range, 84.0–188.0 mmHg), diastolic blood pressure was 80.6 ± 11.3 mmHg (range, 53.5–109.5 mmHg), plasma LDL cholesterol level was 2.84 ± 0.85 mmol/L (range, 0.28–7.07 mmol/L), plasma triglyceride was 2.18 ± 1.31 mmol/L (range, 0.31–11.19 mmol/L).
During a follow-up of 13.06 ± 3.59 years (range, 0.36–16.63 years) for macrovascular complications and 12.77 ± 3.90 years (range, 0.69–16.62 years) for microvascular complications, 1,457 were diagnosed with macrovascular complications at follow-up, and 1,635 were diagnosed with microvascular complications at follow-up. Specifically, 1,149, 750, and 563 participants were diagnosed with CHD, HF, and stroke at follow-up, respectively. In addition, 1,102, 406, and 1,059 participants were diagnosed with DKD, DN, and DR at follow-up, respectively. The baseline characteristics were further described in Supplementary Materials 1 Table S14 and S15.
Metabolic biomarkers associated with incident diabetic complications
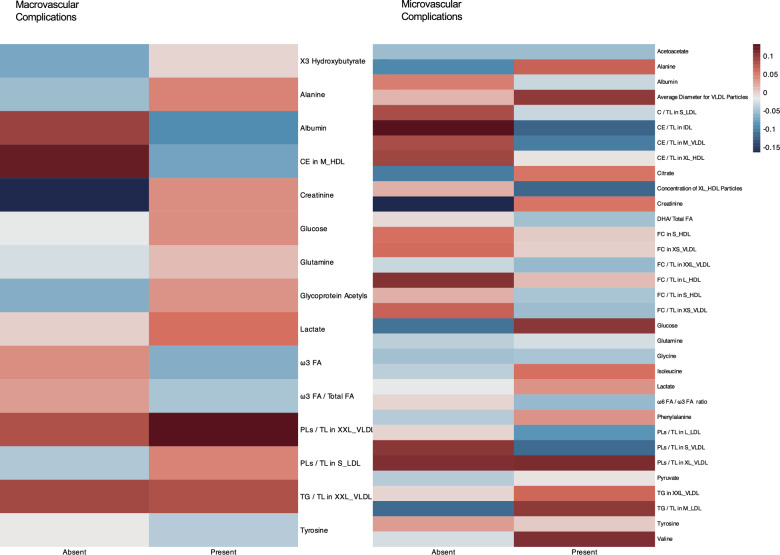
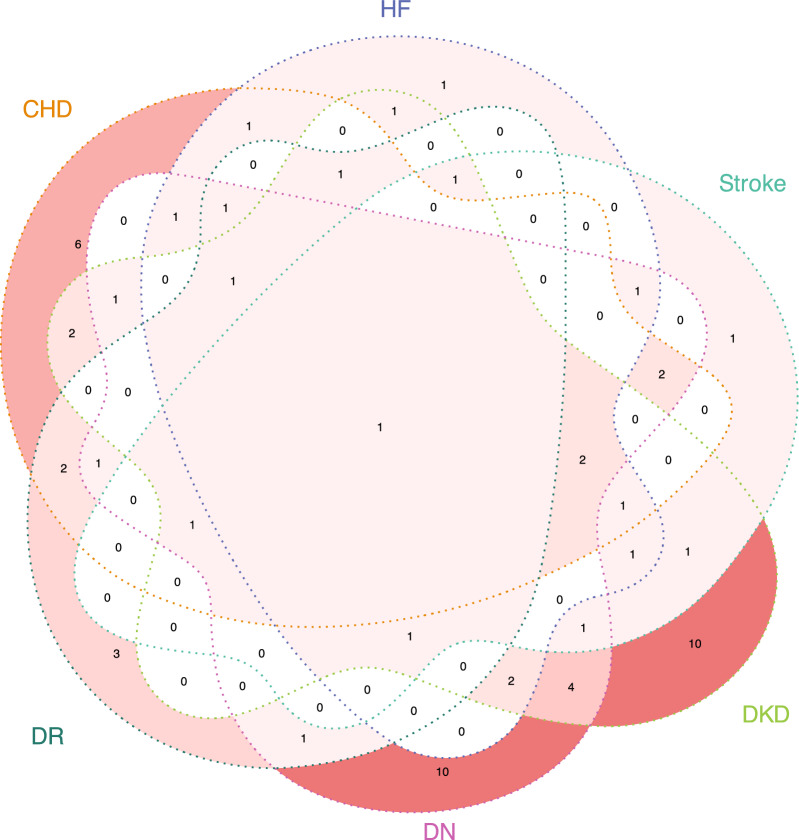
In phase I analysis, among 249 metabolites quantified by NMR spectrum, a total of 15 and 33 metabolites were found to be significantly correlated with macrovascular or microvascular complications in the LASSO-Cox regression, respectively. A heatmap was created to demonstrate the correlations between selected metabolites and both macrovascular and microvascular complications across participants (Fig. 2). Additionally, a Venn diagram was constructed to illustrate the overlapping patterns of metabolites selected by LASSO-Cox across the different complications (Fig. 3). Several metabolites demonstrated broad-spectrum associations across multiple diabetic complications. Notably, acetate exhibited universal associations with all complication types examined. Alanine demonstrated significant correlations with CHD, HF, DKD, DN, and DR, while albumin and creatinine shared identical association profiles with CHD, HF, stroke, DKD, and DN. Glucose metabolism alterations were associated with HF, stroke, DKD, DN, and DR, and tyrosine showed consistent relationships with CHD, stroke, DKD, DN, and DR.
Fig. 2.
Heatmap showing the correlations between selected metabolites and macrovascular/microvascular complications. Colors represent the mean level of corresponding metabolites after natural logarithmic transformation(ln[x + 1]) and scaled by Z transformation. To simplify complex terminology, we standardized data labels by using abbreviations. Lipid size categories were abbreviated as follows: "very small" to "XS", "small" to "S", "medium" to "M", "large" to "L", "very large" to "XL", and "extremely large" to "XXL". For lipid types, "free cholesterol" to "FC","cholesterol" to "C", "cholesteryl esters" to "CE" , "triglycerides" to "TG", "total lipids" to "TL","high-density lipoprotein" to "HDL", "low-density lipoprotein" to "LDL", "very low-density lipoprotein" to "VLDL" and "phospholipids" to "PLs". For fatty acids, "fatty acids" to "FA", "docosahexaenoic acid" to “DHA".
Fig. 3.
Venn diagram showing the LASSO-Cox selected metabolites in different diabetic vascular complications. DKD = diabetic kidney disease; DN = diabetic neuropathy; DR = diabetic retinopathy; CHD = coronary heart disease; HF = heart failure.
Further, our analysis revealed complication-specific metabolites. For DKD, the unique metabolites included acetoacetate, very low-density lipoprotein (VLDL) particle average diameter, cholesteryl esters-to-total lipids ratio in large LDL, free cholesterol-to-total lipids ratio in chylomicrons and extremely large VLDL, isoleucine, leucine, ω-6/ω-3 fatty acid ratio, phospholipids-to-total lipids ratio in small VLDL, triglycerides-to-total lipids ratio in very large high-density lipoprotein (HDL), and triglycerides-to-total lipids ratio in very small VLDL. In DN, the distinctive metabolites consisted of cholesterol-to-total lipids ratio in large HDL, cholesteryl esters-to-total lipids ratio in large HDL, docosahexaenoic acid(DHA)-to-total fatty acids ratio, absolute phospholipid content in chylomicrons and extremely large VLDL, phospholipids-to-total lipids ratio in very large HDL, polyunsaturated fatty acids-to-total fatty acids ratio, total branched-chain amino acids (BCAAs) pool (leucine/isoleucine/valine), triglyceride content in large HDL, triglycerides-to-total lipids ratio in small VLDL, and triglycerides-to-total lipids ratio in very large VLDL. Significant metabolites for each complication were also selected, detailed metabolic biomarkers were provided in Supplementary Materials 1 Table S16-23.
The following metabolites were positively associated with the incidence of macrovascular complications in the multivariate Cox proportional hazards regression: creatinine (HR = 1.32, 95% confidence interval [CI]: 1.17–1.50, P < 0.001), glutamine (HR = 1.08, 95% CI 1.01–1.15, P = 0.020), lactate (HR = 1.07, 95% CI 1.01–1.14, P = 0.023), and phospholipids to total lipids in small LDL (HR = 1.10, 95% CI 1.01–1.19, P = 0.023). Conversely, albumin (HR = 0.87, 95% CI 0.81–0.94, P < 0.001), tyrosine (HR = 0.91, 95% CI 0.85–0.96, P = 0.001) were negatively linked with the incidence of macrovascular complications. The following metabolites were positively associated with its incidence in the multivariate Cox proportional hazards regression: glucose (HR = 1.25, 95% CI 1.18–1.33, P < 0.001), valine (HR = 1.21, 95% CI 1.08–1.36, P = 0.001), free cholesterol to total lipids in very small VLDL (HR = 1.28, 95% CI 1.10–1.49, P = 0.001), alanine (HR = 1.08, 95% CI 1.01–1.15, P = 0.022). Conversely, tyrosine (HR = 0.86, 95% CI 0.80–0.92, P < 0.001), concentration of very large HDL particles (HR = 0.78, 95% CI 0.68–0.90, P = 0.001), albumin (HR = 0.92, 95% CI 0.86–0.99, P = 0.027), and isoleucine (HR = 0.89, 95% CI 0.80–1.00, P = 0.041) were negatively linked with the incidence of microvascular complications (Fig. 4).
Fig. 4.
Hazard ratios of significant metabolites on the incidence of diabetic complications in Cox proportional hazards regression analyses. DKD = diabetic kidney disease; DN = diabetic neuropathy; DR = diabetic retinopathy; CHD = coronary heart disease; HF = heart failure. To simplify complex terminology, we standardized data labels by using abbreviations. Lipid size categories were abbreviated as follows: "very small" to "XS", "small" to "S", "medium" to "M", "large" to "L", "very large" to "XL", and "extremely large" to "XXL". For lipid types, "free cholesterol" to "FC", "cholesterol" to "C", "cholesteryl esters" to "CE", "triglycerides" to "TG", "total lipids" to "TL", "high-density lipoprotein" to "HDL", "low-density lipoprotein" to "LDL", "very low-density lipoprotein" to "VLDL", and "phospholipids" to "PLs".For fatty acids, "fatty acids" to "FA", "monounsaturated fatty acids" to "MUFA", "docosahexaenoic acid" to "DHA" and "polyunsaturated fatty acids" to"PUFA".
The inclusion of metabolites improved the predictive effectiveness of the conventional models for all diabetic complications (all P < 0.05, Fig. 5). In macrovascular complications, the merged model improved predictive accuracy (P < 0.001), with the AUC increasing from 0.672 (95% CI 0.656–0.687) to 0.687 (95% CI 0.672–0.702), NRI of 0.112, relative IDI of 0.222, absolute IDI of 0.005, and C-index rising from 0.649 (95% CI 0.635–0.663) to 0.662 (95% CI 0.649–0.672). For microvascular complications, AUC increased from 0.639 (95% CI 0.623–0.657) to 0.680 (95% CI 0.665–0.695, P < 0.001), NRI reached 0.140, relative IDI was 0.588, absolute IDI was 0.012, and C-index improved from 0.614 (95% CI 0.600–0.628) to 0.649 (95% CI 0.635–0.662). Specific diseases including CHD, HF, stroke, DKD, DN, and DR all showed varying degrees of improved predictive performance, with notable enhancements in DKD (AUC 0.760, 95% CI 0.745–0.776; 0.787, 95% CI 0.782–0.802, P < 0.001), DN (AUC 0.637, 95% CI 0.610–0.665; 0.726, 95% CI 0.703–0.750, P < 0.001), and DR (AUC 0.568, 95% CI 0.550–0.587; 0.631, 95% CI 0.613–0.648, P < 0.001). Detailed NRIs, relative and absolute IDIs, and AUCs for different Cox models after adding metabolomics as a predictor were shown in Supplementary Materials 1 Table S24.
Fig. 5.
The conventional and merged Cox proportional hazards regression models predicting the incidence of diabetic complications. Conventional models were developed by using conventional risk factors: age, sex, smoking status, race, diet, blood pressure, body mass index, blood lipids (combined plasma triglycerides and LDL cholesterol), plasma creatinine, eGFR, and Townsend deprivation index. The merged models incorporated both conventional risk factors and selected metabolomics. AUC = area under the curve; DKD = diabetic kidney disease; DN = diabetic neuropathy; DR = diabetic retinopathy; CHD = coronary heart disease; HF = heart failure.
Metabolic biomarkers causally related to the diabetic vascular complications
It showed that metabolomics had a more noteworthy effect on the development of CHD after FDR correction. Detailed information was demonstrated in Table 1. To be specific, for CHD, the ratio of phospholipids to total lipids in small LDL (odds ratio [OR] = 1.96, 95% CI 1.33–2.88, P = 0.015) proved to be substantially hazardous.
Table 1.
Results of main Mendelian randomization analyses
| Exposure | Outcome | OR | 95% CI | P value | Method |
|---|---|---|---|---|---|
| Phospholipids to total lipids ratio in small LDL | Coronary heart disease | 1.96 | 1.33–2.88 | 0.015 | Inverse variance weighted |
| Acetone | Heart Failure | 0.40 | 0.17–0.95 | 0.038 | Wald ratio |
| Diabetic neuropathy | Ratio of docosahexaenoic acid to total fatty acids | 0.97 | 0.95–0.99 | 0.019 | Inverse variance weighted |
| Diabetic neuropathy | Ratio of docosahexaenoic acid to total fatty acids | 0.97 | 0.95–0.99 | 0.043 | Weighted median |
| Diabetic neuropathy | Albumin | 0.97 | 0.94–0.99 | 0.049 | Weighted median |
| Diabetic neuropathy | Pyruvate | 1.03 | 1.01–1.06 | 0.049 | Weighted median |
| Diabetic neuropathy | Pyruvate | 1.03 | 1.01–1.05 | 0.046 | Inverse variance weighted |
| Diabetic neuropathy | Triglycerides to total lipids ratio in very large VLDL | 1.03 | 1.01–1.05 | 0.049 | Weighted median |
| Diabetic neuropathy | Triglycerides to total lipids ratio in very large VLDL | 1.03 | 1.01–1.05 | 0.019 | Inverse variance weighted |
| Diabetic retinopathy | Phospholipids to total lipids ratio in very large VLDL | 0.96 | 0.94–0.99 | 0.041 | Weighted median |
OR=odds ratio; CI= confidence interval;VLDL=very low-density lipoprotein.
Reverse MR demonstrated the reverse causal effect of complications on metabolomics levels. DN was relevant to decreased level of serum ratio of DHA to total fatty acids (OR = 0.97, 95% CI 0.95–0.99, P = 0.019), increased ratio of triglycerides to total lipids in very large VLDL (OR = 1.03, 95% CI 1.01–1.05, P = 0.019), and pyruvate (OR = 1.03, 95% CI 1.01–1.05, P = 0.046). The main results of MR analysis were demonstrated in Supplementary Materials 2 S3.
To ensure the assumptions of MR analysis were satisfied, analyses including a heterogeneity test, Steiger filtering analysis, and a pleiotropy test were conducted. The results were demonstrated in Supplementary Materials 2 S4-S6. Further, sensitivity analyses including leave-one-out analysis, forest plots, funnel plots, and scatter plots were calculated and demonstrated in Supplementary Materials 1 Figure S1-S47.
Discussion
The findings suggested that certain plasma metabolites, including acetate, alanine, creatinine, glucose, phospholipid ratios, fatty acids, albumin, and tyrosine and so on, may contribute to the development of diabetic vascular complications and could serve as common biomarkers or therapeutic targets for these conditions.
Acetate demonstrated significant associations with all diabetic complications examined in this study. This finding was supported by the established research indicating an inverse relationship between circulating acetate levels and insulin resistance [23]. Further, studies have found that acetate can prevent heart and kidney from nicotine-induced cardiorenal dysmetabolism [24]. Collectively, these observations suggest that acetate dysregulation may play a fundamental role in the pathogenesis of diverse diabetic complications through its modulation of systemic metabolism.
Alanine was found to be associated with CHD, HF, NEP, NEU and DR. There was established evidence linking impaired alanine catabolism to metabolic dysregulation [25, 26]. Specifically, hepatic alanine catabolism contributes to gluconeogenesis, thereby exacerbating hyperglycemia [25]. Also, the ratio of alanine to glycine was reported as a significant predictive factor for diabetes [26]. Therefore, serum level of alanine may affect the level of serum glucose, thus influencing the prognosis of diabetes.
Creatinine emerged as a prominent risk factor for CHD, HF, Stroke, DN and DKD. As serum creatinine is a well-established diagnostic marker for renal dysfunction, including DKD, its association with macrovascular complications likely reflects the known pathophysiological interplay between diabetic microvascular and macrovascular disease [27, 28]. Furthermore, prior studies have demonstrated that hyperglycemia contributes to elevated serum creatinine levels [29]. These collective mechanisms—renal impairment, systemic vascular dysfunction, and metabolic derangements—may jointly account for creatinine's association with multiple diabetic complications.
High level of glucose was reported to be toxic to neurons, which appeared to be highly sensitive to hyperglycemia [30]. Previous studies have found that the consumption of glucose by neurons are independent from plasma insulin [31], indicating that hyperglycemia are directly leading to the damage of neurons by glucose-driven oxidative stress and protein glycation [32]. Due to the abundance of microglia and neuron synapses in retina, retinopathy also appears to be highly sensitive to hyperglycemia [33].
The ratios of phospholipids across different lipoprotein subfractions were significantly associated with multiple diabetic complications, including CHD, DKD, DN and DR. This aligns with existing evidence demonstrating a well-established link between oxidized phospholipids and increased CHD risk [34, 35]. Furthermore, a recent study highlighted distinct phospholipid metabolic profiles in individuals with diabetes compared to non-diabetic controls, reinforcing the potential role of phospholipid dysregulation in diabetes-related vascular pathology [36].
The dysregulation of fatty acid metabolism was a consistent feature across multiple diabetic complications in this study. Specifically, elevated fatty acid ratios in very-small VLDL particles were significantly associated with increased risk of HF, DN, and DKD. Monounsaturated fatty acids (MUFAs) consistently demonstrated adverse associations, whereas polyunsaturated fatty acids (PUFAs) showed protective effects. Furthermore, a higher ω-3 to ω-6 fatty acid ratio was positively associated with DKD progression, suggesting potential pathway-specific mechanisms in renal complications. These findings collectively implicate altered lipid metabolism in the pathogenesis of diabetic vascular complications through multiple interacting pathways. These findings are supported by established studies concerning the correlation between lipid profiles and lipophilic index of serum phospholipids. Such shifts promote impaired glycemic control, endothelial dysfunction, and chronic inflammation, all hallmarks of diabetic vasculopathy [37]. Collectively, our results validate a robust association between the dysregulation of fatty acids and the progression of diabetic micro and macrovascular complications.
Also, albumin was found to be widely correlated with both macrovascular and microvascular complications significantly as a protective factor. Established studies have found a firm correlation of glycated albumin (GA) with serum HbA1c and the risk of incidence of diabetes complications [38, 39]. Therefore, a rising level of serum albumin may indicate a decreasing level of serum glucose and glycated albumin, acting as a protective factor.
Besides, tyrosine was also found to be a significant protective factor for almost all complications, which was consistent with previous studies [40, 41]. Considering tyrosine is the precursor of dopamine, patients with a low level of tyrosine may face inadequate synthesis of dopamine [42], leading to the incidence of DR [40]. Furthermore, the kidney is crucial for the absorption of phenylalanine, its conversion to tyrosine, and the subsequent release. Impaired transformation of phenylalanine into tyrosine has been noted in renal failure [43]. Consequently, lower tyrosine levels may indicate renal failure, potentially leading to other microvascular complications. Also, tyrosine appears to be inversely correlated with HbA1c levels, which is a known risk factor for diabetic vascular complications [41].
Our study observed an increase in plasma triglyceride levels in macrovascular complications, which serves as a critical predisposing factor for atherosclerosis development, the pathological foundation of macrovascular complications. Although the intake of plasma cholesterol by foam cells was considered to be a protective reaction [44], the rising level of circulating lipoproteins still leads to foam cell apoptosis in localized areas lacking oxygen and thus accelerates the development and progression of atherosclerosis [45].
Apart from those molecules mentioned above, it’s interesting to dig into the mechanisms related to these metabolites, most of which are lipids and ketone bodies. Several pathways were reported to be related to the progression of dyslipidemia. Due to the impact of insulin on regulating the expression of low-density lipoprotein receptors, it’s common for those patients with poorly controlled T1D to have increased levels of LDL cholesterol, LDL particles, and apolipoprotein B [46]. Animal models have also proved that the level of plasma LDL-cholesterol in those with diabetes increased compared to those without [47].
Dyslipidemia can damage the circulatory system, especially the macrovascular system. The pathophysiology of how these altered lipid profiles interact with the vascular system was reported previously. First, these can lead to endothelial dysfunction, a precursor to atherosclerosis, by reducing nitric oxide availability and increasing oxidative stress. Dyslipidemia can impair endothelial function via various mechanisms, such as boosting oxygen-derived free radicals, activating protein kinase C (PKC), and worsening lipid imbalances [48]. Second, dyslipidemia is associated with systemic inflammation, which promotes atherosclerosis and plaque instability. Furthermore, elevated triglycerides and altered lipoproteins can enhance coagulation pathways, increasing the risk of thrombosis.
Due to the similar pathological mechanisms of diabetic vascular complications, primarily characterized by endothelial dysfunction and atherosclerosis [49], there has been a growing recommendation for comprehensive management of diabetic vascular complications across multiple organs to improve outcomes and prognoses [30]. Several models within individual studies that forecast multiple diabetes-related vascular complications, covering both macro- and microvascular issues, including RECODe models [50], UKPDS outcomes model 1 and 2 [51], as well as models by other research groups [52, 53]. Notably, the RECODe system demonstrates predictive generalizability across macrovascular sequelae, DR, DN, and DKD, relying on overlapping conventional biomarkers. The performance of these models, indicated by C index values between 0.54 and 0.79, was comparable to our traditional models' predictive capabilities (C index 0.649 for macrovascular complications; 0.614 for microvascular complications). Furthermore, we identified significant plasma metabolites for each condition, which were found to enhance the predictive performance of the respective disease models.
However, before applying them to clinical care, crucial challenges remain unsolved. Though current NMR-quantified metabolomics appears to be more economical and rapid than other mass-spectrum alternatives, the setbacks as sensitivity and limitations exist. Current NMR-spectrum mainly focuses on lipids and is limited by accessible endpoints information [19, 54]. At the same time, however, our study must acknowledge some shortcomings and limitations. First, the metabolic data of our study are from the UK Biobank, and the subjects in the sample are most British people from developed countries in Western Europe, which may limit the generality of our results to countries with other geographical and socioeconomic backgrounds. Secondly, due to the limitations of the database, our study mainly focused on elderly and middle-aged patients with diabetes or those with a history of diabetes, and there was a lack of research and discussion on young people. Thirdly, to ensure the robustness of the overall analysis, the threshold of SNP selection in MR analysis was set to a rather strict value. However, in some analyses, including the reverse MR analysis of HF and DN, there were not enough SNPs for MR-PRESSO analysis or leave-one-out analysis. This constraint may potentially impact the precision of these specific analyses. Fourthly, while multiple ascertainment methods for endpoint diagnosis were employed, the potential incompleteness of data for all participants was not fully addressed, which might lead to bias. Fifthly, the improvements observed in the AUC and C-index were relatively modest. This limitation may stem from the fact that NMR-based metabolites might not fully capture the most critical pathological processes underlying diabetic vascular complications. Consequently, future studies should employ additional methodologies to investigate the roles of other metabolites or omics profiles in diabetic vascular complications. Finally, due to the limited source of longitudinal data, we have not completed external validation of the model, even if we use MR analysis to explain the causal relationship, so we cannot know its universality and make further corrections.
Conclusions
In conclusion, our study revealed that several metabolites were found to be significantly correlated with incident diabetic complications. Genetic predisposition to screened metabolites was also linked to diabetic complications. Future research is needed to explore and verify our findings in different ethnicities and larger populations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This research used the UK Biobank resource (Application Number 99029) and the FinnGen study. We thank the participants, contributors and researchers of the UK Biobank and the FinnGen study for making data available for this study. We thank the Guangdong Basic Research Center of Excellence for Major Blinding Eye Diseases Prevention and Treatment.
Author contributions
Z Li, Y Zheng and M He were involved in the design of the study. Y Ren and Z Li wrote the manuscript. Z Li, Y Zheng and M He critically revised the manuscript for important intellectual content. All authors revised and edited the manuscript. Z Li is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by the National Natural Science Foundation of China (82301249), the Natural Science Foundation of Guangdong Province (2024A1515010338), the Science and Technology Projects in Guangzhou (3030901006202), the Research Funds of the State Key Laboratory of Ophthalmology, the Lumitin Vision to Brightness Research Funding for the Young and middle-aged Ophthalmologists and the Global STEM Professorship Scheme (P0046113). This work only represented the viewpoint of the authors, the funding sources had no involvement in the study design or implementation.
Availability of data and materials
Data from UK Biobank are available on application at www.ukbiobank.ac.uk/register-apply. Data from the FinnGen study are available at www.finngen.gitbook.io/documentation/v/r5.
Declarations
Ethics approval and consent to participate
UK Biobank obtained ethical approval from the North West Multi-Centre Research Ethics Committee to collect and utilize the data. The FinnGen Biobank study was likewise approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District. All participants provided written informed consent. Furthermore, since the data was publicly available and deidentified, institutional review board approval was waived for this analysis.
Consent for publication
Not applicable.
Competing interests
The authors have no financial or other conflicts of interest concerning this study. The funders had no role in the study design or implementation; data collection, management, analysis, or interpretation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication. The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuhan Ren, Yingfeng Zheng and Zhixi Li have contributes equally to this work.
Contributor Information
Zhixi Li, Email: lzx11-11@163.com.
Yingfeng Zheng, Email: zhengyingfeng@gzzoc.com.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. [DOI] [PubMed] [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. [DOI] [PubMed] [Google Scholar]
- 3.Kaplovitch E, Eikelboom JW, Dyal L, Aboyans V, Abola MT, Verhamme P, et al. Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease: a subanalysis of the COMPASS randomized clinical trial. JAMA Cardiol. 2021;6(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li CI, Lin CC, Cheng HM, Liu CS, Lin CH, Lin WY, et al. Derivation and validation of a clinical prediction model for assessing the risk of lower extremity amputation in patients with type 2 diabetes. Diabetes Res Clin Pract. 2020;165: 108231. [DOI] [PubMed] [Google Scholar]
- 6.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93(6):1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handzlik MK, Gengatharan JM, Frizzi KE, McGregor GH, Martino C, Rahman G, et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature. 2023;614(7946):118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225–32. [DOI] [PubMed] [Google Scholar]
- 9.Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2007;30(7):1834–9. [DOI] [PubMed] [Google Scholar]
- 10.Croyal M, Saulnier PJ, Aguesse A, Gand E, Ragot S, Roussel R, et al. Plasma trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J Clin Endocrinol Metab. 2020. 10.1210/clinem/dgaa188. [DOI] [PubMed] [Google Scholar]
- 11.SCORE2-Diabetes: 10-year cardiovascular risk estimation in type 2 diabetes in Europe. Eur Heart J. 2023;44(28):2544–56. [DOI] [PMC free article] [PubMed]
- 12.Jin Q, Ma RCW. Metabolomics in diabetes and diabetic complications: insights from epidemiological studies. Cells. 2021. 10.3390/cells10112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wozniak JM, Mills RH, Olson J, Caldera JR, Sepich-Poore GD, Carrillo-Terrazas M, et al. Mortality Risk Profiling of Staphylococcus aureus Bacteremia by Multi-omic Serum Analysis Reveals Early Predictive and Pathogenic Signatures. Cell. 2020;182(5):1311-27.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Gui Y, Wang MS, Zhang L, Xu T, Pan Y, et al. Serum integrative omics reveals the landscape of human diabetic kidney disease. Mol Metab. 2021;54: 101367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Liu R, Xin Z, Zhu Z, Chu J, Zhong P, et al. Plasma metabolomics identifies key metabolites and improves prediction of diabetic retinopathy: development and validation across multinational cohorts. Ophthalmology. 2024;131(12):1436–46. [DOI] [PubMed] [Google Scholar]
- 16.Pereira PR, Carrageta DF, Oliveira PF, Rodrigues A, Alves MG, Monteiro MP. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med Res Rev. 2022;42(4):1518–44. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Zhu F, Chen L, Chen K. Proteomics, metabolomics and metagenomics for type 2 diabetes and its complications. Life Sci. 2018;212:194–202. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buergel T, Steinfeldt J, Ruyoga G, Pietzner M, Bizzarri D, Vojinovic D, et al. Metabolomic profiles predict individual multidisease outcomes. Nat Med. 2022;28(11):2309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julkunen H, Cichońska A, Tiainen M, Koskela H, Nybo K, Mäkelä V, et al. Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK Biobank. Nat Commun. 2023;14(1):604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton AR, Sherman MA, Mukamel RE, Loh PR. Whole-exome imputation within UK Biobank powers rare coding variant association and fine-mapping analyses. Nat Genet. 2021;53(8):1260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Spiegeleer M, De Paepe E, Van Meulebroek L, Gies I, De Schepper J, Vanhaecke L. Paediatric obesity: a systematic review and pathway mapping of metabolic alterations underlying early disease processes. Mol Med. 2021;27(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michael OS, Dibia CL, Soetan OA, Adeyanju OA, Oyewole AL, Badmus OO, et al. Sodium acetate prevents nicotine-induced cardiorenal dysmetabolism through uric acid/creatine kinase-dependent pathway. Life Sci. 2020;257: 118127. [DOI] [PubMed] [Google Scholar]
- 25.Okun JG, Rusu PM, Chan AY, Wu Y, Yap YW, Sharkie T, et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat Metab. 2021;3(3):394–409. [DOI] [PubMed] [Google Scholar]
- 26.Lee KS, Lee YH, Lee SG. Alanine to glycine ratio is a novel predictive biomarker for type 2 diabetes mellitus. Diabetes Obes Metab. 2024;26(3):980–8. [DOI] [PubMed] [Google Scholar]
- 27.Januzzi JL, Mohebi R, Liu Y, Sattar N, Heerspink HJL, Tefera E, et al. Cardiorenal biomarkers, canagliflozin, and outcomes in diabetic kidney disease: the CREDENCE trial. Circulation. 2023;148(8):651–60. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Zhao S, Huang Y, Ma M, Li B, Li C, et al. Diabetes-related macrovascular complications are associated with an increased risk of diabetic microvascular complications: a prospective study of 1518 patients with type 1 diabetes and 20 802 patients with type 2 diabetes in the UK Biobank. J Am Heart Assoc. 2024;13(11): e032626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Zhang M, Zeng L, Lai Y, Wu S, Su X. Wogonin upregulates SOCS3 to alleviate the injury in diabetic nephropathy by inhibiting TLR4-mediated JAK/STAT/AIM2 signaling pathway. Mol Med. 2024;30(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel N, Llewelyn J, Wright D, Thomas P. Glucose and leucine uptake by rat dorsal root ganglia is not insulin sensitive. J Neurol Sci. 1994;121(2):159–62. [DOI] [PubMed] [Google Scholar]
- 32.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9(1):36–45. [DOI] [PubMed] [Google Scholar]
- 33.Mills SA, Jobling AI, Dixon MA, Bui BV, Vessey KA, Phipps JA, et al. Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc Natl Acad Sci U S A. 2021. 10.1073/pnas.2112561118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilliland TC, Liu Y, Mohebi R, Miksenas H, Haidermota S, Wong M, et al. Lipoprotein(a), oxidized phospholipids, and coronary artery disease severity and outcomes. J Am Coll Cardiol. 2023;81(18):1780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeang C, Hasanally D, Que X, Hung MY, Stamenkovic A, Chan D, et al. Reduction of myocardial ischaemia-reperfusion injury by inactivating oxidized phospholipids. Cardiovasc Res. 2019;115(1):179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng Y, Mtintsilana A, Goedecke JH, Micklesfield LK, Olsson T, Chorell E. Alterations in the metabolism of phospholipids, bile acids and branched-chain amino acids predicts development of type 2 diabetes in black South African women: a prospective cohort study. Metabolism. 2019;95:57–64. [DOI] [PubMed] [Google Scholar]
- 37.Rostoff P, Drwiła-Stec D, Majda A, Stępień K, Nessler J, Gajos G. Lipophilic index of serum phospholipids in patients with type 2 diabetes and atherosclerotic cardiovascular disease: links with metabolic control, vascular inflammation and platelet activation. Cardiovasc Diabetol. 2024;23(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon HJ, Lee YH, Kim SR, Rim TH, Lee EY, Kang ES, et al. Glycated albumin and the risk of micro- and macrovascular complications in subjects with type 1 diabetes. Cardiovasc Diabetol. 2015;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo HH, Li J, Feng XF, Sun XY, Li J, Yang X, et al. Plasma phenylalanine and tyrosine and their interactions with diabetic nephropathy for risk of diabetic retinopathy in type 2 diabetes. BMJ Open Diabetes Res Care. 2020. 10.1136/bmjdrc-2019-000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welsh P, Rankin N, Li Q, Mark PB, Würtz P, Ala-Korpela M, et al. Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: results from the ADVANCE trial. Diabetologia. 2018;61(7):1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137(6 Suppl 1):1539S-S1547. [DOI] [PubMed] [Google Scholar]
- 43.Kopple JD. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr. 2007;137(6 Suppl 1):1586S-S1590. [DOI] [PubMed] [Google Scholar]
- 44.Sorci-Thomas MG, Thomas MJ. Microdomains, inflammation, and atherosclerosis. Circ Res. 2016;118(4):679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Öörni K, Rajamäki K, Nguyen SD, Lähdesmäki K, Plihtari R, Lee-Rueckert M, et al. Acidification of the intimal fluid: the perfect storm for atherogenesis. J Lipid Res. 2015;56(2):203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobs MJ, Kleisli T, Pio JR, Malik S, L’Italien GJ, Chen RS, et al. Prevalence and control of dyslipidemia among persons with diabetes in the United States. Diabetes Res Clin Pract. 2005;70(3):263–9. [DOI] [PubMed] [Google Scholar]
- 47.Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol. 1999;19(12):2981–92. [DOI] [PubMed] [Google Scholar]
- 48.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108(12):1527–32. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X, Yu L, Zhao Y, Ge J. Panvascular medicine: an emerging discipline focusing on atherosclerotic diseases. Eur Heart J. 2022;43(43):4528–31. [DOI] [PubMed] [Google Scholar]
- 50.Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of risk equations for complications of type 2 diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol. 2017;5(10):788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka S, Tanaka S, Iimuro S, Yamashita H, Katayama S, Akanuma Y, et al. Predicting macro- and microvascular complications in type 2 diabetes: the Japan Diabetes Complications Study/the Japanese Elderly Diabetes Intervention Trial risk engine. Diabetes Care. 2013;36(5):1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dagliati A, Marini S, Sacchi L, Cogni G, Teliti M, Tibollo V, et al. Machine learning methods to predict diabetes complications. J Diabetes Sci Technol. 2018;12(2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on -omic technologies. Am J Epidemiol. 2017;186(9):1084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from UK Biobank are available on application at www.ukbiobank.ac.uk/register-apply. Data from the FinnGen study are available at www.finngen.gitbook.io/documentation/v/r5.