Abstract
Objective
To investigate the protective effect of ammonium trichloride tellurate (AS101) on doxorubicin induced ovarian function damage in rats.
Methods
Eighteen female SD rats were randomly divided into three groups with 6 rats in each group: group A blank control group (0.9% normal saline 0.1 ml intraperitoneally for 21 consecutive days), group B (0.9% normal saline 0.1 ml intraperitoneally for 5 days, doxorubicin 10 mg / kg intraperitoneally on day 6, and continued to be injected with normal saline for 15 days), group C (0.9% normal saline 0.1 ml intraperitoneally for 5 days, doxorubicin 10 mg / kg intraperitoneally on day 6, and continued to be injected with AS101 twice every other day). The contents to be recorded by the operator team include: (1) before operation: ① observe the general state of rats in each group and record the weight of rats. ② Vaginal smears of rats in each group were observed at the same time every day to observe the estrous cycle of rats. (2) After operation: ① observe the general state of rats in each group, read the weight of rats, and record the data Vaginal smears of rats in each group were observed at the same time every day after operation to observe the estrous cycle of rats. ③ At the end of the postoperative observation period, blood samples were taken from the tail of rats to measure the serum levels of estrogen 2 (E2), follicle stimulating hormone (FSH), and anti-M ü llerian hormone (AMH). ④ At the end of the observation period, the animals were sacrificed to observe the development of bilateral ovarian follicles. ⑤ At the end of the observation period, blood was taken from the abdominal aorta of rats after anesthesia, and the upper serum was separated after centrifugation at 3000 rpm. The levels of malondialdehyde (MDA), superoxide dismutase (SOD) and reactive oxygen species (ROS) were measured according to the operation methods of the kit instructions.
Result
The body weight of rats in group B was significantly lower than that in group A, while that in group C was significantly higher than that in group B (P < 0.05). The estrous cycle of rats in group B was disordered, and the estrous cycle of rats in group C was restored regularly (P < 0.05). The serum estradiol(E2) and AMH levels in group C were significantly higher than those in group B, and the FSH level was significantly lower than that in group B. Compared with group B, the number of primary follicles increased and the number of atretic follicles decreased in group C (P < 0.05). The SOD level in group B was lower than that in group A, and that in group C was higher than that in group B (P < 0.05). The levels of MDA and ROS in group B were higher than those in group A (P < 0.05), while those in group C were lower than those in group B (P < 0.05).
Conclusion
AS101 can protect the damage of doxorubicin on ovarian function in rats, and its mechanism is related to its antioxidant effect.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-025-01709-z.
Keywords: AS101, Chemotherapy, Ovarian, Oxidative stress, Reproductive endocrine
Introduction
Malignant tumors in the female reproductive system, primarily endometrial, cervical, and ovarian cancers, significantly contribute to global female mortality [1]. Furthermore, the incidence of these malignant tumors has been on a yearly rise [2]. In contemporary medicine, chemotherapy is a crucial treatment for malignant tumors. While it effectively inhibits tumor growth and spread, it can also impair women’s normal physiological functions. Notably, damage to ovarian function is a significant concern [3]. The ovary, a vital component of the female reproductive system, directly influences women’s physiological health, and any functional impairment can have significant effects. For instance, ovarian dysfunction can result in menstrual irregularities, infertility, and premature menopause [4]. This can severely compromise a woman’s quality of life. Additionally, ovarian dysfunction can precipitate psychological issues, including anxiety and depression [5]. This further disrupts the patients’ normal life. Consequently, addressing ovarian dysfunction has emerged as a focal challenge in current medical research. Developing strategies to safeguard against chemotherapy-induced ovarian dysfunction is an urgent research priority.
Doxorubicin, an anthracycline anti-tumor antibiotic, operates primarily by inserting into the base pairs of the DNA double helix. This action inhibits the synthesis of DNA and RNA, thereby curbing the proliferation of tumor cells. It is currently a prevalent choice in chemotherapy for treating malignant tumors in the female reproductive system [6, 7]. Studies indicate that female gonads exhibit sensitivity to doxorubicin, and chemotherapy can readily lead to ovarian dysfunction [6]. The damage mechanism is multifaceted and complex, encompassing irreversible DNA damage, cell apoptosis, overactivation of primordial follicles, and heightened oxidative stress capacity, among others [8, 9]. While some researchers have employed mouse models to investigate protectants against these mechanisms, the efficacy of these protectants remains limited. Conventional ovarian function protection methods, like embryo cryopreservation, also present numerous limitations, including high costs and low success rates [10]. Consequently, there is an urgent need to explore novel protectants that can mitigate ovarian dysfunction during chemotherapy, offering better therapeutic effects at a lower cost.
AS101, a synthetically produced tellurium compound of low molecular weight, exhibits antioxidant and anti-apoptotic properties. It possesses the capability to bolster the immune function in cancer patients. Additional research indicates that AS101 can mitigate the damage inflicted by chemotherapy drugs on organs and tissues. By inhibiting the apoptosis of growing follicles induced by oxidative stress, it also curbs the recruitment of primordial follicles [11, 12]. Recent studies have identified oxidative stress as a significant factor in ovarian damage induced by chemotherapy drugs [13]. Investigating the potential protective effects of AS101 on chemotherapy-induced ovarian dysfunction holds substantial significance.
Materials and methods
Drugs and reagents
AS101 (catalog number: A872502) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Doxorubicin hydrochloride (catalog number: MB1087) was obtained from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China). Enzyme-linked immunosorbent assay (ELISA) kits for E2 (catalog number: CEA441Ra96T), FSH (catalog number: CEA830Ra96T), AMH (catalog number: CEA228Ra96T), were acquired from Wuhan Saver Biological Technology Co., Ltd. (Wuhan, China). MDA (catalog number: BC0025) and SOD (catalog number: BC5165) were acquired from Beijing Solarbio Science and Technology Co., Ltd. (Beijing); ROS (catalog number: MA0219) was obtained from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China).
Tissue processing and embedding were performed using a KD-BMIV automatic tissue dehydrator and a BLIV tissue embedding machine (Wuhan Servicebio Technology Co., Ltd., Wuhan, China). Centrifugation was carried out with a room temperature tabletop high-speed centrifuge (model: 5424R; Eppendorf AG, Hamburg, Germany). Histological observations were conducted using a ci-1 biological microscope (Nikon Corporation, Tokyo, Japan) and a CKX-41 inverted microscope (Olympus Corporation, Tokyo, Japan). Absorbance measurements were recorded on a RT-6100 microplate reader (Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, China).
Animals
We procured 18 female SD rats, each 8 weeks old and weighing approximately 210 ± 10 g, from Beijing Huafukang Biotechnology Co., Ltd. The rats were housed in our institution’s experimental animal center, with each group accommodated in identically sized cages. The rats were maintained under standard conditions, including a 12-hour light/dark cycle, a temperature of 25℃, adequate ventilation, and dryness. They were provided with ample and equal quantities of food and water. Regular replacement and disinfection of the rat cages and bedding were ensured. All animal experiments and procedures involved in this study received approval from our institution’s Animal Welfare Ethics Review Committee. This study was approved by the Experimental Animal Welfare Ethics Committee of Hebei University (Ref:202328SR).
Study design
Animals grouping and treatment
All experimental animals underwent a one-week acclimatization period in our institution’s animal experiment room. The 18 rats were randomly allocated into three groups, each containing six individuals. Group A, designated as the blank control group, received intraperitoneal injections of 0.9% saline (0.1mL) for 21 consecutive days. Group B, referred to as the modeling group, was pre-treated with intraperitoneal injections of 0.9% saline (0.1mL) for five days, followed by an intraperitoneal injection of doxorubicin (10 mg/kg) on the sixth day, and continued saline injections for the subsequent 15 days. Group C, the treatment group, followed the same initial protocol as Group B but received AS101 (prepared in PBS, pH = 7.4) (3 mg/kg) injections every other day after the doxorubicin injection, for a total of two times. Notably, no fatalities were observed among the rats in any of the three groups.
Data collection
The rats are weighed every two days, with the data duly recorded.Each rat undergoes a vaginal smear procedure every morning between 7 and 8 o’clock. Specific steps include inserting a sterilized cotton swab, moistened with physiological saline, approximately 0.5 cm into the rat’s vagina and gently rotating it 2–3 times. (1) The proestrus phase typically spans 12–14 h, during which most cells in the rat vaginal smear are nucleated epithelial cells, interspersed occasionally with keratinocytes and leukocytes. (2) The estrus phase generally endures for 25–27 h, with all cells in the rat vaginal smear being anucleated keratinized epithelial cells, and a small number of oval epithelial cells visible occasionally. (3) The metestrus phase typically lasts for 55–57 h, characterized predominantly by the presence of numerous leukocytes in the vaginal smear, along with a few nucleated epithelial cells. (4) The diestrus phase generally persists for 6–8 h, during which the vaginal smear exhibits a variety of cells concurrently, including leukocytes, keratinocytes, and nucleated epithelial cells [14]. Following the removal of secretions, they are smeared on a glass slide, stained with HE, and observed under an optical microscope to ascertain the rat’s estrous cycle.
Serum test index
Upon completion of the observation period, tail blood samples were collected from each rat group for testing serum levels of E2, FSH, and AMH. Concurrently, blood samples were drawn from the rats’ femoral arteries to assess the serum levels of SOD, MDA, and ROS. The collected blood was transferred into a 5 ml centrifuge tube, centrifuged at 3000 r/min for 20 min to separate the serum, and subsequently stored in a refrigerator at -4℃. Following this, an enzyme-linked immunosorbent assay (ELISA) was employed to quantify the serum hormone levels as well as the serum concentrations of SOD, MDA, and ROS. The specific procedures were meticulously executed in accordance with the instructions provided with the reagent kit.
Hematoxylin-eosin (HE) staining
Following the observation period, the rats were euthanized via carbon dioxide asphyxiation. The skin and subcutaneous tissue were dissected sequentially to locate both ovaries, which were then completely excised for histological analysis. The intact ovarian tissue was fixed in a tissue fixative, dehydrated, embedded in paraffin, and cooled. Finally, 5-µm-thick tissue sections were prepared on slides. One in every four consecutive sections was selected, with an average of five sections per group subjected to deparaffinization and HE staining. The slides were sealed with neutral balsam, and follicular development was evaluated under a light microscope. Under the microscope, the total number of primordial and atretic follicles in five fields of view was counted from HE-stained sections of the entire fragment, and the average number was determined.
Tissue collection
After a 14-day observation period, rats were euthanized by carbon dioxide asphyxiation. The skin and subcutaneous tissues were sequentially incised to expose and completely dissect both ovaries for histological evaluation. The intact ovarian tissues were fixed in fixative solution, dehydrated, embedded in paraffin, cooled, and then sectioned into 5 μm-thick slices, which were mounted on glass slides. One section was selected from every four consecutive slices, with an average of five sections per group. These sections were deparaffinized, stained with HE, mounted with neutral balsam, and assessed for follicular development under an optical microscope. Primordial follicles throughout the entire section were counted on HE-stained slides under the microscope. The total numbers of primordial and atretic follicles were recorded across five fields of view, and the averages were calculated.
The euthanasia procedure
The specific steps are as follows: (1) Check the carbon dioxide cylinder pressure to ensure an adequate gas supply. (2) Adjust the CO₂ flow rate to the recommended level. 3) Place the rats in the euthanasia chamber. (4) Open the CO₂ cylinder valve and introduce CO₂ into the chamber at the predetermined flow rate. (5) Monitor the rats until respiration ceases, pupils are dilated, and no muscular movement is observed. (6) After confirming death, continue CO₂ infusion for at least one additional minute to ensure complete euthanasia.
Statistical methods
The collected data, encompassing hormone levels of Group A, Group B, and Group C, ovarian SOD, MDA, ROS, and follicle development, among others, underwent statistical analysis using SPSS26.0 software. The measurement data are presented as mean ± standard deviation (—x±s). One-way ANOVA is employed for comparisons among multiple groups, while the LSD test is utilized for pairwise mean comparisons between multiple groups. P < 0.05 indicates that the difference is statistically significant.
Result
Comparison of the general state of rats
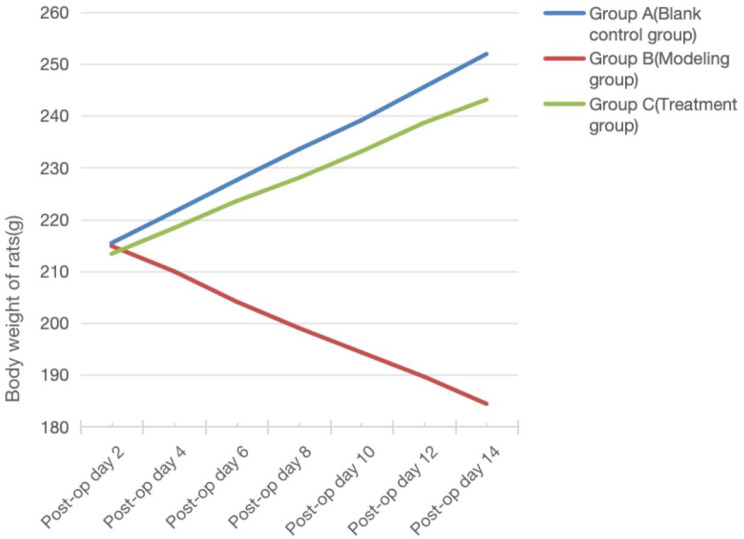
Prior to surgery, the rats in each group were in good general health, exhibiting consistent and satisfactory weight gain. Post-surgery, the rats exhibited dull, lusterless fur, poor dietary intake, heightened irritability, and diminished responsiveness to external stimuli. Compared to Group A, Group B experienced a significant decrease in weight, while Group C showed a substantial and significant weight increase compared to Group B (Figs. 1 and 2).
Fig. 1.
General state of rats in each groups. (A) Group A (saline)(n = 6); (B) Group B (saline + doxorubicin) (n = 6); (C) Group C (saline + doxorubicin AS101) (n = 6). After collecting blood samples from each group of rats for further analysis, the 6 samples were uniformly measured using the ELISA in a single test. The experiment was repeated 3 times, and since the conclusions were consistent, the dataset with the best results was selected for statistical analysis. Moreover, the data presented in this study (tables and bar charts) represent the mean value of 6 samples per group of rats
Fig. 2.
Trends in body weight of rats in each group
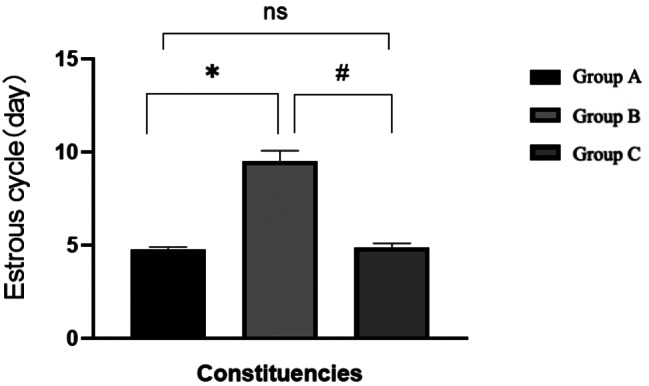
Estrous cycle
Prior to administration, the estrous cycle of the rats in each group was normal, with no statistically significant differences observed between the groups (P > 0.05). Following the doxorubicin injection, the rats’ estrous cycle was disrupted. However, in Group C, where rats were injected with AS101, the estrous cycle recovered. This difference was statistically significant when compared to Group B (P < 0.05)(Figs. 3, 4 and 5). For further details, please refer to Table 1.
Fig. 3.
HE staining of vaginal smear cells from normal rat estrous cycles. (A) Proestrus: The duration typically ranges from 12 to 14 h, with nucleated epithelial cells being the predominant cell type observed in rat vaginal smears, along with occasional keratinized cells and leukocytes; (B) Estrus: The duration typically ranges from 25 to 27 h, with rat vaginal smears consisting entirely of anucleate keratinized epithelial cells, occasionally accompanied by a few oval-shaped epithelial cells; (C) Interestrus: The duration typically ranges from 55 to 57 h, with the most notable characteristic of the vaginal smear being the presence of numerous leukocytes and a small number of nucleated epithelial cells; (D) Late estrus: The duration typically ranges from 6 to 8 h, with the vaginal smear displaying a mixture of cell types, including leukocytes, keratinized cells, and nucleated epithelial cells. Blue arrow: unnucleated keratinocyte; Orange arrows: nucleated epithelial cells; Green arrows: white blood cells
Fig. 4.

Estrous cycle of rats in each group before intervention
Fig. 5.
Estrous cycle of rats in each group after intervention
Table 1.
Estrous cycle of rats in each group before and after intervention
| Group | Before intervention | After intervention |
|---|---|---|
| Group A | 4.51 ± 0.36 | 4.77 ± 0.13 |
| Group B | 4.78 ± 0.29 | 9.52 ± 0.56* |
| Group C | 4.83 ± 0.33 | 4.90 ± 0.20# |
| F | 1.590 | 350.517 |
| P | 0.236 | 0.000 |
*: Comparison between group A and group B after intervention: P < 0.05 #:Comparison between group B and group C after intervention: P < 0.05
Serum test index
FSH, E2 and AMH
Following the observation period, a comparison of serum E2, AMH, and FSH levels across the three rat groups revealed that, relative to Group A, Group B rats exhibited an increase in follicle-stimulating hormone and a decrease in estrogen and anti-Müllerian hormone, all of which were statistically significant (P < 0.05). When compared to Group B, Group C demonstrated a decrease in follicle-stimulating hormone and an increase in estrogen and anti-Müllerian hormone, changes that were statistically significant (P < 0.05). The hormone levels in Group C, when compared to Group A, did not exhibit statistically significant differences (P > 0.05)(Figs. 6, 7 and 8). For further details, please refer to Table 2.
Fig. 6.
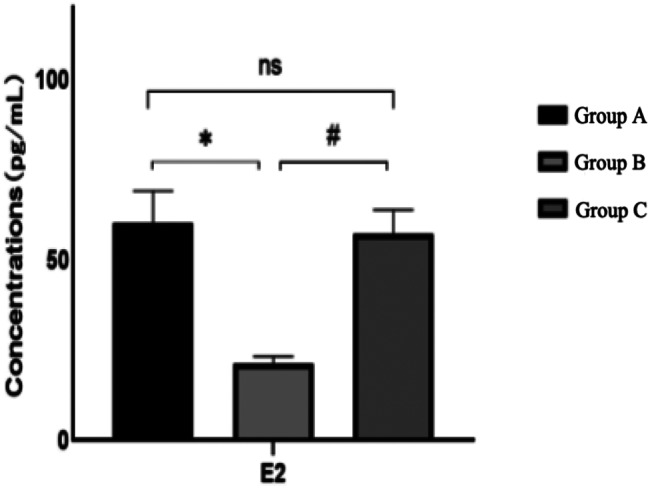
Comparison of serum E2 levels of rats in each group after intervention
Fig. 7.
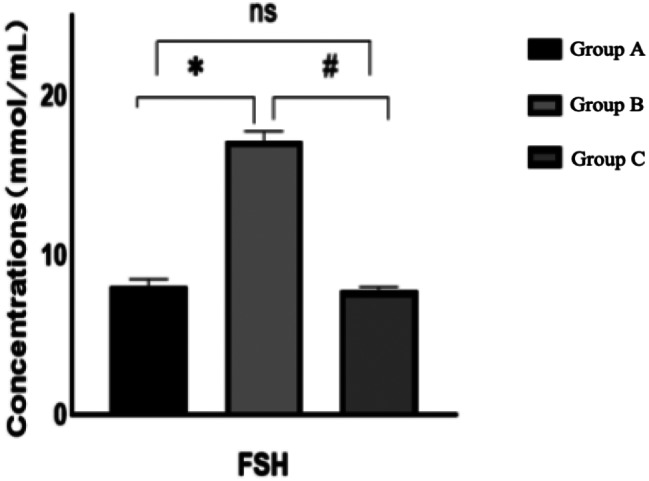
Comparison of serum FSH levels of rats in each group after intervention
Fig. 8.

Comparison of serum AMH levels of rats in each group after intervention
Table 2.
Comparison of serum hormone levels in each group after intervention
| Group | E2/(pg/mL) | FSH/(lU/L) | AMH/(Pg/ml) |
|---|---|---|---|
| Group A | 60.15 ± 9.00 | 8.03 ± 0.47 | 2002.25 ± 40.55 |
| Group B | 21.13 ± 2.05* | 17.14 ± 0.61* | 1257.15 ± 111.16* |
| Group C | 57.22 ± 6.65# | 7.77 ± 0.25# | 2002.00 ± 79.52# |
*: Comparison between group A and group B after intervention: P < 0.05 #:Comparison between group B and group C after intervention: P < 0.05
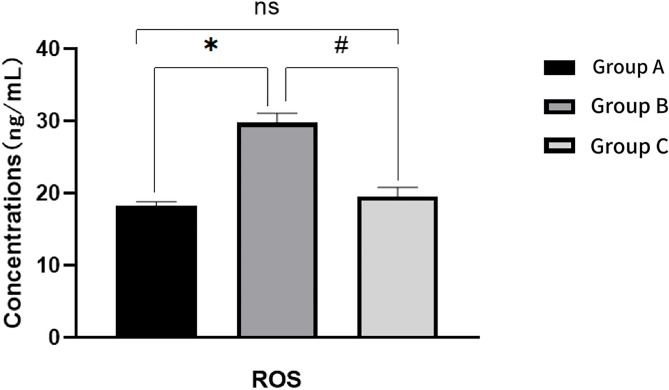
SOD, MDA, and ROS
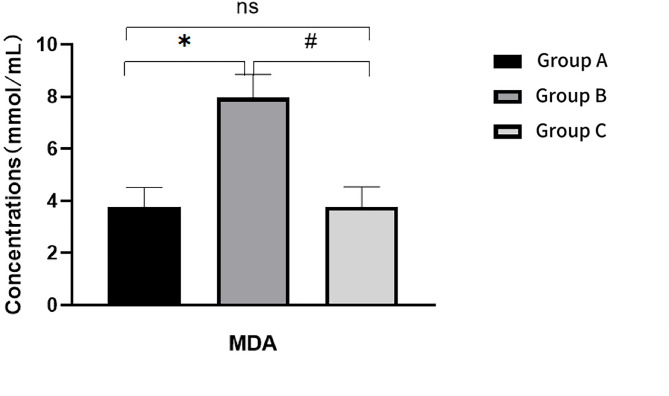
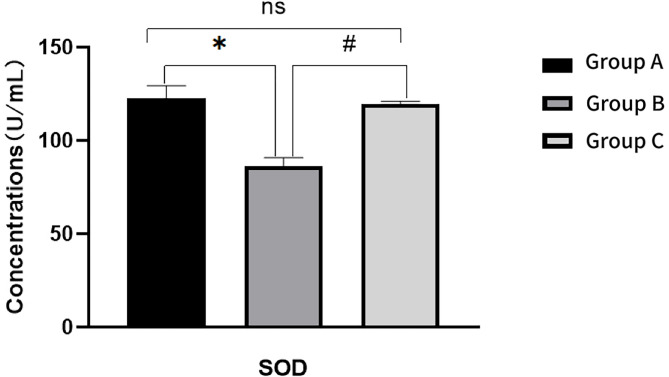
Relative to Group A, Group B rats exhibited a significant increase in serum MDA and ROS levels (P < 0.05), along with a significant decrease in SOD level (P < 0.05). When compared to Group B, Group C demonstrated a significant decrease in MDA and ROS levels (P < 0.05), and a significant increase in SOD level (P < 0.05)(Figs. 9, 10 and 11). For further details, please refer to Table 3.
Fig. 9.
Comparison of MDA levels in ovarian tissue of rats after intervention
Fig. 10.
Comparison of SOD levels in ovarian tissue of rats in each group after intervention
Fig. 11.
Comparison of ROS levels in ovarian tissue of rats in each group after intervention
Table 3.
Comparison of MDA, SOD and ROS levels in ovarian tissue of rats in each group after intervention
| Group | MDA(mmol/L) | SOD(U/mL) | ROS(ng/mL) |
|---|---|---|---|
| Group A | 3.77 ± 0.74 | 122.89 ± 6.60 | 18.30 ± 0.54 |
| Group B | 7.96 ± 0.89* | 86.28 ± 4.56* | 29.76 ± 1.34* |
| Group C | 3.78 ± 0.76# | 119.80 ± 1.21# | 19.55 ± 1.23# |
| F | 54.725 | 112.96 | 197.36 |
| P | 0.000 | 0.000 | 0.000 |
*: Comparison between group A and group B after intervention: P < 0.05 #:Comparison between group B and group C after intervention: P < 0.05
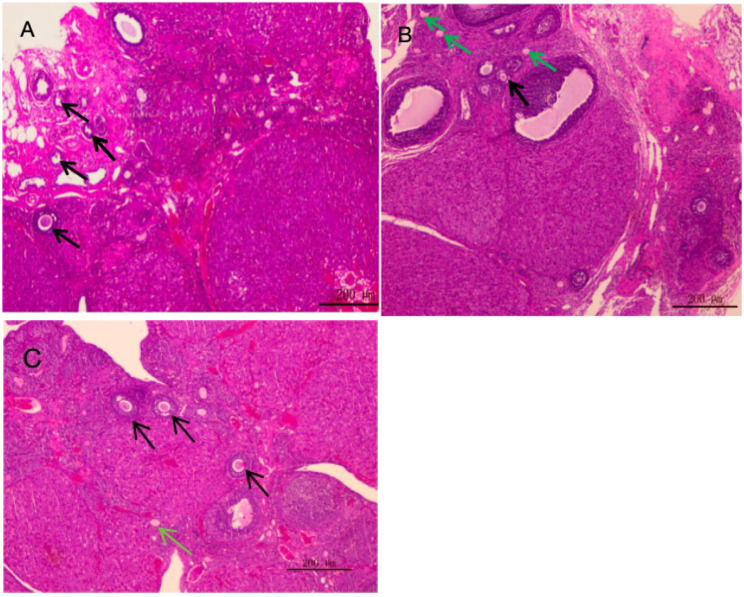
Development of follicles in ovarian tissue of rats in each group
Primordial follicles (black arrows) are numerous, small, and dormant, located in the ovarian cortex and derived from germline stem cells. Atretic follicles (green arrows) lose their round or oval shape, with irregularly shaped oocytes and disordered granulosa cell arrangement. A higher number of primordial follicles indicates enhanced ovarian reserve function, whereas a higher number of atretic follicles suggests its decline (Fig. 12).
Fig. 12.
HE staining of ovarian tissue in each groups. (A) HE staining of ovarian tissue in Ggroup A; (B) HE staining of ovarian tissue in Group B; (C) HE staining of ovarian tissue in Group C. Black arrow: Primordial follicle. Green arrow: Atretic follicle
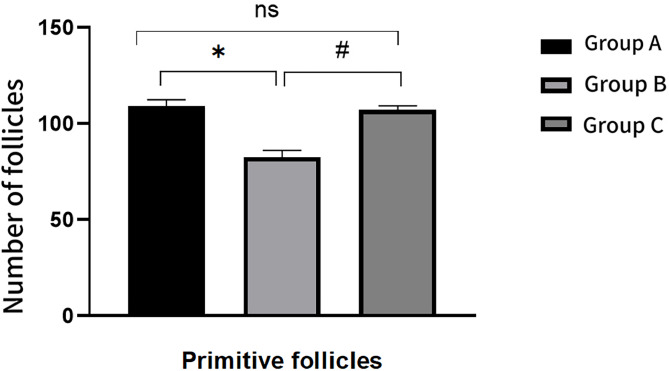
Following the AS101 intervention, the comparison of primordial and atretic follicles between Group C and Group A revealed no statistically significant differences (P > 0.05). Relative to Group A, Group B exhibited a decrease in the number of primordial follicles and an increase in the number of atretic follicles, both changes being statistically significant (P < 0.05). When compared to Group B, Group C demonstrated an increase in the number of normal follicles and a decrease in the number of atretic follicles, both changes being statistically significant (P < 0.05)(Figs. 13 and 14). For further details, please refer to Table 4.
Fig. 13.
Comparison of the number of primordial follicles in each group after intervention
Fig. 14.
Comparison of the number of atretic follicles in each group after intervention
Table 4.
Comparison of ovarian tissue in each group after intervention
| Group | Primordial follicles | Atretic follicles |
|---|---|---|
| Group A | 109.17 ± 3.312 | 4.17 ± 1.169 |
| Group B | 82.5 ± 3.619* | 15.50 ± 1.871* |
| Group C | 107.17 ± 2.137# | 6.00 ± 1.414# |
| F | 138.673 | 97.015 |
| P | 0.000 | 0.000 |
*: Comparison between group A and group B after intervention: P < 0.05 #:Comparison between group B and group C after intervention: P < 0.05
Discussion
While chemotherapy is a standard treatment for various malignant tumors, it also inflicts damage on the female reproductive system. The primary indicators of chemotherapy-induced ovarian damage in female patients include weight loss, loss of primary follicles, follicular dysfunction, and endocrine hormone disorders, all of which contribute to overall ovarian damage. Consequently, strategies to mitigate ovarian damage induced by chemotherapy drugs have emerged as a prominent topic in contemporary medical research.AS101 holds the distinction of being the first tellurium compound subjected to clinical efficacy testing. This compound functions as an effective immunomodulator in both in vitro and in vivo settings, boasting a range of potential therapeutic applications, including its antioxidant and anti-apoptotic effects [15]. Studies have established a link between oxidative stress and ovarian damage, with oxidative stress induced by chemotherapy factors also implicated in causing ovarian damage [16]. Hence, this experiment set up a doxorubicin-induced rat ovarian damage model and monitored a series of indicators to evaluate the impact of AS101 on ovarian function, aiming to provide a reference for ameliorating chemotherapy-induced ovarian damage.
Chemotherapy-induced ovarian damage manifests as a decline in ovarian reserve function, which is directly indicated by the number of growing follicles [17]. Some researchers propose that cyclophosphamide-induced damage to rat ovarian function leads to a significant decrease in the number of ovaries in the normal development stage and a significant increase in the number of atretic follicles [18]. AMH, a member of the transforming growth factor-β (TGF-β) superfamily, is primarily produced by granulosa cells as well as small antral and pre-antral follicles [19]. It serves as an indirect measure of ovarian reserve function. In this experiment, the addition of AS101 resulted in a significant increase in the number of original follicles in rat ovarian tissue, a significant decrease in the number of atretic follicles, and a significant increase in serum AMH levels in rats. This implies that AS101 has the potential to not only directly enhance ovarian reserve function but also indirectly safeguard it by elevating AMH levels.
FSH is a glycoprotein hormone, secreted by basophilic cells in the anterior pituitary, that primarily promotes the development and maturation of ovarian follicles [20]. E2 is a steroid hormone and a natural estrogen, predominantly derived from the follicles of female ovaries [21]. It plays a crucial role in maintaining female reproductive organs, promoting long bone growth, and other female characteristics. The cyclical changes observed in vaginal exfoliated cells can serve as an indirect indicator of ovarian reproductive function. The experiment’s results demonstrate that following the intraperitoneal injection of doxorubicin in rats, the estrous cycle is disrupted, with significant reductions in serum E2 and AMH levels, and a significant increase in FSH. Following the addition of AS101, the estrous cycle recovers, E2 and AMH levels significantly increase, and serum FSH significantly decreases. This suggests that AS101 may offer protection against doxorubicin-induced damage to ovarian endocrine function.
Studies have established a close relationship between chemotherapy-induced ovarian function damage and oxidative stress [22]. Excessive follicular atresia can expedite ovarian function damage, primarily due to the acceleration of follicular atresia under conditions of oxidative stress in the body. The level of ROS serves as a crucial indicator for assessing oxidative stress damage within the body [23]. MDA, a metabolic product of lipid peroxidation, exhibits a positive correlation with the state of oxidative stress in the body [24]. SOD is an antioxidant enzyme that catalyzes the disproportionation of superoxide anion free radicals to yield oxygen and water molecules. It prevents cell lipid peroxidation, plays a pivotal role in maintaining the balance between oxidation and antioxidation in the body, and its decreased activity serves as a significant marker of oxidative stress [25]. This study’s findings indicate that following doxorubicin injection, the levels of malondialdehyde and reactive oxygen species in rat ovarian tissue significantly increased, while the level of dismutase significantly decreased. This suggests that doxorubicin induces an imbalance in the oxidative stress state within ovarian tissue. Conversely, following the addition of AS101, the levels of malondialdehyde and reactive oxygen species significantly decreased, and the level of dismutase significantly increased. This indicates that AS101 effectively regulates the imbalance of the oxidation and antioxidant system within ovarian tissue. Vishwakarma P and colleagues discovered that, compared to the use of cyclophosphamide alone, the number of original follicles in female CD1 mice treated with AS101 plus cyclophosphamide increased, indicating its known protective effect. Additionally, an increase in SIRT1 was observed in the ovaries of mice treated with cyclophosphamide, revealing the presence of oxidative stress [26]. Similarly, an increase in mitochondrial SIRT3 is observed, while SOD and the mitochondrial biosynthesis activator PGC1-α decrease, indicating the onset of mitochondrial damage [27]. Administering AS101 can prevent the surge of SIRT1, suggesting that maintaining the balance of oxidation and reduction can aid the ovaries in offsetting the damage inflicted by cyclophosphamide [28]. The results demonstrate that AS101 can lower the level of reactive oxygen species in ovarian tissue and stabilize cellular homeostasis within ovarian tissue. Therefore, it is hypothesized that the mechanism through which AS101 protects against doxorubicin-induced ovarian function damage in rats is associated with its antioxidant effect.
Conclusion
In conclusion, this study demonstrates that AS101 protects against doxorubicin-induced ovarian dysfunction through multiple mechanisms. First, AS101 directly preserves ovarian reserve by increasing the number of primordial follicles, reducing atretic follicle loss, and enhancing AMH levels. Second, AS101 regulates endocrine homeostasis by increasing E2 and AMH levels while reducing FSH levels, thereby stabilizing ovarian endocrine function. More importantly, AS101 alleviates oxidative stress in ovarian tissue by enhancing SOD activity, reducing ROS and MDA accumulation, and restoring ovarian antioxidant capacity, thereby mitigating chemotherapy-induced oxidative damage.
Limitation and prospect
The active exploration and development of adjuvant drugs to safeguard against chemotherapy-induced ovarian function damage is a prominent area of research. Despite the many unknown factors associated with new drugs like AS101, including its dosage and potential side effects, the development of adjuvant drugs to protect ovarian function offers a solid theoretical foundation and a novel approach for future clinical practice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Y.Z., Y.Z. and Y.L.: Conceptualization and Methodology; Y.Z. and M.G.: Data curation, Formal Analysis and Writing- Original draft preparation; Y.Z., Y.Z. and G.L.: Validation and Investigation; L.Y. and J.H.: Supervision; Y.D. and Y.Z.: Data Curation and Resources; J.C. and Z.C.:Writing- Reviewing and Project administration.
Funding
Hebei Province Government Funded Clinical Medical Excellence Project (Leader), 361007; Science and Technology Project of Changzhou Health Commission, ZD202314; Top Talent of Changzhou “The 14th Five-Year Plan” High-Level Health Talents Training Project, 2022CZBJ074; The Maternal and Child Health Research Project of Jiangsu Province, F202138.
Data availability
The data which was used and/or analyzed during this study is available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Yumeng Zhai and Yaoyang Zhang are co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiming Chen, Email: cjming@126.com.
Zhihui Cai, Email: czh20106152@163.com.
References
- 1.Han X, Wang Z, Huang D, Deng K, Wang Q, Li C, Zhu J. Analysis of the disease burden trend of malignant tumors of the female reproductive system in China from 2006 to 2020. BMC Womens Health. 2022;22(1):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Liu Q, Chang M, Pan Y, Yahaya BH, Liu Y, Lin J. Chemotherapy impairs ovarian function through excessive ROS-induced ferroptosis. Cell Death Dis. 2023;14(5):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Wu T, Tang X, Wen J, Zhang Y, Zhang J, Wang S. Increased cellular senescence in doxorubicin-induced murine ovarian injury: effect of senolytics. Geroscience. 2023;45(3):1775–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi D, Chen B, Tao H, et al. The risk of depressive and anxiety symptoms in women with premature ovarian insufficiency: a systematic review and meta-analysis. Arch Womens Ment Health. 2023;26:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Zanden SY, Qiao X, Neefjes J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021;288(21):6095–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown PA, Shah B, Advani A et al. Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(9):1079–1109. 10.6004/jnccn.2021.0042. PMID: 34551384. [DOI] [PubMed]
- 8.Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018;25(3):261–9. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Liu M, Johnson SB, Yuan G, Arriba AK, Zubizarreta ME, Chatterjee S, Nagarkatti M, Nagarkatti P, Xiao S. Doxorubicin obliterates mouse ovarian reserve through both primordial follicle Atresia and overactivation. Toxicol Appl Pharmacol. 2019;381:114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–65. [DOI] [PubMed] [Google Scholar]
- 11.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and burnout; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):185ra62. [DOI] [PubMed] [Google Scholar]
- 12.Vallet N, Boissel N, Elefant E, Chevillon F, Pasquer H, Calvo C, Dhedin N, Poirot C. Can some Anticancer Treatments Preserve Ovarian Reserve? Oncologist. 2021;26(6):492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kropp J, Roti Roti EC, Ringelstetter A, Khatib H, Abbott DH, Salih SM. Dexrazoxane diminishes Doxorubicin-Induced acute ovarian damage and preserves ovarian function and fecundity in mice. PLoS ONE. 2015;10(11):e0142588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oktay K. Oktem o.ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience [J].Fertil steril,2010,93 (3):762–8. [DOI] [PubMed]
- 15.Naor Y, Hayun M, Sredni B, Don J. Multiple signal transduction pathways are involved in G2/M growth arrest and apoptosis induced by the Immunomodulator AS101 in multiple myeloma. Leuk Lymphoma. 2013;54(1):160–6. [DOI] [PubMed] [Google Scholar]
- 16.Park SJ, Kim JH, Lee DG, et al. Peroxiredoxin 2 deficiency accelerates age-related ovarian failure through the reactive oxygen species-mediated JNK pathway in mice[J]. Free Radic Biol Med. 2018;123(1):96–106. [DOI] [PubMed] [Google Scholar]
- 17.Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update. 2019;25(6):673–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood NMA. Protective effects of Azilsartan against cyclophosphamide-induced ovarian toxicity in rats model. Toxicol Res (Camb). 2024;13(2):tfae027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moolhuijsen LME, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conforti A, Vaiarelli A, Cimadomo D, Bagnulo F, Peluso S, Carbone L, Di Rella F, De Placido G, Ubaldi FM, Huhtaniemi I, Alviggi C. Pharmacogenetics of FSH action in the female. Front Endocrinol (Lausanne). 2019;10:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueck AO, Römer T. Choice of progestogen for endometrial protection in combination with transdermal estradiol in menopausal women. Horm Mol Biol Clin Investig. 2018;37(2). [DOI] [PubMed]
- 22.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum reprod update. 2012 Sep-Oct;18(5):525–35. [DOI] [PubMed]
- 23.Zhang W. Wu fuju,linoleic acid induces human ovarian granulosa cell inflammation and apoptosis through the ER-FOXO1-ROS-NFκB pathway. [J] Sci Rep. 2024;14:6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordiano R, Di Gioacchino M, Mangifesta R, Panzera C, Gangemi S, Minciullo PL. Malondialdehyde as a potential oxidative stress marker for Allergy-Oriented diseases: an update. Molecules. 2023;28(16):5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng M, Liu Y, Zhang G, Yang Z, Xu W, Chen Q. The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxid (Basel). 2023;12(9):1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vishwakarma P, Parmar N, Chandrakar P, Sharma T. Et al.ammonium Trichloro [1,2-ethanediolato-O,O’]-tellurate cures experimental visceral leishmaniasis by redox modulation of leishmania donovani trypanothione reductase and inhibiting host integrin linked PI3K/Akt pathway. Cell Mol Life Sci. 2018;75(3):563–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danoch H, Kalechman Y, Albeck M, Longo DL, Sredni B. Sensitizing B- and T- cell lymphoma cells to Paclitaxel/Abraxane-Induced death by AS101 via Inhibition of the VLA-4-IL10-Survivin Axis. Mol Cancer Res. 2015;13(3):411–22. [DOI] [PubMed] [Google Scholar]
- 28.Altintas R, Ciftci O, Aydin M, Akpolat N, Oguz F, Beytur A. Quercetin prevents docetaxel-induced testicular damage in rats. Andrologia. 2015;47(3):248–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data which was used and/or analyzed during this study is available from the corresponding author on reasonable request.