Abstract
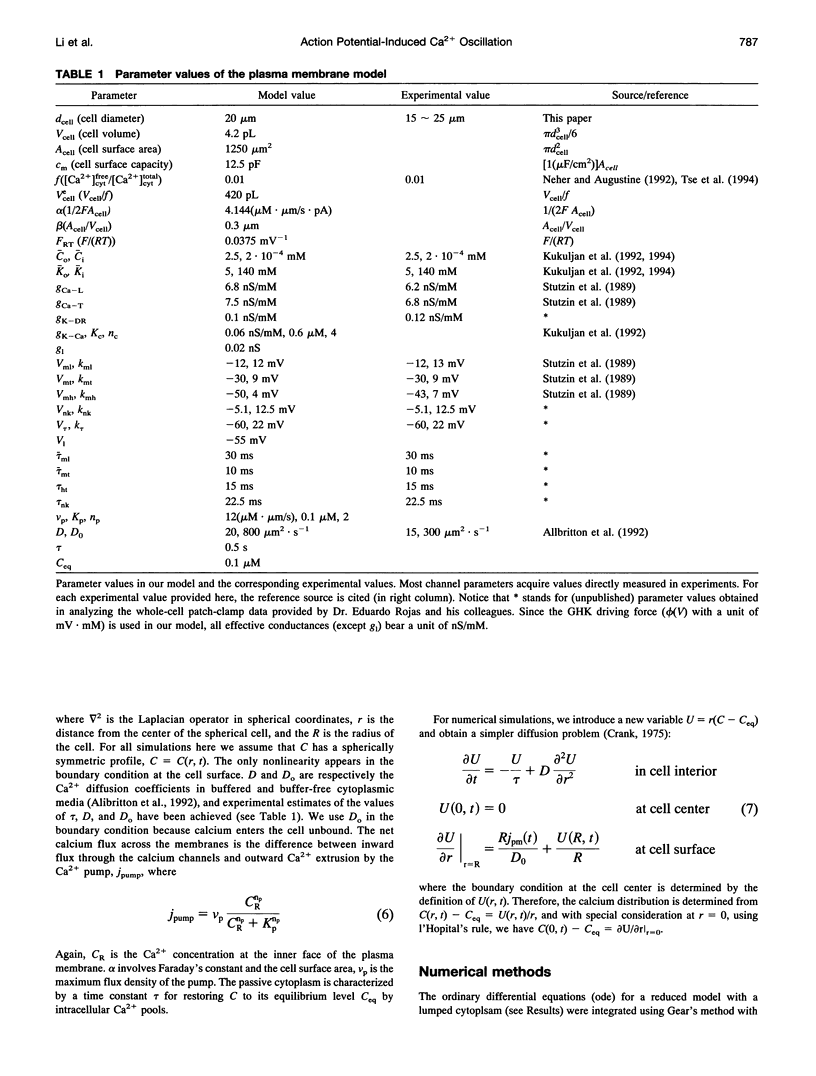
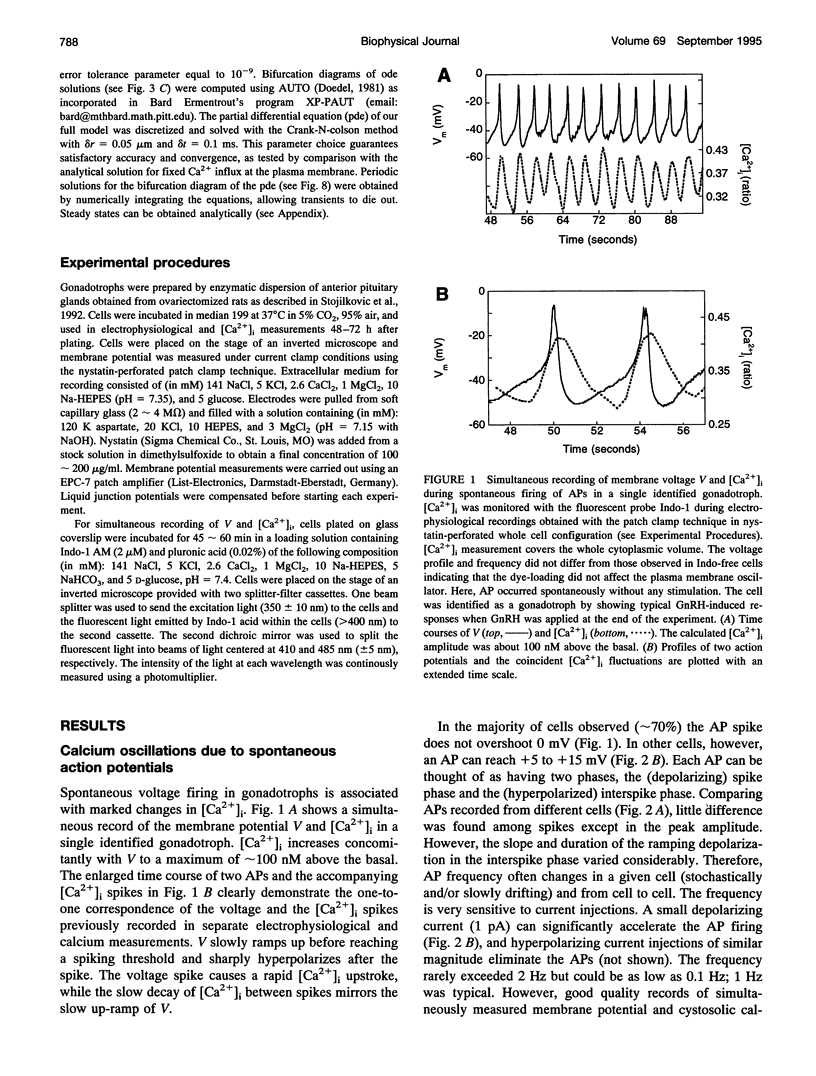
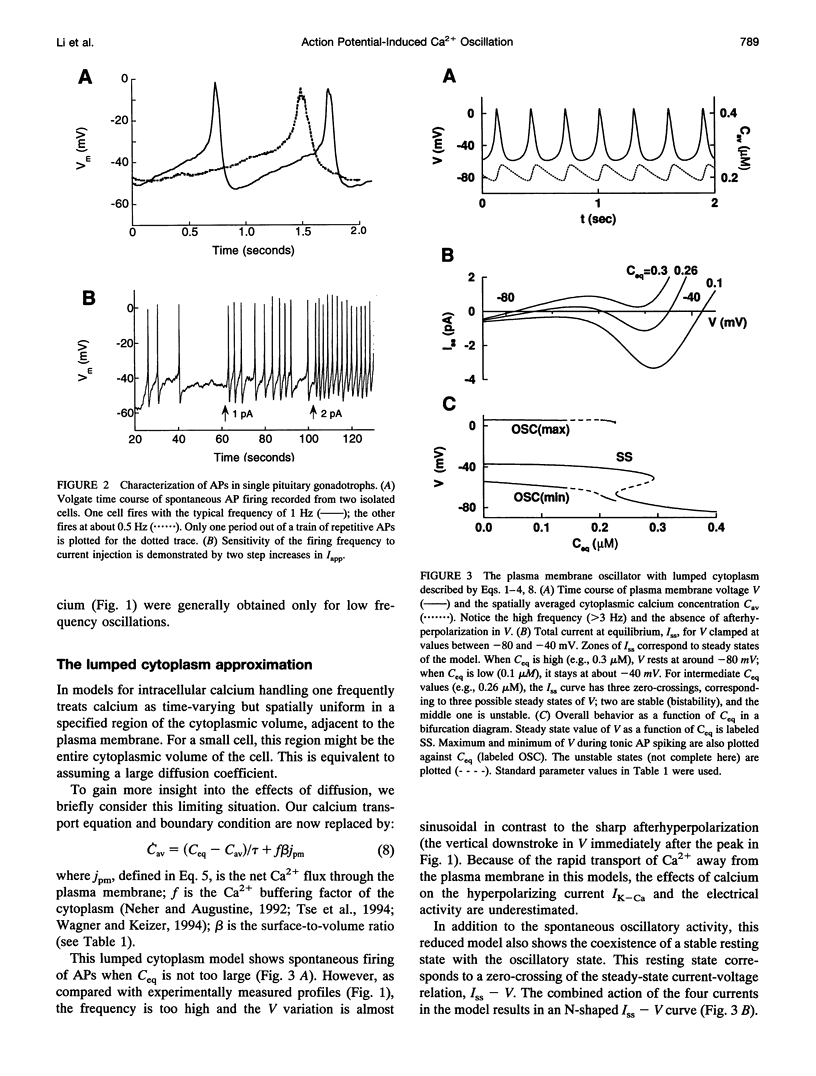
Single pituitary cells often fire spontaneous action potentials (APs), which are believed to underlie spiking fluctuations in cytosolic calcium concentration ([Ca2+]i). To address how these basal [Ca2+]i fluctuations depend on changes in plasma membrane voltage (V), simultaneous measurements of V and [Ca2+]i were performed in rat pituitary gonadotrophs. The data show that each [Ca2+]i spike is produced by the Ca2+ entry during a single AP. Using these and previously obtained patch-clamp data, we develop a quantitative mathematical model of this plasma membrane oscillator and the accompanying spatiotemporal [Ca2+]i oscillations. The model demonstrates that AP-induced [Ca2+]i spiking is prominent only in a thin shell layer neighboring the cell surface. This localized [Ca2+]i spike transiently activates the Ca2(+)- dependent K+ current resulting in a sharp afterhyperpolarization following each voltage spike. In accord with experimental observations, the model shows that the frequency and amplitude of the voltage spikes are highly sensitive to current injection and to the blocking of the Ca(2+)-sensitive current. Computations also predict that leaving the membrane channels intact, the firing rate can be modified by changing the Ca2+ handling parameters: the Ca2+ diffusion rate, the Ca2+ buffering capacity, and the plasma membrane Ca2+ pump rate. Finally, the model suggests reasons that spontaneous APs were seen in some gonadotrophs but not in others. This model provides a basis for further exploring how plasma membrane electrical activity is involved in the control of cytosolic calcium level in unstimulated as well as agonist-stimulated gonadotrophs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Wong B. S., Sabol S. L., Busis N., Jackson M. B., Weight F. F. Action potentials and membrane ion channels in clonal anterior pituitary cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2086–2090. doi: 10.1073/pnas.80.7.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Biales B., Dichter M. A., Tischler A. Sodium and calcium action potential in pituitary cells. Nature. 1977 May 12;267(5607):172–174. doi: 10.1038/267172a0. [DOI] [PubMed] [Google Scholar]
- Bosma M. M., Hille B. Electrophysiological properties of a cell line of the gonadotrope lineage. Endocrinology. 1992 Jun;130(6):3411–3420. doi: 10.1210/endo.130.6.1317783. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérineau N., Corcuff J. B., Tabarin A., Mollard P. Spontaneous and corticotropin-releasing factor-induced cytosolic calcium transients in corticotrophs. Endocrinology. 1991 Jul;129(1):409–420. doi: 10.1210/endo-129-1-409. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Mandell G. L., Sullivan J. A., Sinha Y. N., Leong D. A. Spontaneous oscillations of intracellular calcium and growth hormone secretion. J Biol Chem. 1988 Jul 15;263(20):9682–9685. [PubMed] [Google Scholar]
- Iida T., Stojilković S. S., Izumi S., Catt K. J. Spontaneous and agonist-induced calcium oscillations in pituitary gonadotrophs. Mol Endocrinol. 1991 Jul;5(7):949–958. doi: 10.1210/mend-5-7-949. [DOI] [PubMed] [Google Scholar]
- Ingram C. D., Bicknell R. J., Mason W. T. Intracellular recordings from bovine anterior pituitary cells: modulation of spontaneous activity by regulators of prolactin secretion. Endocrinology. 1986 Dec;119(6):2508–2518. doi: 10.1210/endo-119-6-2508. [DOI] [PubMed] [Google Scholar]
- Keizer J., De Young G. Effect of voltage-gated plasma membrane Ca2+ fluxes on IP3-linked Ca2+ oscillations. Cell Calcium. 1993 May;14(5):397–410. doi: 10.1016/0143-4160(93)90044-7. [DOI] [PubMed] [Google Scholar]
- Keja J. A., Kits K. S. Single-channel properties of high- and low-voltage-activated calcium channels in rat pituitary melanotropic cells. J Neurophysiol. 1994 Mar;71(3):840–855. doi: 10.1152/jn.1994.71.3.840. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Bolden A., Horn R. Control of action potentials and Ca2+ influx by the Ca(2+)-dependent chloride current in mouse pituitary cells. J Physiol. 1991 Aug;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuljan M., Rojas E., Catt K. J., Stojilkovic S. S. Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J Biol Chem. 1994 Feb 18;269(7):4860–4865. [PubMed] [Google Scholar]
- Kukuljan M., Stojilković S. S., Rojas E., Catt K. J. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett. 1992 Apr 13;301(1):19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Goodman M. B., St John P. A., Barker J. L. Calcium currents and fura-2 signals in fluorescence-activated cell sorted lactotrophs and somatotrophs of rat anterior pituitary. Endocrinology. 1988 Jul;123(1):611–621. doi: 10.1210/endo-123-1-611. [DOI] [PubMed] [Google Scholar]
- Li Y. X., Rinzel J., Keizer J., Stojilković S. S. Calcium oscillations in pituitary gonadotrophs: comparison of experiment and theory. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):58–62. doi: 10.1073/pnas.91.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Childs G. V., Brown A. M. Voltage-dependent calcium currents in rat gonadotropes separated by centrifugal elutriation. Am J Physiol. 1990 Apr;258(4 Pt 1):E589–E596. doi: 10.1152/ajpendo.1990.258.4.E589. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Sikdar S. K. Characterization of voltage-gated sodium channels in ovine gonadotrophs: relationship to hormone secretion. J Physiol. 1988 May;399:493–517. doi: 10.1113/jphysiol.1988.sp017093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. J., Hinkle P. M. Characteristics of the Ca2+ spike and oscillations induced by different doses of thyrotropin-releasing hormone (TRH) in individual pituitary cells and nonexcitable cells transfected with TRH receptor complementary deoxyribonucleic acid. Endocrinology. 1994 Sep;135(3):1084–1092. doi: 10.1210/endo.135.3.8070350. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electrophysiology of excitable endocrine cells. Physiol Rev. 1986 Oct;66(4):887–952. doi: 10.1152/physrev.1986.66.4.887. [DOI] [PubMed] [Google Scholar]
- Rawlings S. R., Berry D. J., Leong D. A. Evidence for localized calcium mobilization and influx in single rat gonadotropes. J Biol Chem. 1991 Nov 25;266(33):22755–22760. [PubMed] [Google Scholar]
- Roberts W. M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994 May;14(5 Pt 2):3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Simasko S. M., Weiland G. A., Oswald R. E. Pharmacological characterization of two calcium currents in GH3 cells. Am J Physiol. 1988 Mar;254(3 Pt 1):E328–E336. doi: 10.1152/ajpendo.1988.254.3.E328. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Catt K. J. Calcium oscillations in anterior pituitary cells. Endocr Rev. 1992 May;13(2):256–280. doi: 10.1210/edrv-13-2-256. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Izumi S., Catt K. J. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem. 1988 Sep 15;263(26):13054–13061. [PubMed] [Google Scholar]
- Stojilković S. S., Kukuljan M., Iida T., Rojas E., Catt K. J. Integration of cytoplasmic calcium and membrane potential oscillations maintains calcium signaling in pituitary gonadotrophs. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4081–4085. doi: 10.1073/pnas.89.9.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzin A., Stojilković S. S., Catt K. J., Rojas E. Characteristics of two types of calcium channels in rat pituitary gonadotrophs. Am J Physiol. 1989 Nov;257(5 Pt 1):C865–C874. doi: 10.1152/ajpcell.1989.257.5.C865. [DOI] [PubMed] [Google Scholar]
- Thorner M. O., Holl R. W., Leong D. A. The somatotrope: an endocrine cell with functional calcium transients. J Exp Biol. 1988 Sep;139:169–179. doi: 10.1242/jeb.139.1.169. [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992 Jan 24;255(5043):462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. Role of voltage-gated Na+ and Ca2+ channels in gonadotropin-releasing hormone-induced membrane potential changes in identified rat gonadotropes. Endocrinology. 1993 Apr;132(4):1475–1481. doi: 10.1210/endo.132.4.8384989. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn J. A., Louiset E., Vaudry H., Cazin L. Dopamine-induced inhibition of action potentials in cultured frog pituitary melanotrophs is mediated through activation of potassium channels and inhibition of calcium and sodium channels. Neuroscience. 1991;42(1):29–39. doi: 10.1016/0306-4522(91)90147-g. [DOI] [PubMed] [Google Scholar]
- Wagner J., Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys J. 1994 Jul;67(1):447–456. doi: 10.1016/S0006-3495(94)80500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]