Abstract
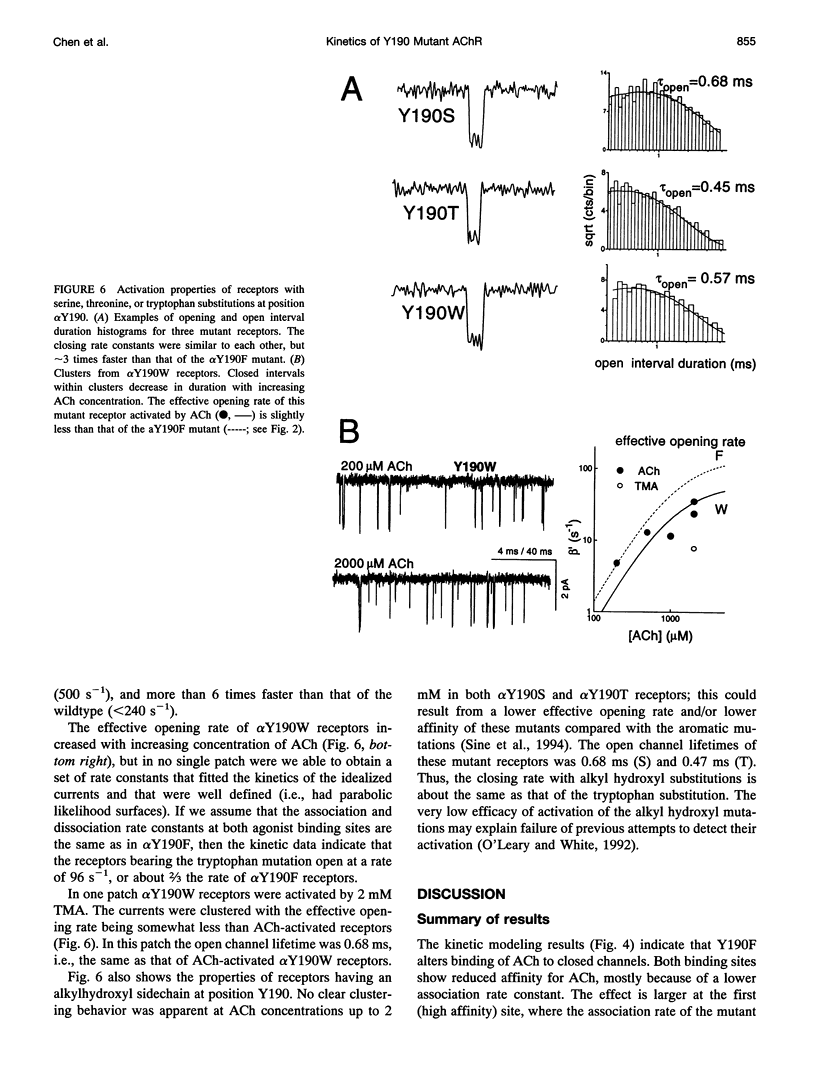
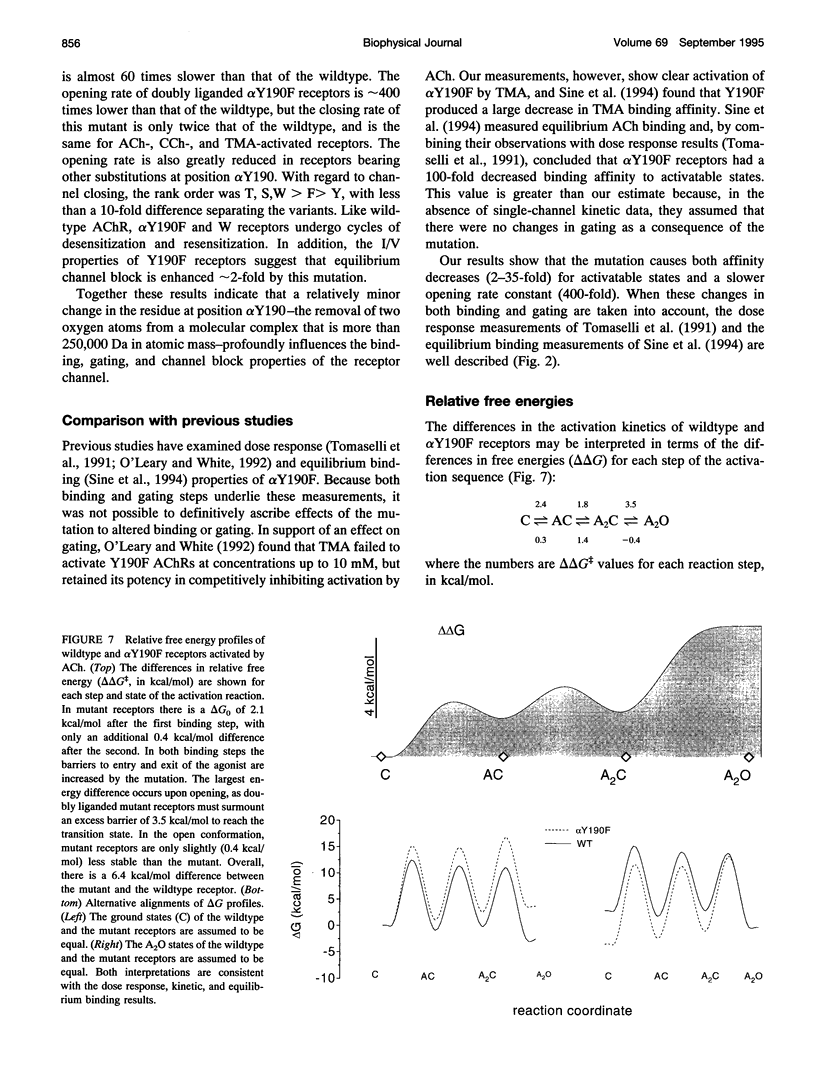
Affinity labeling and mutagenesis studies have demonstrated that the conserved tyrosine Y190 of the acetylcholine receptor (AChR) alpha-subunit is a key determinant of the agonist binding site. Here we describe the binding and gating kinetics of embryonic mouse AChRs with mutations at Y190. In Y190F the dissociation constant for ACh binding to closed channels was reduced approximately 35-fold at the first binding site and only approximately 2-fold at the second site. At both binding sites the association and dissociation rate constants were decreased by the mutation. Compared with wildtype AChRs, doubly-liganded alpha Y190F receptors open 400 times more slowly but close only 2 times more rapidly. Considering the overall activation reaction (vacant-closed to fully occupied-open), there is an increase of approximately 6.4 kcal/mol caused by the Y-to-F mutation, of which at least 2.1 and 0.3 kcal/mol comes from altered agonist binding to the first and second binding sites, respectively. The closing rate constant of alpha Y190F receptors was the same with ACh, carbamoylcholine, or tetramethylammonium as the agonist. This rate constant was approximately 3 times faster in ACh-activated S, W, and T mutants. The equilibrium dissociation constant for channel block by ACh was approximately 2-fold lower in alpha Y190F receptors compared with in wildtype receptors, suggesting that there are changes in the pore region of the receptor as a consequence of the mutation. The activation reaction is discussed with regard to energy provided by agonist-receptor binding contacts, and by the intrinsic folding energy of the receptor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball F. G., Sansom M. S. Ion-channel gating mechanisms: model identification and parameter estimation from single channel recordings. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):385–416. doi: 10.1098/rspb.1989.0029. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Galzi J. L., Devillers-Thiéry A., Bertrand D. The functional architecture of the acetylcholine nicotinic receptor explored by affinity labelling and site-directed mutagenesis. Q Rev Biophys. 1992 Nov;25(4):395–432. doi: 10.1017/s0033583500004352. [DOI] [PubMed] [Google Scholar]
- Czajkowski C., Kaufmann C., Karlin A. Negatively charged amino acid residues in the nicotinic receptor delta subunit that contribute to the binding of acetylcholine. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6285–6289. doi: 10.1073/pnas.90.13.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D. A., Stauffer D. A. Acetylcholine binding by a synthetic receptor: implications for biological recognition. Science. 1990 Dec 14;250(4987):1558–1560. doi: 10.1126/science.2274786. [DOI] [PubMed] [Google Scholar]
- Gao J., Chou L. W., Auerbach A. The nature of cation-pi binding: interactions between tetramethylammonium ion and benzene in aqueous solution. Biophys J. 1993 Jul;65(1):43–47. doi: 10.1016/S0006-3495(93)81031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao P. N., Karlin A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J Biol Chem. 1986 Jun 25;261(18):8085–8088. [PubMed] [Google Scholar]
- O'Leary M. E., White M. M. Mutational analysis of ligand-induced activation of the Torpedo acetylcholine receptor. J Biol Chem. 1992 Apr 25;267(12):8360–8365. [PubMed] [Google Scholar]
- Pedersen S. E., Cohen J. B. d-Tubocurarine binding sites are located at alpha-gamma and alpha-delta subunit interfaces of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2785–2789. doi: 10.1073/pnas.87.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Sine S. M. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine curare selectivity. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9436–9440. doi: 10.1073/pnas.90.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Quiram P., Papanikolaou F., Kreienkamp H. J., Taylor P. Conserved tyrosines in the alpha subunit of the nicotinic acetylcholine receptor stabilize quaternary ammonium groups of agonists and curariform antagonists. J Biol Chem. 1994 Mar 25;269(12):8808–8816. [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Tomaselli G. F., McLaughlin J. T., Jurman M. E., Hawrot E., Yellen G. Mutations affecting agonist sensitivity of the nicotinic acetylcholine receptor. Biophys J. 1991 Sep;60(3):721–727. doi: 10.1016/S0006-3495(91)82102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995 Jan 5;373(6509):37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Valenzuela C. F., Weign P., Yguerabide J., Johnson D. A. Transverse distance between the membrane and the agonist binding sites on the Torpedo acetylcholine receptor: a fluorescence study. Biophys J. 1994 Mar;66(3 Pt 1):674–682. doi: 10.1016/s0006-3495(94)80841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]