Abstract
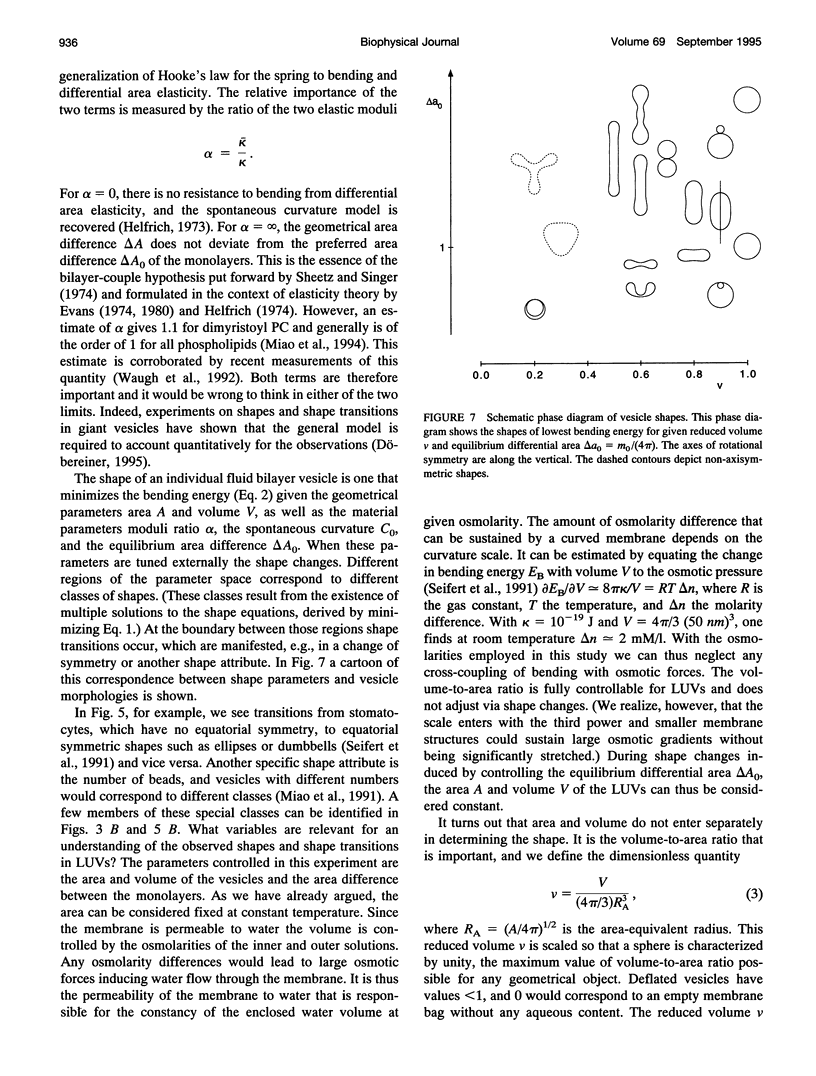
The morphological consequences of differences in the monolayer surface areas of large unilamellar vesicles (LUVs) have been examined employing cryoelectron microscopy techniques. Surface area was varied by inducing net transbilayer transport of dioleoylphosphatidylglycerol (DOPG) in dioleoylphosphatidylcholine (DOPC):DOPG (9:1, mol:mol) LUVs in response to transmembrane pH gradients. It is shown that when DOPG is transported from the inner to the outer monolayer, initially invaginated LUVs are transformed to long narrow tubular structures, or spherical structures with one or more protrusions. Tubular structures are also seen in response to outward DOPG transport in DOPC:DOPG:Chol (6:1:3, mol:mol:mol) LUV systems, and when lyso-PC is allowed to partition into the exterior monolayer of DOPC:DOPG (9:1, mol:mol) LUVs in the absence of DOPG transport. Conversely, when the inner monolayer area is expanded by the transport of DOPG from the outer monolayer to the inner monolayer of non-invaginated LUVs, a reversion to invaginated structures is observed. The morphological changes are well described by an elastic bending theory of the bilayer. Identification of the difference in relaxed monolayer areas and of the volume-to-area ratio of the LUVs as the shape-determining factors allows a quantitative classification of the observed morphologies. The morphology seen in LUVs supports the possibility that factors leading to differences in monolayer surface areas could play important roles in intracellular membrane transport processes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Canham P. B. The minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cell. J Theor Biol. 1970 Jan;26(1):61–81. doi: 10.1016/s0022-5193(70)80032-7. [DOI] [PubMed] [Google Scholar]
- Cooper M. S., Cornell-Bell A. H., Chernjavsky A., Dani J. W., Smith S. J. Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-golgi elements into a reticulum. Cell. 1990 Apr 6;61(1):135–145. doi: 10.1016/0092-8674(90)90221-y. [DOI] [PubMed] [Google Scholar]
- Dabora S. L., Sheetz M. P. The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988 Jul 1;54(1):27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Devaux P. F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991 Feb 5;30(5):1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Döbereiner H. G., Käs J., Noppl D., Sprenger I., Sackmann E. Budding and fission of vesicles. Biophys J. 1993 Oct;65(4):1396–1403. doi: 10.1016/S0006-3495(93)81203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman S. J., Hope M. J., Cullis P. R. Transbilayer transport of phosphatidic acid in response to transmembrane pH gradients. Biochemistry. 1991 Feb 19;30(7):1740–1745. doi: 10.1021/bi00221a002. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Bending resistance and chemically induced moments in membrane bilayers. Biophys J. 1974 Dec;14(12):923–931. doi: 10.1016/S0006-3495(74)85959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Minimum energy analysis of membrane deformation applied to pipet aspiration and surface adhesion of red blood cells. Biophys J. 1980 May;30(2):265–284. doi: 10.1016/S0006-3495(80)85093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E., Devaux P. F. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys J. 1992 Feb;61(2):347–357. doi: 10.1016/S0006-3495(92)81841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C., Gruler H., Sackmann E. On domain structure and local curvature in lipid bilayers and biological membranes. Z Naturforsch C. 1977 Jul-Aug;32(7-8):581–596. doi: 10.1515/znc-1977-7-817. [DOI] [PubMed] [Google Scholar]
- Heinrich V, V, Svetina S, Zeks B. Nonaxisymmetric vesicle shapes in a generalized bilayer-couple model and the transition between oblate and prolate axisymmetric shapes. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993 Oct;48(4):3112–3123. doi: 10.1103/physreve.48.3112. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Blocked lipid exchange in bilayers and its possible influence on the shape of vesicles. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):510–515. doi: 10.1515/znc-1974-9-1010. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Redelmeier T. E., Wong K. F., Rodrigueza W., Cullis P. R. Phospholipid asymmetry in large unilamellar vesicles induced by transmembrane pH gradients. Biochemistry. 1989 May 16;28(10):4181–4187. doi: 10.1021/bi00436a009. [DOI] [PubMed] [Google Scholar]
- Jin A. J., Nossal R. Topological mechanisms involved in the formation of clathrin-coated vesicles. Biophys J. 1993 Oct;65(4):1523–1537. doi: 10.1016/S0006-3495(93)81189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülicher F, Lipowsky R. Domain-induced budding of vesicles. Phys Rev Lett. 1993 May 10;70(19):2964–2967. doi: 10.1103/PhysRevLett.70.2964. [DOI] [PubMed] [Google Scholar]
- Käs J., Sackmann E. Shape transitions and shape stability of giant phospholipid vesicles in pure water induced by area-to-volume changes. Biophys J. 1991 Oct;60(4):825–844. doi: 10.1016/S0006-3495(91)82117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Chen L. B. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988 Jul 1;54(1):37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- Lipowsky R. The conformation of membranes. Nature. 1991 Feb 7;349(6309):475–481. doi: 10.1038/349475a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Madden T. D., Tilcock C. P., Wong K., Cullis P. R. Spontaneous vesiculation of large multilamellar vesicles composed of saturated phosphatidylcholine and phosphatidylglycerol mixtures. Biochemistry. 1988 Nov 29;27(24):8724–8730. doi: 10.1021/bi00424a006. [DOI] [PubMed] [Google Scholar]
- Markin V. S. Lateral organization of membranes and cell shapes. Biophys J. 1981 Oct;36(1):1–19. doi: 10.1016/S0006-3495(81)84713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R., Janoff A. S. Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985 Jul 11;817(1):193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- Miao L, Fourcade B, Rao M, Wortis M, Zia RK. Equilibrium budding and vesiculation in the curvature model of fluid lipid vesicles. Phys Rev A. 1991 Jun 15;43(12):6843–6856. doi: 10.1103/physreva.43.6843. [DOI] [PubMed] [Google Scholar]
- Miao L, Seifert U, Wortis M, Döbereiner HG. Budding transitions of fluid-bilayer vesicles: The effect of area-difference elasticity. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994 Jun;49(6):5389–5407. doi: 10.1103/physreve.49.5389. [DOI] [PubMed] [Google Scholar]
- Oster G. F., Cheng L. Y., Moore H. P., Perelson A. S. Vesicle formation in the Golgi apparatus. J Theor Biol. 1989 Dec 19;141(4):463–504. doi: 10.1016/s0022-5193(89)80231-0. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
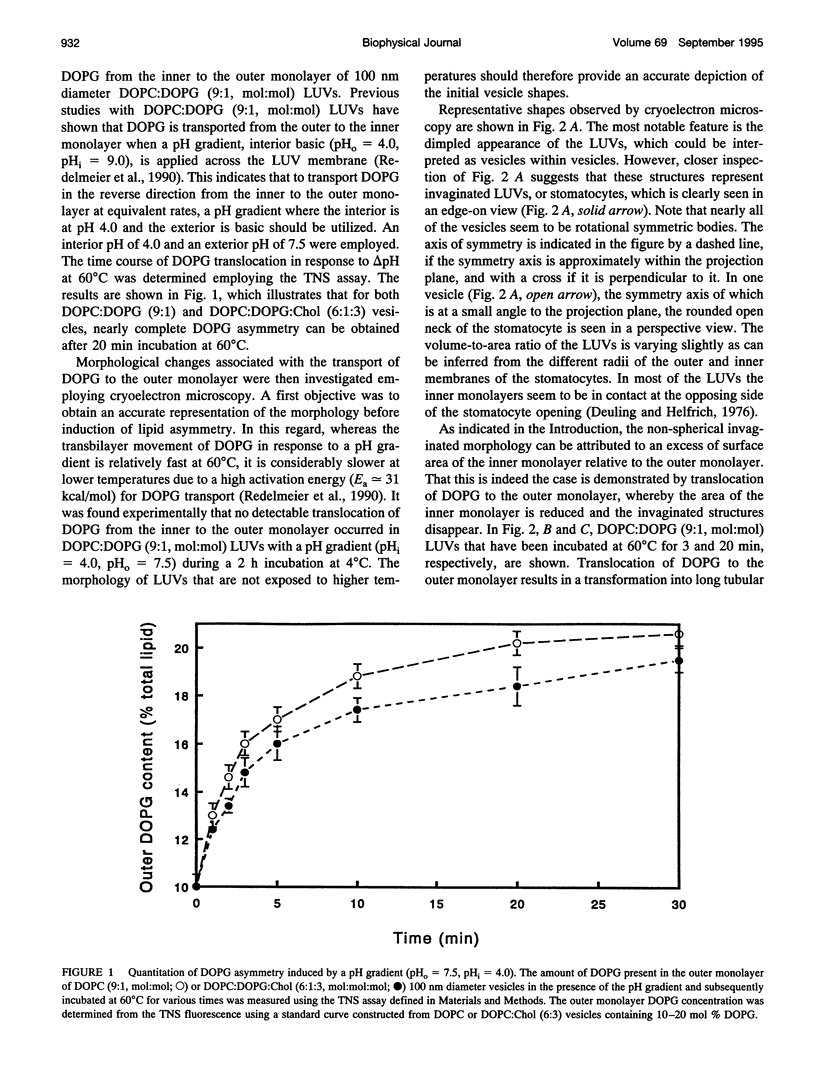
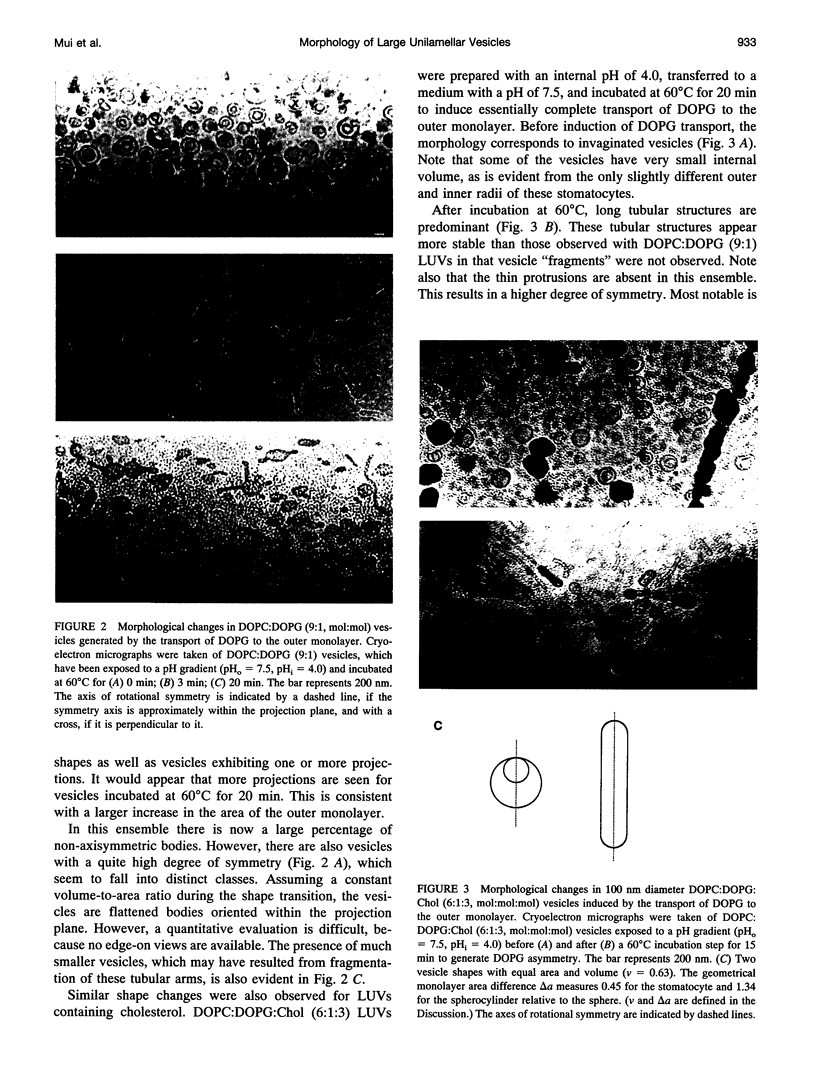
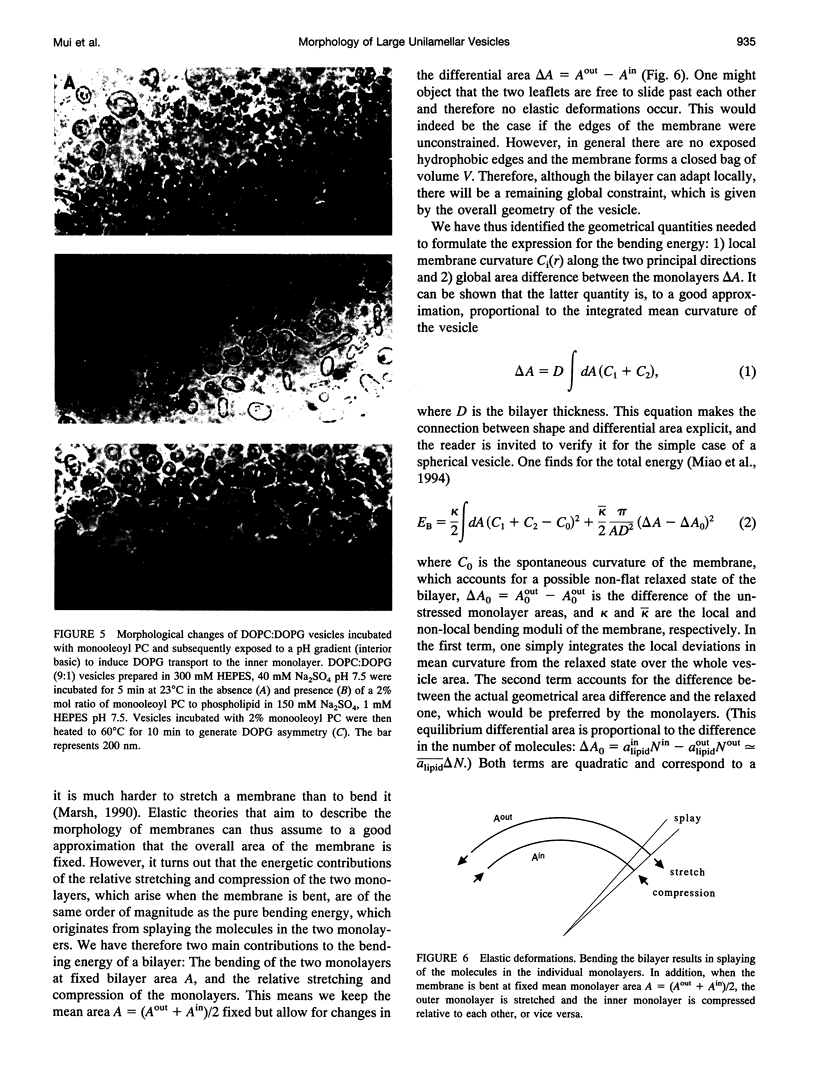
- Redelmeier T. E., Hope M. J., Cullis P. R. On the mechanism of transbilayer transport of phosphatidylglycerol in response to transmembrane pH gradients. Biochemistry. 1990 Mar 27;29(12):3046–3053. doi: 10.1021/bi00464a022. [DOI] [PubMed] [Google Scholar]
- Sackmann E., Duwe H. P., Engelhardt H. Membrane bending elasticity and its role for shape fluctuations and shape transformations of cells and vesicles. Faraday Discuss Chem Soc. 1986;(81):281–290. doi: 10.1039/dc9868100281. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Jefferson J. R., Kier A. B., Knittel J., Scallen T. J., Wood W. G., Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc Soc Exp Biol Med. 1991 Mar;196(3):235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- Seifert U, Berndl K, Lipowsky R. Shape transformations of vesicles: Phase diagram for spontaneous- curvature and bilayer-coupling models. Phys Rev A. 1991 Jul 15;44(2):1182–1202. doi: 10.1103/physreva.44.1182. [DOI] [PubMed] [Google Scholar]
- Seifert U. Curvature-induced lateral phase segregation in two-component vesicles. Phys Rev Lett. 1993 Mar 1;70(9):1335–1338. doi: 10.1103/PhysRevLett.70.1335. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetina S., Zeks B. Membrane bending energy and shape determination of phospholipid vesicles and red blood cells. Eur Biophys J. 1989;17(2):101–111. doi: 10.1007/BF00257107. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Song J., Svetina S., Zeks B. Local and nonlocal curvature elasticity in bilayer membranes by tether formation from lecithin vesicles. Biophys J. 1992 Apr;61(4):974–982. doi: 10.1016/S0006-3495(92)81904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]