Abstract
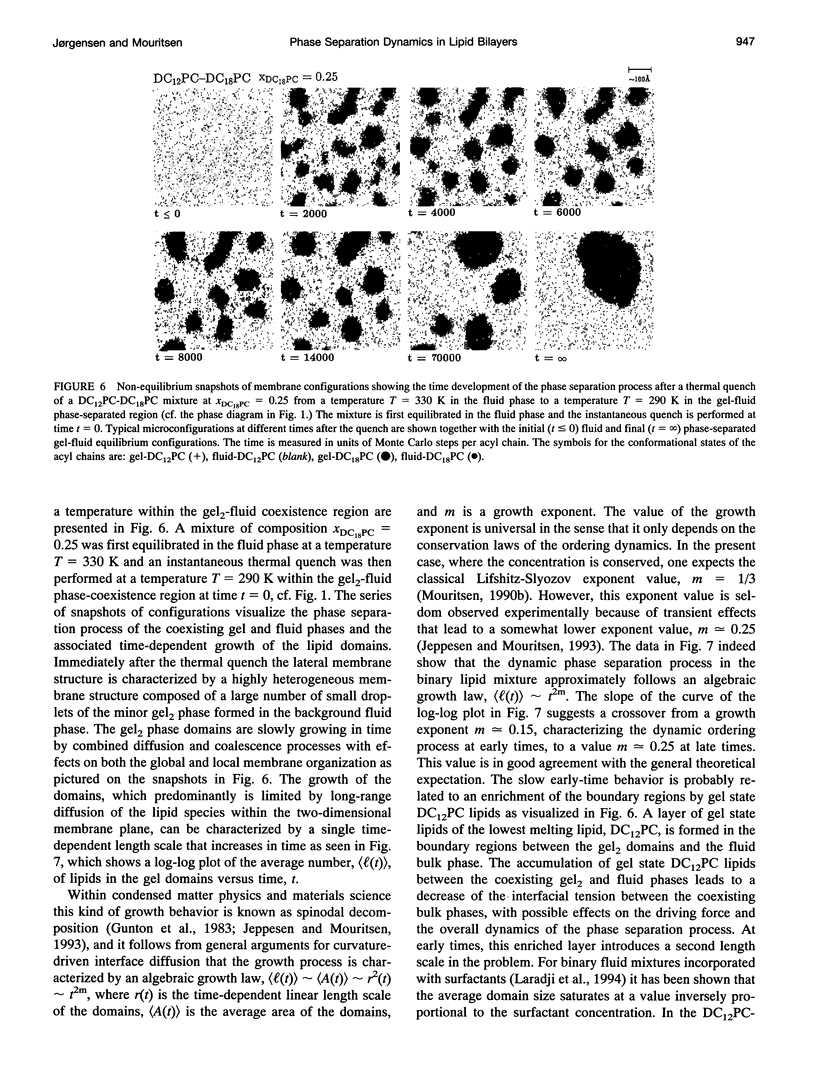
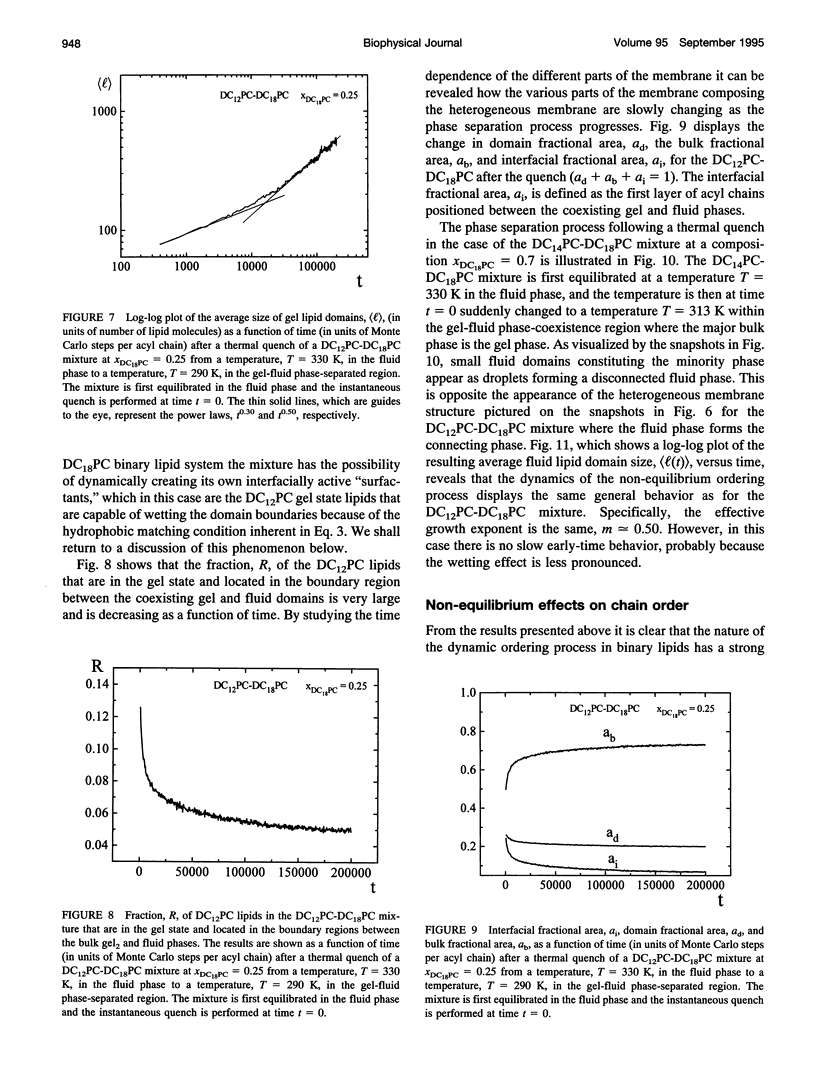
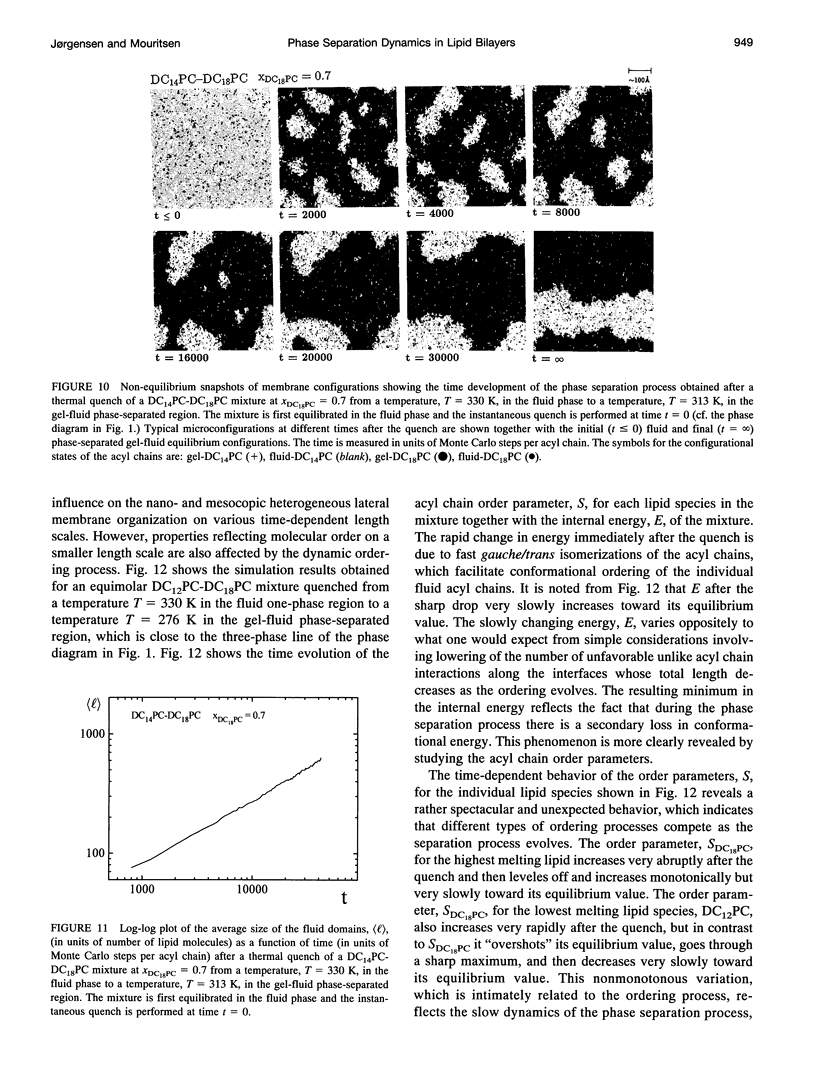
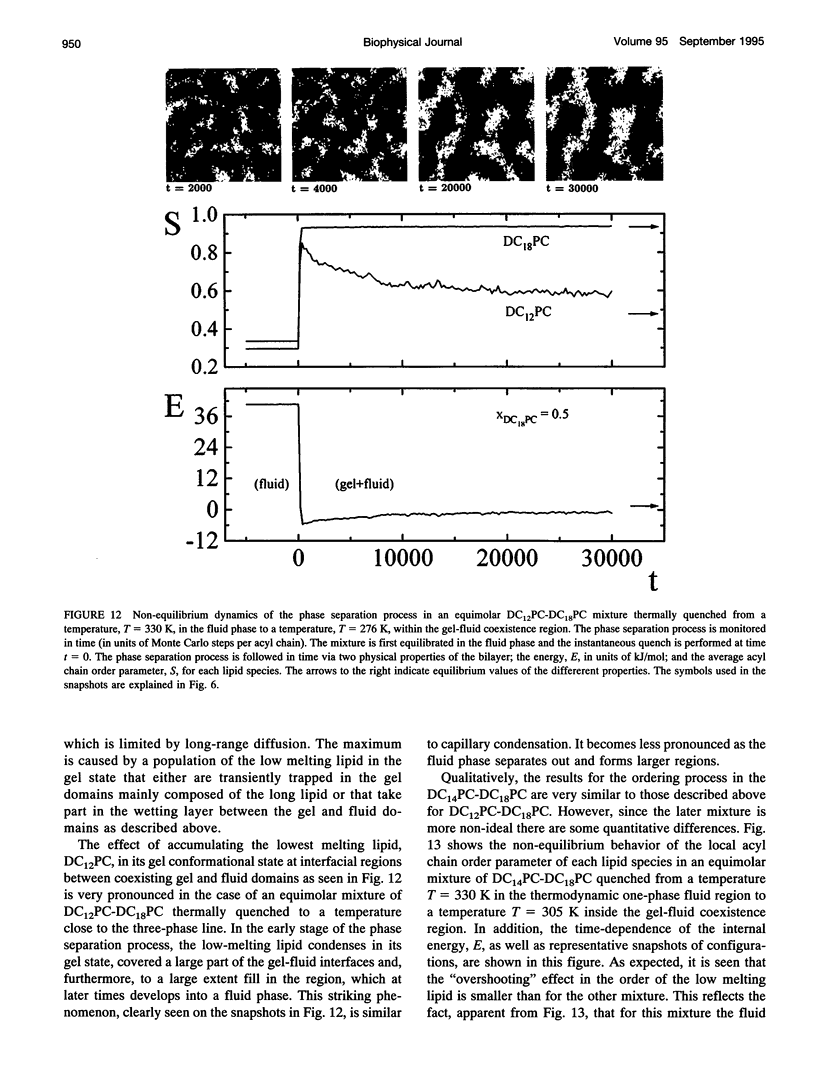
The non-equilibrium dynamic ordering process of coexisting phases has been studied for two-component lipid bilayers composed of saturated di-acyl phospholipids with different acyl chain lengths, such as DC14PC-DC18PC and DC12PC-DC18PC. By means of a microscopic interaction model and computer-simulation techniques the non-equilibrium properties of these two mixtures have been determined with particular attention paid to the effects of the non-equilibrium ordering process on membrane heterogeneity in terms of local and global lateral membrane organization. The results reveal that a sudden temperature change that takes the lipid mixture from the fluid one-phase region into the gel-fluid phase-coexistence region leads to the formation of a large number of small lipid domains which slowly are growing in time. The growth of the lipid domains, which is limited by long-range diffusion of the lipid molecules within the two-dimensional membrane plane, gives rise to the existence of a highly heterogeneous percolative-like structure with a network of interfacial regions that have properties different from those of the phase-separated gel and fluid bulk phases. The results, which are discussed in relation to recent experimental observations interpreted in terms of a percolative-like membrane structure within the two phase region (Almeida, P.F.F., Vaz, W.L.C., and T.E. Thompson. 1992. Biochemistry 31:7198-7210), suggest that non-equilibrium effects may influence lipid domain formation and membrane organization on various length and time scales. Such effects might be of importance in relation to membrane processes that require molecular mobility of the membrane components in restricted geometrical environments of the compartmentalized lipid membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion and percolation in two-phase, two-component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 1992 Aug 11;31(31):7198–7210. doi: 10.1021/bi00146a024. [DOI] [PubMed] [Google Scholar]
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 1992 Jul 28;31(29):6739–6747. doi: 10.1021/bi00144a013. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D. Lipid domain reorganization and receptor events. Results obtained with new fluorescent lipid probes. FEBS Lett. 1992 Feb 10;297(3):212–215. doi: 10.1016/0014-5793(92)80540-w. [DOI] [PubMed] [Google Scholar]
- Bloom M., Evans E., Mouritsen O. G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991 Aug;24(3):293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Briggs J., Caffrey M. The temperature-composition phase diagram of monomyristolein in water: equilibrium and metastability aspects. Biophys J. 1994 Mar;66(3 Pt 1):573–587. doi: 10.1016/s0006-3495(94)80847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack W. R., Yuan Q., Biltonen R. L. Role of lateral phase separation in the modulation of phospholipase A2 activity. Biochemistry. 1993 Jan 19;32(2):583–589. doi: 10.1021/bi00053a025. [DOI] [PubMed] [Google Scholar]
- Cruzeiro-Hansson L., Mouritsen O. G. Passive ion permeability of lipid membranes modelled via lipid-domain interfacial area. Biochim Biophys Acta. 1988 Sep 15;944(1):63–72. doi: 10.1016/0005-2736(88)90316-1. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Chemically induced phase separation in mixed vesicles containing phosphatidic acid. An optical study. J Am Chem Soc. 1975 Jul 9;97(14):4114–4120. doi: 10.1021/ja00847a040. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G., Bloom M. Relationships between lipid membrane area, hydrophobic thickness, and acyl-chain orientational order. The effects of cholesterol. Biophys J. 1990 Mar;57(3):405–412. doi: 10.1016/S0006-3495(90)82557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen C, Mouritsen OG. Universality of ordering dynamics in conserved multicomponent systems. Phys Rev B Condens Matter. 1993 Jun 1;47(22):14724–14733. doi: 10.1103/physrevb.47.14724. [DOI] [PubMed] [Google Scholar]
- Jørgensen K., Ipsen J. H., Mouritsen O. G., Zuckermann M. J. The effect of anaesthetics on the dynamic heterogeneity of lipid membranes. Chem Phys Lipids. 1993 Oct;65(3):205–216. doi: 10.1016/0009-3084(93)90018-x. [DOI] [PubMed] [Google Scholar]
- Jørgensen K., Sperotto M. M., Mouritsen O. G., Ipsen J. H., Zuckermann M. J. Phase equilibria and local structure in binary lipid bilayers. Biochim Biophys Acta. 1993 Oct 10;1152(1):135–145. doi: 10.1016/0005-2736(93)90240-z. [DOI] [PubMed] [Google Scholar]
- Knoll W., Ibel K., Sackmann E. Small-angle neutron scattering study of lipid phase diagrams by the contrast variation method. Biochemistry. 1981 Oct 27;20(22):6379–6383. doi: 10.1021/bi00525a015. [DOI] [PubMed] [Google Scholar]
- Laradji M, Mouritsen OG, Toxvaerd S, Zuckermann MJ. Molecular dynamics simulations of phase separation in the presence of surfactants. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994 Aug;50(2):1243–1252. doi: 10.1103/physreve.50.1243. [DOI] [PubMed] [Google Scholar]
- Leckband D. E., Helm C. A., Israelachvili J. Role of calcium in the adhesion and fusion of bilayers. Biochemistry. 1993 Feb 2;32(4):1127–1140. doi: 10.1021/bi00055a019. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo E. C., Lourtie I. M., Sankaram M. B., Thompson T. E., Vaz W. L. Effects of domain connection and disconnection on the yields of in-plane bimolecular reactions in membranes. Biophys J. 1992 Dec;63(6):1506–1512. doi: 10.1016/S0006-3495(92)81735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Dynamic lipid-bilayer heterogeneity: a mesoscopic vehicle for membrane function? Bioessays. 1992 Feb;14(2):129–136. doi: 10.1002/bies.950140211. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Dynamical order and disorder in lipid bilayers. Chem Phys Lipids. 1994 Sep 6;73(1-2):3–25. doi: 10.1016/0009-3084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G. Theoretical models of phospholipid phase transitions. Chem Phys Lipids. 1991 Mar;57(2-3):179–194. doi: 10.1016/0009-3084(91)90075-m. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Pink D. A., Green T. J., Chapman D. Raman scattering in bilayers of saturated phosphatidylcholines. Experiment and theory. Biochemistry. 1980 Jan 22;19(2):349–356. doi: 10.1021/bi00543a016. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Thompson T. E. Determination of fluid and gel domain sizes in two-component, two-phase lipid bilayers. An electron spin resonance spin label study. Biophys J. 1992 Aug;63(2):340–349. doi: 10.1016/S0006-3495(92)81619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Deuterium magnetic resonance study of phase equilibria and membrane thickness in binary phospholipid mixed bilayers. Biochemistry. 1992 Sep 8;31(35):8258–8268. doi: 10.1021/bi00150a020. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Muderhwa J. M., Brockman H. L. Is lateral phase separation required for fatty acid to stimulate lipases in a phosphatidylcholine interface? Biochemistry. 1994 Feb 22;33(7):1915–1922. doi: 10.1021/bi00173a039. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Translational diffusion and fluid domain connectivity in a two-component, two-phase phospholipid bilayer. Biophys J. 1989 Nov;56(5):869–876. doi: 10.1016/S0006-3495(89)82733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T. X. A computer simulation of free-volume distributions and related structural properties in a model lipid bilayer. Biophys J. 1993 Sep;65(3):1108–1120. doi: 10.1016/S0006-3495(93)81156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Lee G. M., Jacobson K. Protein lateral mobility as a reflection of membrane microstructure. Bioessays. 1993 Sep;15(9):579–588. doi: 10.1002/bies.950150903. [DOI] [PubMed] [Google Scholar]