Abstract
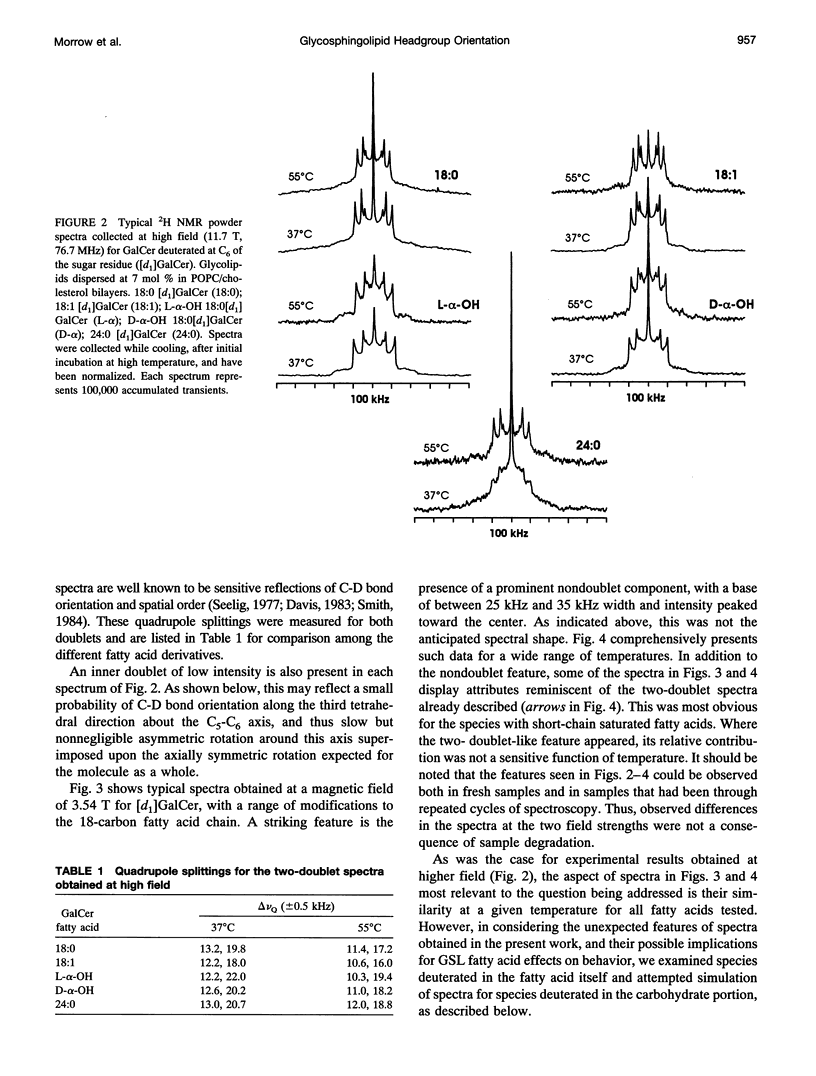
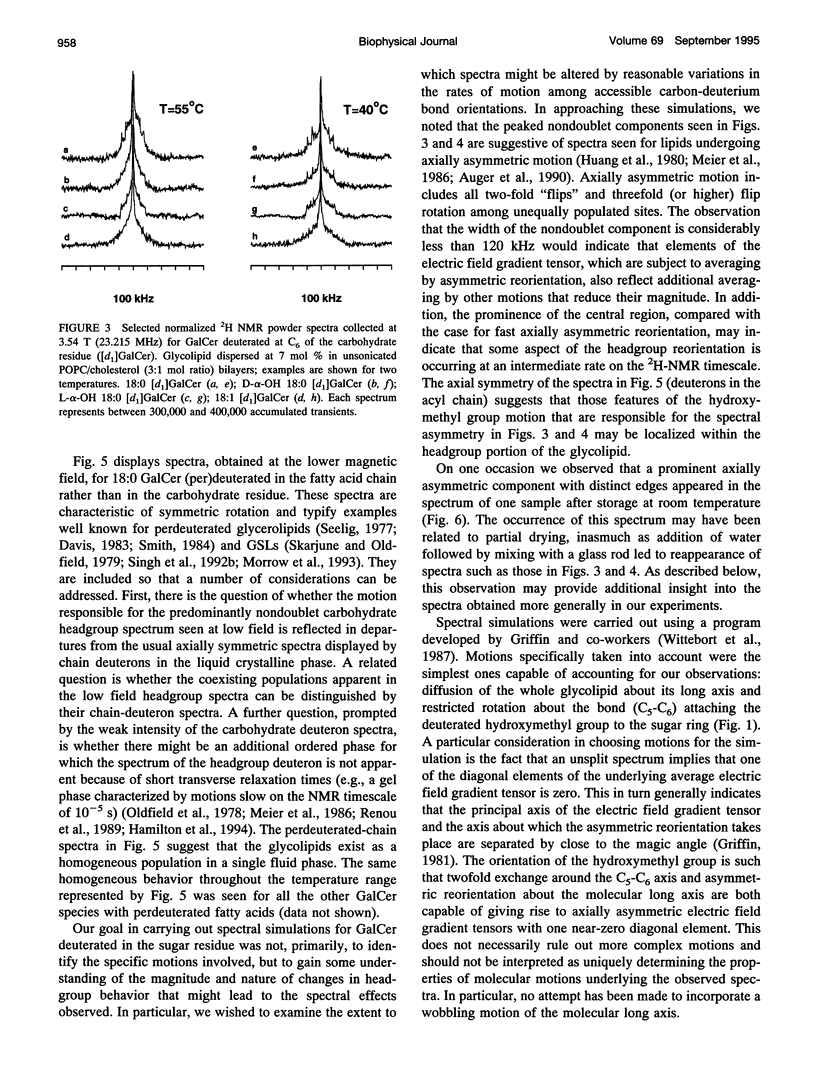
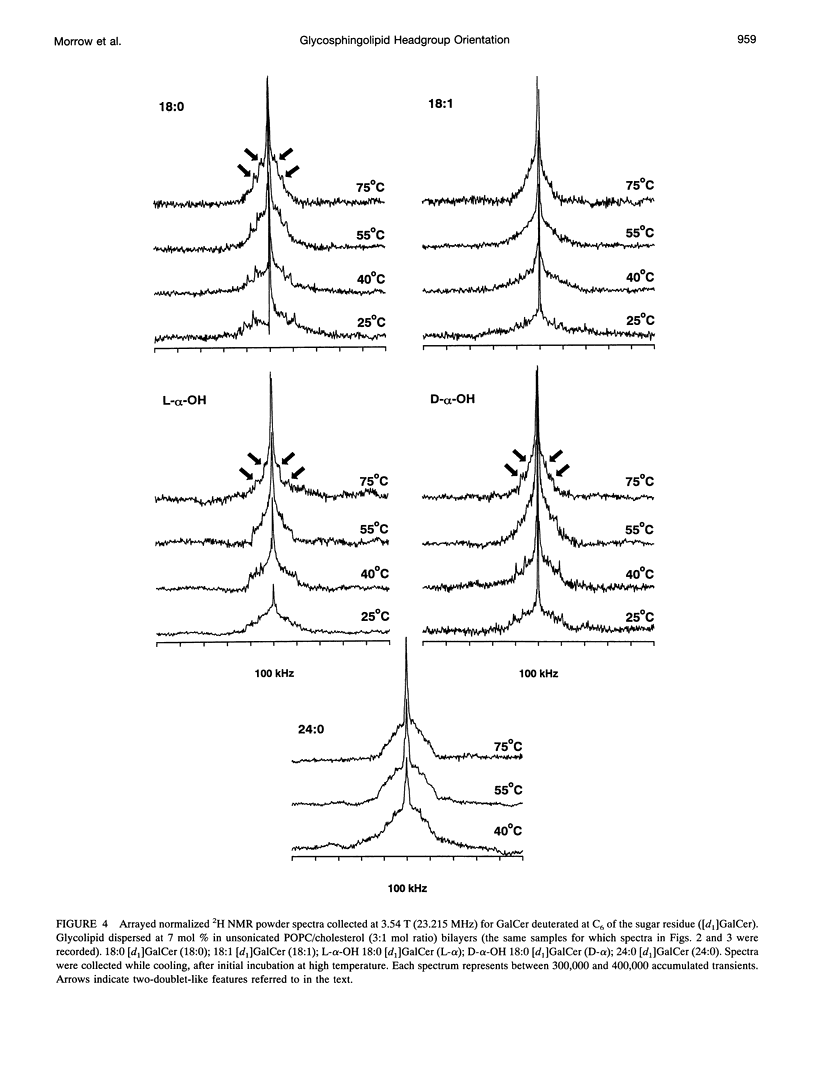
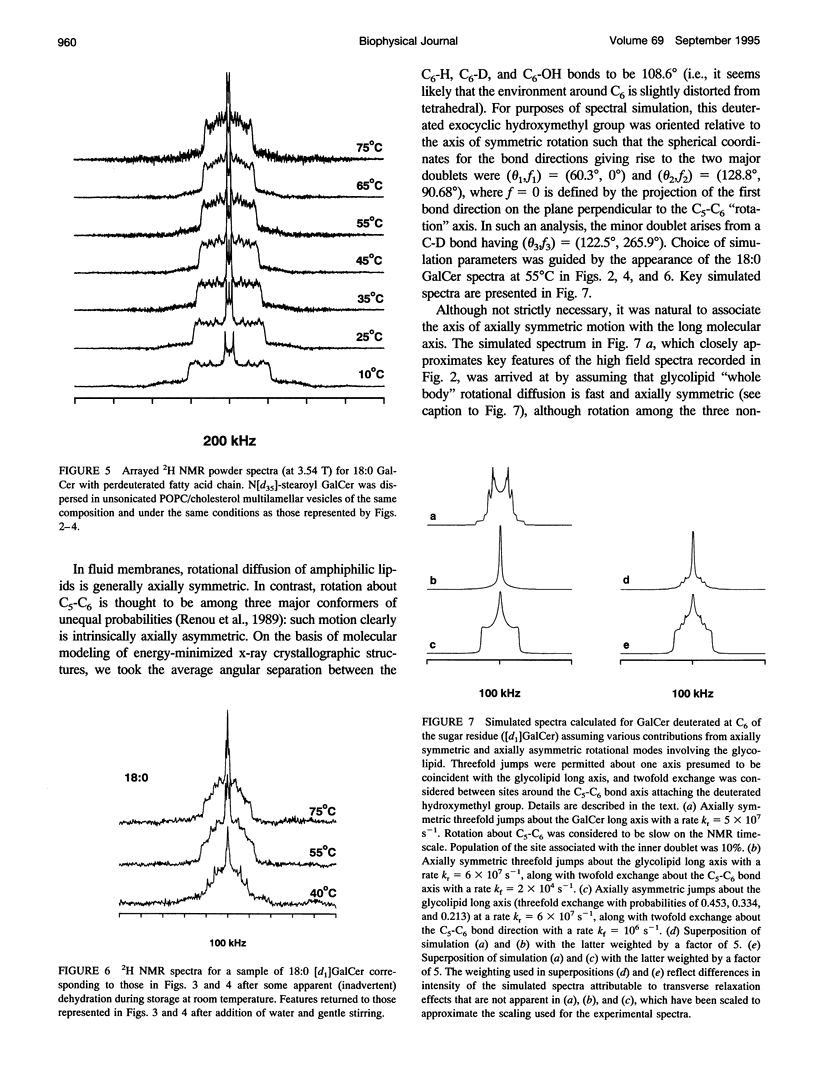
Galactosyl ceramide (GalCer) was labeled for nuclear magnetic resonance (NMR) spectroscopy by replacement of a hydrogen atom at C6 of the galactose residue with deuterium. Wideline 2H NMR of [d1]GalCer permitted consideration of a mechanism traditionally entertained for cell surface recognition site modulation: that the nature of the fatty acid attached to the sphingosine backbone of glycosphingolipids (GSLs) importantly influences carbohydrate headgroup orientation. Comparison was made among various glycolipid fatty acids by altering hydroxylation, saturation, and chain length. Studies were carried out in unsonicated bilayer membranes mimicking several important characteristics of cell plasma membranes: fluidity, low GSL content, predominant [sn-2]monounsaturated phosphatidylcholine (PC) (1-palmitoyl-2-oleoyl PC), and the presence of cholesterol. Spectroscopy was performed on samples over a range of temperatures, which included the physiological. 2H NMR spectra of [d1]GalCer having 18-carbon saturated fatty acid (stearic acid), cis-9-unsaturated fatty acid (oleic acid), D- and L-stereoisomers of alpha-OH stearic acid, or 24-carbon saturated fatty acid (lignoceric acid) were importantly similar. This argues that for GSLs dispersed as minor components in fluid membranes, variation of the glycolipid fatty acid does not provide as much potential for direct conformational modulation of the carbohydrate portion as has sometimes been assumed. However, there was some evidence of motional differences among the species studied. The 2H NMR spectra that were obtained proved to be more complex than was anticipated. Their features could be approximated by assuming a combination of axially symmetric and axially asymmetric glycolipid motions. Presuming the appropriateness of such a analysis, at a magnetic field of 3.54 T (23.215 MHz), the experimental spectra suggested predominantly asymmetric motional contributions. At the higher field of 11.7 T (76.7 MHz, equivalent to a proton frequency of 500 MHz), spectra indicated dominance by axially symmetric rotational modes. There was also evidence of some bilayer orientation in the stronger magnetic field. The unusual observation of spectral differences between the two magnetic field strengths may involve a diamagnetic response to high field on the part of some liposome physical characteristics.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Urban K. A., Richards R. L. Influence of temperature on complement-dependent immune damage to liposomes. Biochim Biophys Acta. 1980 Jul 16;600(1):117–125. doi: 10.1016/0005-2736(80)90417-4. [DOI] [PubMed] [Google Scholar]
- Auger M., Van Calsteren M. R., Smith I. C., Jarrell H. C. Glycerolipids: common features of molecular motion in bilayers. Biochemistry. 1990 Jun 19;29(24):5815–5821. doi: 10.1021/bi00476a024. [DOI] [PubMed] [Google Scholar]
- Curatolo W. Glycolipid function. Biochim Biophys Acta. 1987 Jun 24;906(2):137–160. doi: 10.1016/0304-4157(87)90009-8. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Jungalwala F. B. Phase behavior of galactocerebrosides from bovine brain. Biochemistry. 1985 Nov 5;24(23):6608–6613. doi: 10.1021/bi00344a046. [DOI] [PubMed] [Google Scholar]
- Curatolo W. The physical properties of glycolipids. Biochim Biophys Acta. 1987 Jun 24;906(2):111–136. doi: 10.1016/0304-4157(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Keough K. M. Chain arrangements in the gel state and the transition temperatures of phosphatidylcholines. Biophys J. 1985 Dec;48(6):915–918. doi: 10.1016/S0006-3495(85)83854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., Jansen J. W., van Dijck P. W., van Deenen L. L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977 Feb 14;465(1):1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- Fenske D. B., Hamilton K., Jarrell H. C., Florio E., Barber K. R., Grant C. W. Glycosphingolipids: 2H NMR study of the influence of carbohydrate headgroup structure on ceramide acyl chain behavior in glycolipid-phospholipid bilayers. Biochemistry. 1991 May 7;30(18):4503–4509. doi: 10.1021/bi00232a019. [DOI] [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hamilton K. S., Briere K., Jarrell H. C., Grant C. W. Acyl chain length effects related to glycosphingolipid crypticity in phospholipid membranes: probed by 2H-NMR. Biochim Biophys Acta. 1994 Mar 23;1190(2):367–375. doi: 10.1016/0005-2736(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Hamilton K. S., Jarrell H. C., Brière K. M., Grant C. W. Glycosphingolipid backbone conformation and behavior in cholesterol-containing phospholipid bilayers. Biochemistry. 1993 Apr 20;32(15):4022–4028. doi: 10.1021/bi00066a024. [DOI] [PubMed] [Google Scholar]
- Johnston D. S., Chapman D. A calorimetric study of the thermotropic behaviour of mixtures of brain cerebrosides with other brain lipids. Biochim Biophys Acta. 1988 Apr 22;939(3):603–614. doi: 10.1016/0005-2736(88)90108-3. [DOI] [PubMed] [Google Scholar]
- Kiarash A., Boyd B., Lingwood C. A. Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J Biol Chem. 1994 Apr 15;269(15):11138–11146. [PubMed] [Google Scholar]
- Mehlhorn I. E., Florio E., Barber K. R., Lordo C., Grant C. W. Evidence that trans-bilayer interdigitation of glycosphingolipid long chain fatty acids may be a general phenomenon. Biochim Biophys Acta. 1988 Mar 22;939(1):151–159. doi: 10.1016/0005-2736(88)90056-9. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Singh D., Lu D., Grant C. W. Glycosphingolipid acyl chain orientational order in unsaturated phosphatidylcholine bilayers. Biophys J. 1993 Mar;64(3):654–664. doi: 10.1016/S0006-3495(93)81424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow M. R., Singh D., Lu D., Grant C. W. Glycosphingolipid phase behaviour in unsaturated phosphatidylcholine bilayers: a 2H-NMR study. Biochim Biophys Acta. 1992 Apr 29;1106(1):85–93. doi: 10.1016/0005-2736(92)90225-b. [DOI] [PubMed] [Google Scholar]
- Needham D., McIntosh T. J., Evans E. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochemistry. 1988 Jun 28;27(13):4668–4673. doi: 10.1021/bi00413a013. [DOI] [PubMed] [Google Scholar]
- Nyholm P. G., Pascher I., Sundell S. The effect of hydrogen bonds on the conformation of glycosphingolipids. Methylated and unmethylated cerebroside studied by X-ray single crystal analysis and model calculations. Chem Phys Lipids. 1990 Jan;52(1):1–10. doi: 10.1016/0009-3084(90)90002-9. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Meadows M., Rice D., Jacobs R. Spectroscopic studies of specifically deuterium labeled membrane systems. Nuclear magnetic resonance investigation of the effects of cholesterol in model systems. Biochemistry. 1978 Jul 11;17(14):2727–2740. doi: 10.1021/bi00607a006. [DOI] [PubMed] [Google Scholar]
- Opella S. J. Protein dynamics by solid state nuclear magnetic resonance. Methods Enzymol. 1986;131:327–361. doi: 10.1016/0076-6879(86)31048-6. [DOI] [PubMed] [Google Scholar]
- Prosser R. S., Davis J. H., Dahlquist F. W., Lindorfer M. A. 2H nuclear magnetic resonance of the gramicidin A backbone in a phospholipid bilayer. Biochemistry. 1991 May 14;30(19):4687–4696. doi: 10.1021/bi00233a008. [DOI] [PubMed] [Google Scholar]
- Qiu X., Mirau P. A., Pidgeon C. Magnetically induced orientation of phosphatidylcholine membranes. Biochim Biophys Acta. 1993 Apr 8;1147(1):59–72. doi: 10.1016/0005-2736(93)90316-r. [DOI] [PubMed] [Google Scholar]
- Reed R. A., Shipley G. G. Effect of chain unsaturation on the structure and thermotropic properties of galactocerebrosides. Biophys J. 1989 Feb;55(2):281–292. doi: 10.1016/S0006-3495(89)82803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinl H., Brumm T., Bayerl T. M. Changes of the physical properties of the liquid-ordered phase with temperature in binary mixtures of DPPC with cholesterol: A H-NMR, FT-IR, DSC, and neutron scattering study. Biophys J. 1992 Apr;61(4):1025–1035. doi: 10.1016/S0006-3495(92)81910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou J. P., Giziewicz J. B., Smith I. C., Jarrell H. C. Glycolipid membrane surface structure: orientation, conformation, and motion of a disaccharide headgroup. Biochemistry. 1989 Feb 21;28(4):1804–1814. doi: 10.1021/bi00430a057. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Silvius J. R. Cholesterol modulation of lipid intermixing in phospholipid and glycosphingolipid mixtures. Evaluation using fluorescent lipid probes and brominated lipid quenchers. Biochemistry. 1992 Apr 7;31(13):3398–3408. doi: 10.1021/bi00128a014. [DOI] [PubMed] [Google Scholar]
- Simatos G. A., Forward K. B., Morrow M. R., Keough K. M. Interaction between perdeuterated dimyristoylphosphatidylcholine and low molecular weight pulmonary surfactant protein SP-C. Biochemistry. 1990 Jun 19;29(24):5807–5814. doi: 10.1021/bi00476a023. [DOI] [PubMed] [Google Scholar]
- Singh D. M., Shan X., Davis J. H., Jones D. H., Grant C. W. Oligosaccharide behavior of complex natural glycosphingolipids in multicomponent model membranes. Biochemistry. 1995 Jan 17;34(2):451–463. doi: 10.1021/bi00002a009. [DOI] [PubMed] [Google Scholar]
- Singh D., Jarrell H. C., Barber K. R., Grant C. W. Glycosphingolipids: 2H NMR study of the influence of ceramide fatty acid characteristics on the carbohydrate headgroup in phospholipid bilayers. Biochemistry. 1992 Mar 17;31(10):2662–2669. doi: 10.1021/bi00125a005. [DOI] [PubMed] [Google Scholar]
- Singh D., Jarrell H. C., Florio E., Fenske D. B., Grant C. W. Effects of fatty acid alpha-hydroxylation on glycosphingolipid properties in phosphatidylcholine bilayers. Biochim Biophys Acta. 1992 Jan 31;1103(2):268–274. doi: 10.1016/0005-2736(92)90096-5. [DOI] [PubMed] [Google Scholar]
- Skarjune R., Oldfield E. Physical studies of cell surface and cell membrane structure. Deuterium nuclear magnetic resonance investigation of deuterium-labelled N-hexadeconoylgalactosylceramides (cerebrosides). Biochim Biophys Acta. 1979 Sep 21;556(2):208–218. doi: 10.1016/0005-2736(79)90043-9. [DOI] [PubMed] [Google Scholar]
- Speyer J. B., Sripada P. K., Das Gupta S. K., Shipley G. G., Griffin R. G. Magnetic orientation of sphingomyelin-lecithin bilayers. Biophys J. 1987 Apr;51(4):687–691. doi: 10.1016/S0006-3495(87)83394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton S. G., Kantor A. B., Petrossian A., Owicki J. C. Location and dynamics of a membrane-bound fluorescent hapten. A spectroscopic study. Biochim Biophys Acta. 1984 Oct 3;776(2):228–236. doi: 10.1016/0005-2736(84)90212-8. [DOI] [PubMed] [Google Scholar]
- Stewart R. J., Boggs J. M. Exposure of galactosylceramide to galactose oxidase in liposomes: dependence on lipid environment and ceramide composition. Biochemistry. 1993 Jun 1;32(21):5605–5614. doi: 10.1021/bi00072a016. [DOI] [PubMed] [Google Scholar]
- Strömberg N., Nyholm P. G., Pascher I., Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewalt J. L., Bloom M. Phosphatidylcholine: cholesterol phase diagrams. Biophys J. 1992 Oct;63(4):1176–1181. doi: 10.1016/S0006-3495(92)81681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. E., Tillack T. W. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Utsumi H., Suzuki T., Inoue K., Nojima S. Haptenic activity of galactosyl ceramide and its topographical distribution on liposomal membranes. Effects of temperature and phospholipid composition. J Biochem. 1984 Jul;96(1):97–105. doi: 10.1093/oxfordjournals.jbchem.a134835. [DOI] [PubMed] [Google Scholar]


