Abstract
Activation of the phagocyte NADPH oxidase complex requires assembly of the cytosolic factors p47PHOX, p67PHOX, p40PHOX, and Rac with the membrane-bound cytochrome b558. We recently established a direct interaction between p67PHOX and cytochrome b558. In the present study, we show that removal of the C-terminal domain of p67PHOX increased its binding to cytochrome b558. Whereas phosphorylated p40PHOX alone did not bind to cytochrome b558, phosphorylated p47PHOX did, and, moreover, it allowed the binding of p40PHOX to the cytochrome. Furthermore, both increased the binding of p67PHOX to the cytochrome. Phosphorylated p47PHOX thus appears to increase the binding of p67PHOX to cytochrome b558 by serving as an adapter, bringing p67PHOX into proximity with cytochrome b558, whereas phosphorylated p40PHOX may increase the binding by inducing a conformational change that allows p67PHOX to interact fully with cytochrome b558.
The NADPH oxidase of phagocytes plays a pivotal role in host defense against microbial infection. It catalyzes the reduction of oxygen to O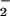 at the expense of NADPH. The O
at the expense of NADPH. The O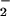 is the precursor of potent oxidants used to kill the invading microorganisms (1–4).
is the precursor of potent oxidants used to kill the invading microorganisms (1–4).
The NADPH oxidase is composed of a number of cytosolic and membrane-bound proteins—in the cytosol, p67PHOX, p47PHOX, p40PHOX, and Rac1/Rac2; in the membrane, gp91PHOX and p22PHOX—which, together, comprise cytochrome b558. When activation takes place, the cytosolic components migrate to the membranes, where they associate with the membrane-bound components to assemble the catalytically active oxidase (5). Cytochrome b558, which contains both flavin and heme groups (6), likely is responsible for electron transfer from NADPH to oxygen upon activation of the cells, although it was demonstrated that p67PHOX also possesses an NADPH-binding site (7). P47PHOX, which is phosphorylated extensively during the activation of the oxidase (8), probably initiates the assembly of the enzyme, whereas the function of p67PHOX appears to be to activate catalysis by cytochrome b558, although the mechanism of activation is obscure (9). Rac appears to promote the interaction between p67PHOX and cytochrome b558 (10).
During the NADPH oxidase activation process, a number of defined protein–protein binding interactions occur (11). For example, p47PHOX and p67PHOX each contain two SH3 domains, the C-terminal SH3 domain of p67PHOX interacts with p47PHOX, and the N-terminal SH3 domain of p47PHOX is able to interact with p22PHOX (12).
In the present study, we established a direct interaction between p67PHOX and cytochrome b558 (10) and showed that this interaction increased when the C-terminal region of the protein was removed and also when the whole protein was incubated in the presence of phosphorylated p47PHOX or phosphorylated p40PHOX.
Experimental Procedures
Expression and Purification of Recombinant Proteins in Escherichia coli.
P67PHOX and its truncated forms were expressed in E. coli as glutathione S-transferase (GST) fusion proteins and purified with glutathione-Sepharose beads (13). The proteins then were separated from GST while on the beads by cleavage with PreScission protease (Amersham Pharmacia) or eluted from the beads by incubating for 20 min at 4°C with 1 ml of 50 mM Tris⋅HCl, pH 8/5 mM glutathione/150 mM NaCl. P47PHOX was expressed in E. coli as GST fusion proteins and purified with glutathione-Sepharose beads (Amersham Pharmacia) followed by thrombin cleavage when needed. P40PHOX cDNA in pET-32a plasmid (a kind gift of A. Fuchs, Centre National de la Recherche Scientifique, Grenoble, France) was transformed in BL21-DE3 (pLysS) E. coli strain and expressed as follows. An overnight culture was diluted 10-fold in fresh Terrific Broth medium containing 100 μg/ml ampicillin and grown for an additional hour at 37°C. The culture then was induced with 0.5 mM isopropyl β-d-thiogalactoside for 4 h at 25°C. Bacteria were harvested by centrifugation and lysed by sonication in 20 mM Hepes, pH 7.9/0.5 M NaCl/10 mM imidazole in the presence of 0.2 mM PMSF/100 μg/ml leupeptin/100 μg/ml pepstatin/0.5 mM diisopropyl fluorophosphate. The homogenate then was centrifuged at 150,000 × g for 15 min, and the supernatant was incubated with Probond resin (Invitrogen) for 1 h at 4°C. The resin then was packed into a gravity column, washed twice in 20 mM Hepes, pH 7.9/0.5 M NaCl/20 mM imidazole, and then again washed twice in 20 mM Hepes, pH 7.9/0.5 M NaCl/30 mM imidazole. The protein then was eluted with 150 mM imidazole and dialyzed against 20 mM Hepes, pH 7.9/0.1 mM DTT/2 mM EGTA. Protein concentrations were determined with the Bio-Rad Assay Kit with BSA as a standard.
Contruction of Truncated p67phox Forms.
P67PHOX truncated forms were constructed as described by Dang et al. (13). Briefly, p67PHOX (1–243), p67PHOX (1–210), p67PHOX (1–199), and p67PHOX (244–526) were obtained by PCR with Pfu polymerase (Stratagene) and wild-type p67PHOX cDNA cloned into the vector pBK-CMV. The PCR products were ligated into the SmaI site of pGEX-6P1 for p67PHOX (1–243), BamHI and EcoRI of pGEX-6P1 for p67PHOX (1–210) and p67PHOX (1–199), and EcoRI and XhoI sites of pGEX-6P3 for p67PHOX (244–526) and transformed into DH5α for expression of the proteins. The sequences all were confirmed by DNA sequencing in the Scripps Research Institute molecular biology facility.
Purification of Cytochrome b558.
Cytochrome b558 was purified from human neutrophil plasma membranes by heparin and hydroxyapatite chromatography and subsequently relipidated and reflavinated as described by Cross et al. (14).
Affinity Precipitation.
For affinity precipitation, 80 pmol of GST, full-length GST-p67PHOX or truncated GST-p67PHOX (1–243), GST-p67PHOX (1–210), GST-p67PHOX (1–199), or GST-P67PHOX (244–526) was incubated in the presence of 4.3 pmol of cytochrome b558 in 200 μl of 20 mM Hepes, pH 7.5/1% Nonidet P-40/10 mM NaCl/1 mM EGTA for 2 h at room temperature. In some assays, phosphorylated GST-p67PHOX was used instead. Then, 40 μl of glutathione-Sepharose 4B beads (Amersham Pharmacia) then was added followed by incubation for 2 h at 4°C. After washing five times with the same buffer, the beads were pelleted, resuspended in 40 μl of Laemmli sample buffer, and boiled for 5 min. Proteins were separated by SDS/PAGE on a 12% Tris-glycine gel (Bio-Rad) and transferred onto nitrocellulose. The cytochrome b558 on the nitrocellulose then was visualized by using an anti-gp91PHOX mAb (15). To determine the effect of p47PHOX and p40PHOX on p67PHOX binding to gp91PHOX, 80 pmol of phosphorylated p47PHOX or unphosphorylated p47PHOX, phosphorylated p40PHOX or unphosphorylated p40PHOX was added to the assay at the same time as p67PHOX.
Affinity precipitation of cytochrome b by p47PHOX was carried out as described above except that GST-p47PHOX was used instead of GST-p67PHOX.
For affinity precipitation of cytochrome b by p40PHOX, 40 pmol of His-tagged p40PHOX or phosphorylated His-tagged p40PHOX was incubated in the presence of 2.15 pmol of cytochrome b558 in the presence of 100 μl of 20 mM Hepes, pH 7.5/1% Nonidet P-40/10 mM NaCl/1 mM EGTA/40 mM imidazole for 2 h at room temperature and then precipitated with nickel-chelating beads.
Western Blotting.
Nitrocellulose membranes were blocked with 5% nonfat dry milk in borate-buffered saline, pH 8.4 (100 mM boric acid/25 mM borax/75 mM NaCl) for 1 h at room temperature and then incubated with 1:1,000 mouse monoclonal anti-gp91PHOX antibody overnight. The membranes then were washed extensively and incubated with horseradish peroxidase-conjugated 1:5,000 anti-mouse IgG for 1 h at room temperature. Blots were visualized by using enhanced chemiluminescence Western blotting reagents (Amersham Pharmacia).
Phosphorylation.
Phosphorylation of p67PHOX by mitogen-activated protein kinase and protein kinase C (Calbiochem) in combination was performed by incubating 80 pmol of p67PHOX in a reaction mixture containing 40 mM Hepes (pH 7.5), 10 mM MgCl2, 10 mM DTT, 0.6 mM CaCl2, 5 μg/ml 1,2-dioleoyl-sn-glycerol (Sigma), 50 μg/ml l-α-phosphatidylserine (Sigma), the indicated kinase, and 50 μM ATP in a total volume of 40 μl for 30 min at 30°C. Phosphorylation of p40PHOX and p47PHOX by protein kinase C was performed the same way. The reactions were stopped by adding 5 μM GF109203X, an inhibitor of conventional and novel protein kinase C.
Statistical Analysis.
All of the experiments were repeated a minimum of three times. Results are expressed as means ± SE. Statistical analysis was performed with one-way ANOVA. P < 0.05 was considered to be statistically significant.
Results
P67PHOX Interacts Directly with Cytochrome b558.
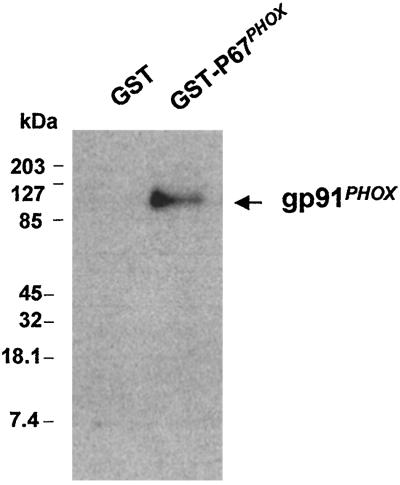
The direct binding of p67PHOX to cytochrome b558 was established by affinity-precipitation experiments. Cytochrome b558 was incubated in the presence of GST-p67PHOX or GST. Fig. 1 shows that cytochrome b558 was precipitated with GST-p67PHOX coupled to glutathione-Sepharose beads but not with GST coupled to glutathione-Sepharose beads. The results demonstrate a direct interaction between cytochrome b558 and p67PHOX.
Figure 1.
Affinity precipitation of cytochrome b558 with GST-p67PHOX. Eighty pmol of GST or full-length GST-p67PHOX was incubated in the presence of 4.3 pmol of cytochrome b558 in 200 μl of 20 mM Hepes, pH 7.5/1% Nonidet P-40/10 mM NaCl/1 mM EGTA for 2 h at room temperature. Then, 40 μl of glutathione-Sepharose 4B beads (Pharmacia) was added, followed by incubation for 2 h at 4°C. After washing five times with the same buffer, the beads were pelleted, resuspended in 40 μl of Laemmli sample buffer (19), and boiled for 5 min. Proteins were resolved by SDS/12% PAGE and transferred onto nitrocellulose. The proteins then were detected with a monoclonal anti-gp91PHOX antibody.
Interaction of Truncated Forms of p67PHOX with Cytochrome b558.
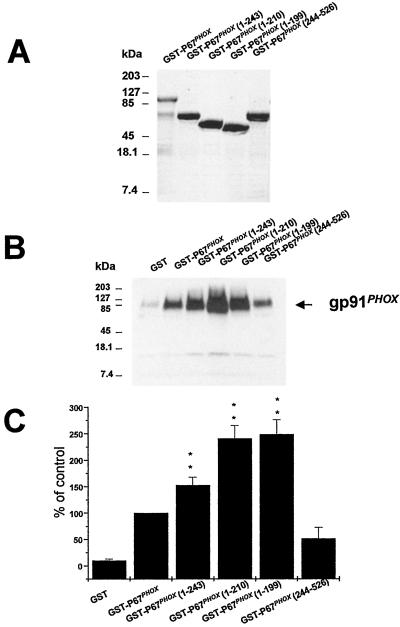
In an attempt to localize the region of p67PHOX responsible for binding to cytochrome b558, a series of truncated GST-p67PHOX forms were generated and expressed (Fig. 2A). Full-length GST-p67PHOX or truncated GST-p67PHOX was used in affinity-precipitation experiments. As shown in Fig. 2 B and C, affinity precipitation of cytochrome b558 was increased when the C-terminal GST truncations of p67PHOX were used. This increase is especially dramatic for GST-p67PHOX (1–210) and GST-p67PHOX (1–199). By quantitation, the increase was 153 ± 15% for GST-p67PHOX (1–243) and 241 ± 27% and 242 ± 24% for GST-p67PHOX (1–210) and GST-p67PHOX (1–199), respectively. The C-terminal fragment GST-p67PHOX (244–526) precipitated cytochrome b558 much less effectively. Indeed, scanning of several experiments showed no significant difference compared with control GST. These results suggest that in the N-terminal forms of p67PHOX, the binding site for cytochrome b558 is unmasked, whereas in the full-length p67PHOX, this site is masked partially by the C-terminal half of the molecule. When this region is removed, the interaction with cytochrome b558 is increased.
Figure 2.
Binding of various truncated forms of p67PHOX to cytochrome b558. (A) SDS/PAGE of full-length and truncated GST-p67PHOX forms expressed and purified as described under Experimental Procedures. The samples were visualized by Coomassie blue staining. (B) Affinity precipitation of cytochrome b558 by full-length GST-p67 PHOX or truncated GST-p67PHOX (1–243), GST-p67PHOX (1–210), GST-p67PHOX (1–199), or GST-p67PHOX (244–526). The precipitation was performed as described in Fig. 1. Quantification of binding was determined by scanning densitometry. The data are expressed as percentage of full-length GST-p67PHOX and are the means ± SE of four experiments.
P67PHOX (1–210) is active in the cell-free NADPH oxidase system, whereas p67PHOX (1–199) is inactive (16). It is interesting that the GST counterparts of those two p67PHOX fragments both are able to bind to cytochrome b558. This result suggests that the binding of GST-p67PHOX (1–199) to cytochrome b558 serves to hold the active portion of p67PHOX to the cytochrome.
Effect of p67PHOX Phosphorylation, p47PHOX, and p40PHOX on the Interaction of p67PHOX with Cytochrome b558.
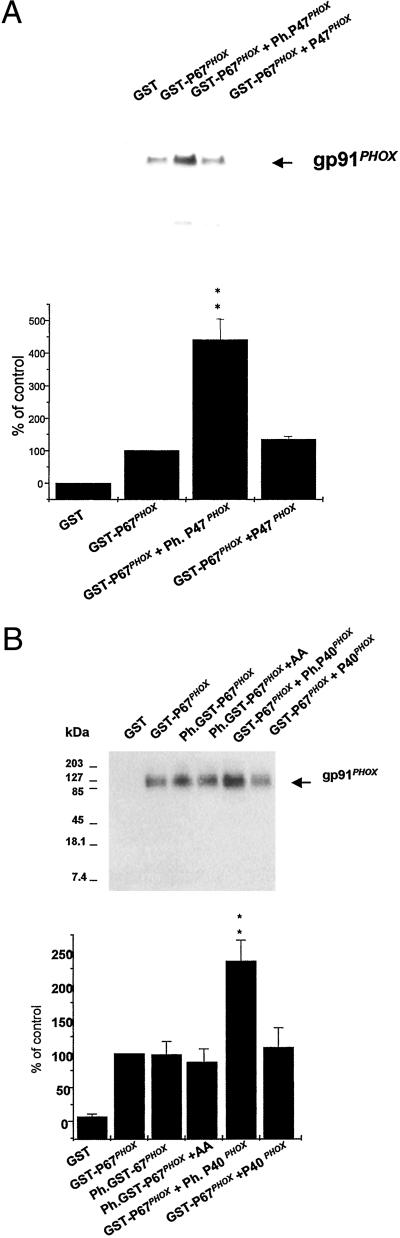
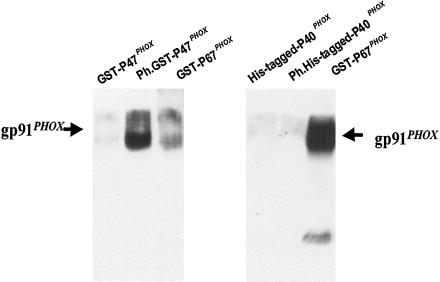
The above results indicate that a change in p67PHOX conformation is required for its interaction with cytochrome b558. We next aimed to determine whether the phosphorylation of p67PHOX or the presence of other components of the NADPH oxidase complex could affect the association of cytochrome b558 with GST-p67PHOX. The phosphorylation of p67PHOX had little effect (Fig. 3B). Even arachidonic acid, which increases the interaction of p47PHOX with the cytosolic tail of p22PHOX (17), had no effect (Fig. 3B). Interestingly, when phosphorylated p47PHOX or phosphorylated p40PHOX was added to the assay, the coprecipitation of cytochrome b558 by GST-p67PHOX was found to be increased (Fig. 3 A and B). On the other hand, unphosphorylated p47PHOX and unphosphorylated p40PHOX had no effect. These data indicate that phosphorylated p47PHOX and phosphorylated p40PHOX are able to increase the binding of p67PHOX to cytochrome b558. Phosphorylated p47PHOX has been shown to interact directly with the cytoplasmic tail of p22PHOX (17), and we established here its interaction with cytochrome b558. Indeed, phosphorylated GST-p47PHOX was able to affinity-precipitate cytochrome b558 in the presence of glutathione-Sepharose beads (Fig. 4 Left), whereas unphosphorylated GST-p47PHOX was not capable of this interaction. Thus, phosphorylated p47PHOX might increase the binding of p67PHOX to cytochrome b by acting as an adapter protein, bringing p67PHOX into proximity with cytochrome b558. In contrast, we were not able to find any interaction of phosphorylated p40PHOX with cytochrome b558. Indeed, phosphorylated or unphosphorylated His-tagged-p40PHOX was not able to affinity-precipitate cytochrome b in the presence of nickel-chelating beads (Fig. 4 Right). Thus, it is probable that phosphorylated p40PHOX increases the binding of p67PHOX with cytochrome b by inducing a conformational change of p67PHOX, allowing it to fully interact with cytochrome b558.
Figure 3.
Phosphorylated p47PHOX and phosphorylated p40PHOX increase the binding of p67PHOX to cytochrome b558. (A) Effect of p47PHOX. (Upper) Affinity precipitation was performed as described in Fig. 1, except that in some assays 80 pmol of phosphorylated p47PHOX (lane 3) or unphosphorylated p47PHOX (lane 4) was added. (Lower) Quantification of the binding was determined by scanning densitometry. The data are expressed as the percentage of full-length GST-p67PHOX and are the means ± SE of three experiments. (B) Effect of phosphorylated p67PHOX, p40PHOX. (Upper) Affinity precipitation was performed as described in Fig. 1. Lanes: 3, phosphorylated GST-p67PHOX was used instead of GST-p67PHOX; 4, phosphorylated GST-p67PHOX and 10 μM arachidonic acid were added; 5, phosphorylated p40PHOX (80 pmol) was added to the assay; 6, unphosphorylated p40PHOX (80 pmol) was added to the assay. (Lower) Quantification of the binding was determined by scanning densitometry. The data are expressed as percentage of full-length GST-p67PHOX and are the means ± SE of three experiments.
Figure 4.
Phosphorylated p47PHOX binds to cytochrome b whereas phosphorylated p40PHOX does not. (Left) Precipitation of cytochrome b by p47PHOX. Affinity precipitation was performed as described in Experimental Procedures in the presence of unphosphorylated GST-p47PHOX (lane 1) or phosphorylated GST-p47PHOX (lane 2). (Right) Precipitation of cytochrome b by p40PHOX. Affinity precipitation was performed as described in Experimental Procedures in the presence of unphosphorylated His-tagged p40PHOX (lane 1) or phosphorylated His-tagged p40PHOX (lane 2). His-tagged P40PHOX was precipitated with nickel-chelating beads.
Discussion
In an earlier study, we demonstrated by several methods that p67PHOX is able to bind directly to cytochrome b558 (10). A direct interaction between p67PHOX and cytochrome b558 is in accord with the idea that p67PHOX regulates the transfer of electrons from NADPH to the flavin (18) because p67PHOX then would be in proximity to the flavin center, enabling it to perform a regulatory function in this part of the protein. Recently, one specific domain of p67PHOX, called the activation domain, has been shown to be involved in this regulation (16). The activation domain, p67PHOX (1–210), binds more strongly to cytochrome b558 than does the complete protein, but so does a domain of similar size, p67PHOX (1–199), which shows no activity in the cell-free NADPH oxidase system. These results suggest that the C truncation that yields p67PHOX (1–210) and p67PHOX (1–199) affects the binding of the fragments to cytochrome b558 but has nothing to do with oxidase activity.
The effect of p47PHOX and p40PHOX on the binding of p67PHOX to cytochrome b558 also was examined. Our results showed that the unphosphorylated proteins had no effect, but that phosphorylated p47PHOX and phosphorylated p40PHOX both increased the binding of p67PHOX to cytochrome b558. Phosphorylated p47PHOX probably serves as an adapter protein, bringing full-length p67PHOX into proximity with cytochrome b558. Phosphorylated p40PHOX appears to induce a conformational change in p67PHOX, although the functional significance of this conformational change is unclear. In conclusion, our data establish a direct interaction between p67PHOX and cytochrome b558, as demonstrated previously. Furthermore, we have shown that full binding requires a conformation change that, in the intact cells, might be induced by phosphorylated p40PHOX.
Acknowledgments
P.M.-C.D. is the recipient of a postdoctoral fellowship from the Arthritis Foundation. This work was supported in part by U.S. Public Health Service Grants AI-24227, AI-28479, AI-24838, and AR42426.
Abbreviation
- GST
glutathione S-transferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Babior B M. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 2.Segal A W. J Clin Invest. 1989;83:1785–1793. doi: 10.1172/JCI114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malech H L, Gallin J I. N Engl J Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 4.Chanock S J, El Benna J, Smith R M, Babior B M. J Biol Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- 5.Clark R A, Volpp B D, Leidal K G, Nauseef W M. J Clin Invest. 1990;85:714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotrosen D, Yeung C L, Leto T L, Malech H L, Kwong C H. Science. 1992;256:1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- 7.Smith R M, Connor J A, Chen L M, Babior B M. J Clin Invest. 1996;98:977–983. doi: 10.1172/JCI118882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa T, Suzuki K, Suzuki S, Andrews P C, Babior B M. J Biol Chem. 1986;261:9109–9115. [PubMed] [Google Scholar]
- 9.Koshkin V, Lotan O, Pick E. J Biol Chem. 1996;271:30326–30329. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- 10.Dang P M-C, Cross A R, Babior B M. Proc Natl Acad Sci USA. 2001;98:3001–3005. doi: 10.1073/pnas.061029698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deleo F R, Quinn M T. J Leukocyte Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 12.Sumimoto H, Kage Y, Nunoi H, Sasaki H, Nose T, Fukumaki Y, Ohno M, Minakami S, Takeshige K. Proc Natl Acad Sci USA. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang P M-C, Johnson J L, Babior B M. Biochemistry. 2000;39:3069–3075. doi: 10.1021/bi9919807. [DOI] [PubMed] [Google Scholar]
- 14.Cross A R, Erickson R W, Ellis B A, Curnutte J T. Biochem J. 1999;338:229–233. [PMC free article] [PubMed] [Google Scholar]
- 15.Burrit J B, Quinn M T, Jutila M A, Bond C W, Jesaitis A J. J Biol Chem. 1995;270:16974–16980. doi: 10.1074/jbc.270.28.16974. [DOI] [PubMed] [Google Scholar]
- 16.Han C-H, Freeman J L R, Lee T, Motalebi S A, Lambeth J D. J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 17.Shiose A, Sumimoto H. J Biol Chem. 2000;275:13793–13801. doi: 10.1074/jbc.275.18.13793. [DOI] [PubMed] [Google Scholar]
- 18.Nisimoto Y, Motalebi S, Han C H, Lambeth J D. J Biol Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]