Abstract
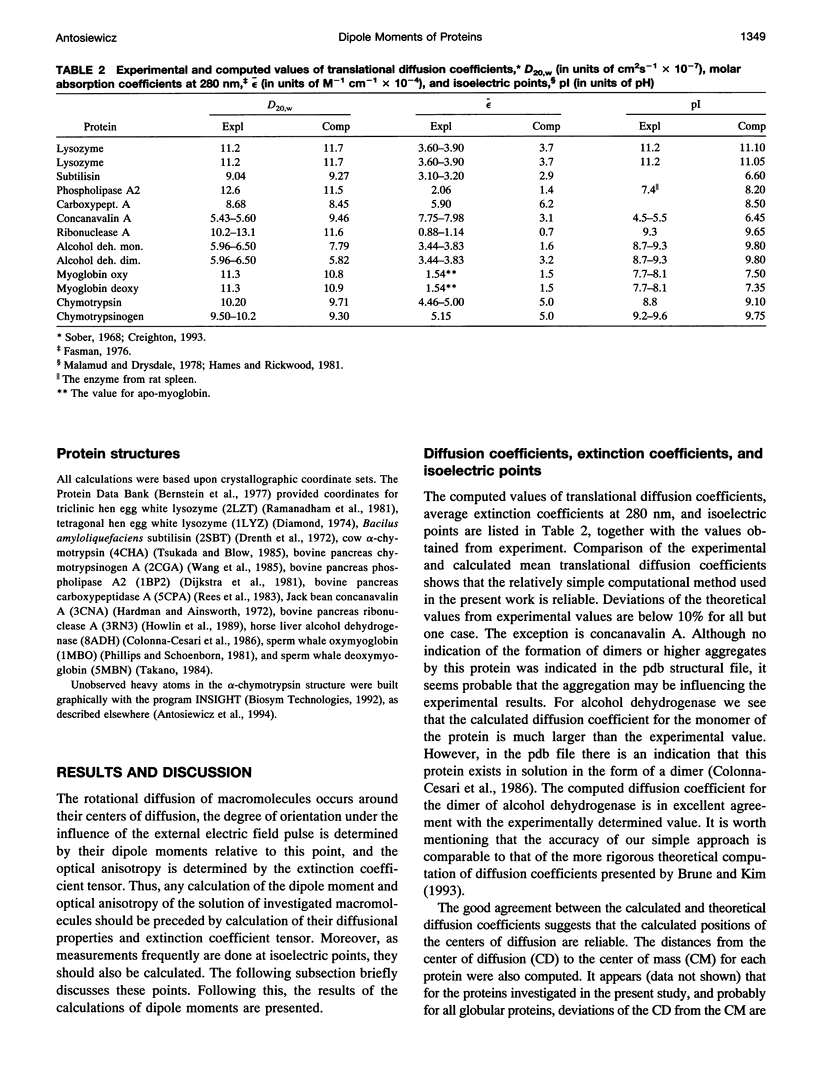
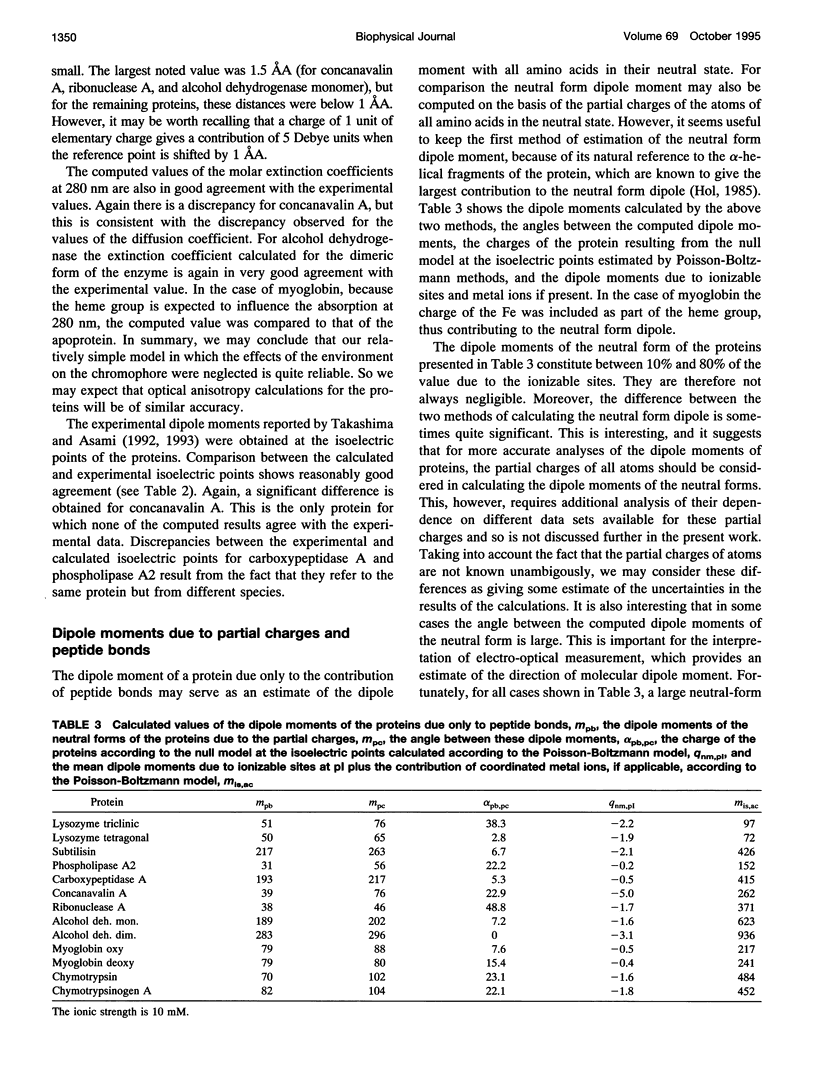
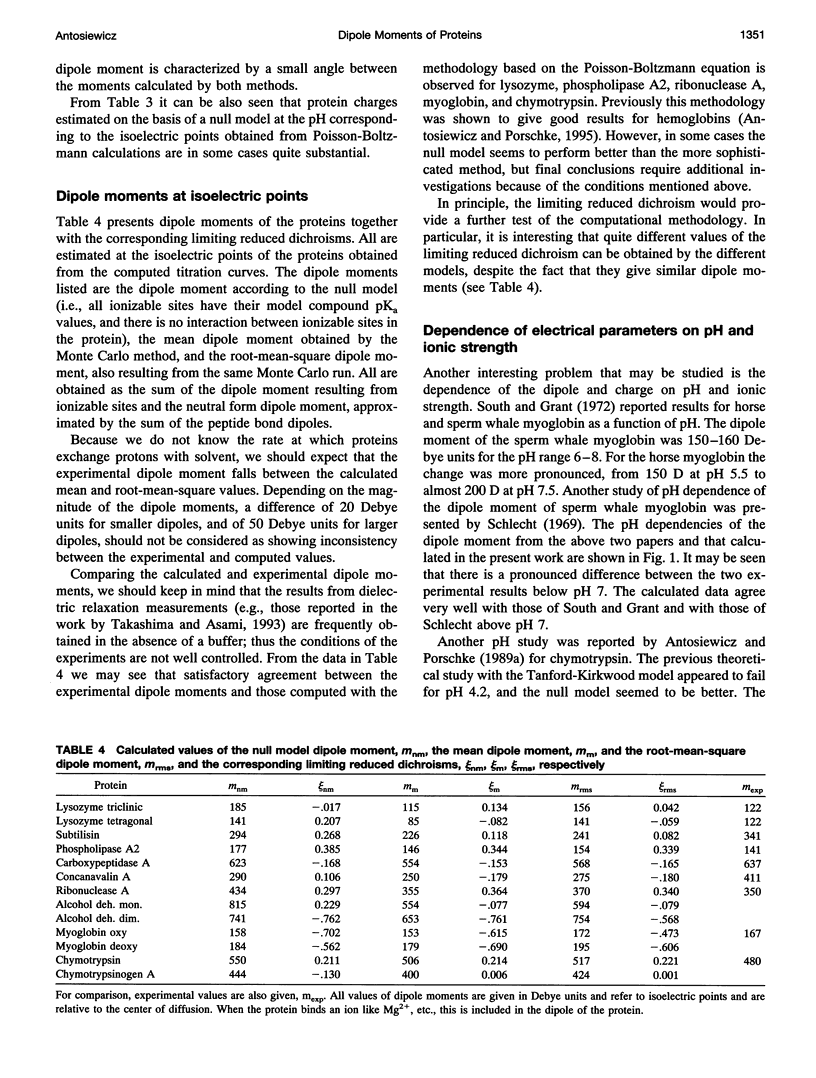
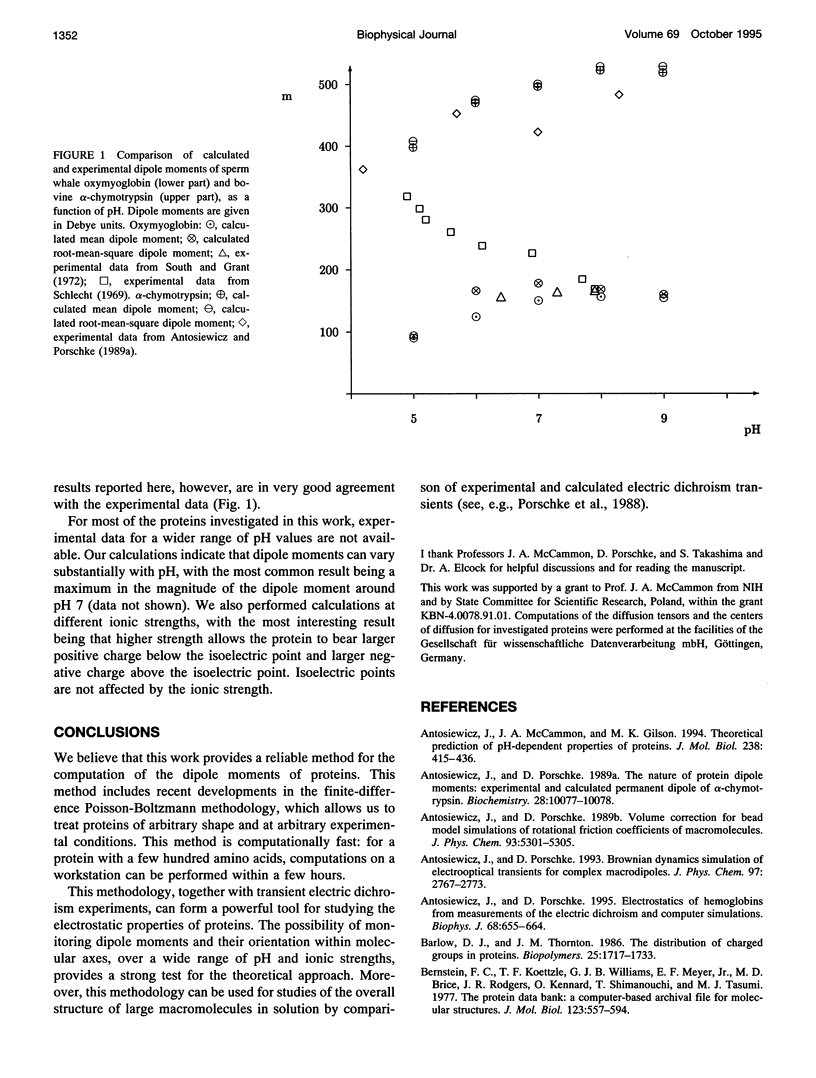
A simple and computationally feasible procedure for the calculation of net charges and dipole moments of proteins at arbitrary pH and salt conditions is described. The method is intended to provide data that may be compared to the results of transient electric dichroism experiments on protein solutions. The procedure consists of three major steps: (i) calculation of self energies and interaction energies for ionizable groups in the protein by using the finite-difference Poisson-Boltzmann method, (ii) determination of the position of the center of diffusion (to which the calculated dipole moment refers) and the extinction coefficient tensor for the protein, and (iii) generation of the equilibrium distribution of protonation states of the protein by a Monte Carlo procedure, from which mean and root-mean-square dipole moments and optical anisotropies are calculated. The procedure is applied to 12 proteins. It is shown that it gives hydrodynamic and electrical parameters for proteins in good agreement with experimental data.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antosiewicz J., McCammon J. A., Gilson M. K. Prediction of pH-dependent properties of proteins. J Mol Biol. 1994 May 6;238(3):415–436. doi: 10.1006/jmbi.1994.1301. [DOI] [PubMed] [Google Scholar]
- Antosiewicz J., Porschke D. Electrostatics of hemoglobins from measurements of the electric dichroism and computer simulations. Biophys J. 1995 Feb;68(2):655–664. doi: 10.1016/S0006-3495(95)80226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosiewicz J., Porschke D. The nature of protein dipole moments: experimental and calculated permanent dipole of alpha-chymotrypsin. Biochemistry. 1989 Dec 26;28(26):10072–10078. doi: 10.1021/bi00452a029. [DOI] [PubMed] [Google Scholar]
- Barlow D. J., Thornton J. M. The distribution of charged groups in proteins. Biopolymers. 1986 Sep;25(9):1717–1733. doi: 10.1002/bip.360250913. [DOI] [PubMed] [Google Scholar]
- Brune D., Kim S. Predicting protein diffusion coefficients. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3835–3839. doi: 10.1073/pnas.90.9.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A. T., Karplus M. Polar hydrogen positions in proteins: empirical energy placement and neutron diffraction comparison. Proteins. 1988;4(2):148–156. doi: 10.1002/prot.340040208. [DOI] [PubMed] [Google Scholar]
- Colonna-Cesari F., Perahia D., Karplus M., Eklund H., Brädén C. I., Tapia O. Interdomain motion in liver alcohol dehydrogenase. Structural and energetic analysis of the hinge bending mode. J Biol Chem. 1986 Nov 15;261(32):15273–15280. [PubMed] [Google Scholar]
- Diamond R. Real-space refinement of the structure of hen egg-white lysozyme. J Mol Biol. 1974 Jan 25;82(3):371–391. doi: 10.1016/0022-2836(74)90598-1. [DOI] [PubMed] [Google Scholar]
- Diekmann S., Hillen W., Jung M., Wells R. D., Pörschke D. Electric properties and structure of DNA-restriction fragments from measurements of the electric dichroism. Biophys Chem. 1982 May;15(2):157–167. doi: 10.1016/0301-4622(82)80028-8. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Kalk K. H., Hol W. G., Drenth J. Structure of bovine pancreatic phospholipase A2 at 1.7A resolution. J Mol Biol. 1981 Mar 25;147(1):97–123. doi: 10.1016/0022-2836(81)90081-4. [DOI] [PubMed] [Google Scholar]
- Drenth J., Hol W. G., Jansonius J. N., Koekoek R. A comparison of the three-dimensional structures of subtilisin BPN' and subtilisin novo. Cold Spring Harb Symp Quant Biol. 1972;36:107–116. doi: 10.1101/sqb.1972.036.01.016. [DOI] [PubMed] [Google Scholar]
- Garcia de la Torre J. G., Bloomfield V. A. Hydrodynamic properties of complex, rigid, biological macromolecules: theory and applications. Q Rev Biophys. 1981 Feb;14(1):81–139. doi: 10.1017/s0033583500002080. [DOI] [PubMed] [Google Scholar]
- Gilson M. K. Multiple-site titration and molecular modeling: two rapid methods for computing energies and forces for ionizable groups in proteins. Proteins. 1993 Mar;15(3):266–282. doi: 10.1002/prot.340150305. [DOI] [PubMed] [Google Scholar]
- Gorbunoff M. J. The interaction of proteins with hydroxyapatite. I. Role of protein charge and structure. Anal Biochem. 1984 Feb;136(2):425–432. doi: 10.1016/0003-2697(84)90239-2. [DOI] [PubMed] [Google Scholar]
- Hardman K. D., Ainsworth C. F. Structure of concanavalin A at 2.4-A resolution. Biochemistry. 1972 Dec 19;11(26):4910–4919. doi: 10.1021/bi00776a006. [DOI] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Howlin B., Moss D. S., Harris G. W. Segmented anisotropic refinement of bovine ribonuclease A by the application of the rigid-body TLS model. Acta Crystallogr A. 1989 Dec 1;45(Pt 12):851–861. doi: 10.1107/s0108767389009177. [DOI] [PubMed] [Google Scholar]
- Keefe S. E., Grant E. H. Dipole moment and relaxation time of ribonuclease. Phys Med Biol. 1974 Sep;19(5):701–707. doi: 10.1088/0031-9155/19/5/010. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. G., Shumaker J. B. The Influence of Dipole Moment Fluctuations on the Dielectric Increment of Proteins in Solution. Proc Natl Acad Sci U S A. 1952 Oct;38(10):855–862. doi: 10.1073/pnas.38.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Malamud D., Drysdale J. W. Isoelectric points of proteins: a table. Anal Biochem. 1978 Jun 1;86(2):620–647. doi: 10.1016/0003-2697(78)90790-x. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Hanania G. I., Gurd F. R. Electrostatic effects in hemoglobin: hydrogen ion equilibria in human deoxy- and oxyhemoglobin A. Biochemistry. 1979 May 15;18(10):1919–1928. doi: 10.1021/bi00577a011. [DOI] [PubMed] [Google Scholar]
- Orttung W. H. Anisotropy of proton fluctuations and the Kerr effect of protein solutions. Theoretical considerations. J Phys Chem. 1968 Nov;72(12):4058–4066. doi: 10.1021/j100858a020. [DOI] [PubMed] [Google Scholar]
- Orttung W. H. Calculation of the mean-square dipole moment and proton fluctuation anisotropy of hemoglobin at low ionic strength. J Phys Chem. 1969 Feb;73(2):418–423. doi: 10.1021/j100722a026. [DOI] [PubMed] [Google Scholar]
- Phillips S. E., Schoenborn B. P. Neutron diffraction reveals oxygen-histidine hydrogen bond in oxymyoglobin. Nature. 1981 Jul 2;292(5818):81–82. doi: 10.1038/292081a0. [DOI] [PubMed] [Google Scholar]
- Porschke D., Antosiewicz J. Permanent dipole moment of tRNA's and variation of their structure in solution. Biophys J. 1990 Aug;58(2):403–411. doi: 10.1016/S0006-3495(90)82386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porschke D., Tovar K., Antosiewicz J. Structure of the Tet repressor and Tet repressor-operator complexes in solution from electrooptical measurements and hydrodynamic simulations. Biochemistry. 1988 Jun 28;27(13):4674–4679. doi: 10.1021/bi00413a014. [DOI] [PubMed] [Google Scholar]
- Pörschke D. Electric, optical and hydrodynamic parameters of lac repressor from measurements of the electric dichroism. High permanent dipole moment associated with the protein. Biophys Chem. 1987 Nov;28(2):137–147. doi: 10.1016/0301-4622(87)80083-2. [DOI] [PubMed] [Google Scholar]
- Rees D. C., Lewis M., Lipscomb W. N. Refined crystal structure of carboxypeptidase A at 1.54 A resolution. J Mol Biol. 1983 Aug 5;168(2):367–387. doi: 10.1016/s0022-2836(83)80024-2. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Rizzo V., Schellman J. A. Matrix-method calculation of linear and circular dichroism spectra of nucleic acids and polynucleotides. Biopolymers. 1984 Mar;23(3):435–470. doi: 10.1002/bip.360230305. [DOI] [PubMed] [Google Scholar]
- Scheider W. Dielectric Relaxation of Molecules with Fluctuating Dipole Moment. Biophys J. 1965 Sep;5(5):617–628. doi: 10.1016/s0006-3495(65)86740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht P. Dielectric properties of hemoglobin and myoglobin. II. Dipole moment of sperm whale myoglobin. Biopolymers. 1969;8(6):757–765. doi: 10.1002/bip.1969.360080606. [DOI] [PubMed] [Google Scholar]
- Takashima S., Asami K. Calculation and measurement of the dipole moment of small proteins: use of protein data base. Biopolymers. 1993 Jan;33(1):59–68. doi: 10.1002/bip.360330107. [DOI] [PubMed] [Google Scholar]
- Takashima S. Use of protein database for the computation of the dipole moments of normal and abnormal hemoglobins. Biophys J. 1993 May;64(5):1550–1558. doi: 10.1016/S0006-3495(93)81524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Roxby R. Interpretation of protein titration curves. Application to lysozyme. Biochemistry. 1972 May 23;11(11):2192–2198. doi: 10.1021/bi00761a029. [DOI] [PubMed] [Google Scholar]
- Tsukada H., Blow D. M. Structure of alpha-chymotrypsin refined at 1.68 A resolution. J Mol Biol. 1985 Aug 20;184(4):703–711. doi: 10.1016/0022-2836(85)90314-6. [DOI] [PubMed] [Google Scholar]
- Wang D., Bode W., Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A resolution. J Mol Biol. 1985 Oct 5;185(3):595–624. doi: 10.1016/0022-2836(85)90074-9. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]



