Abstract
We report here the striking anisotropy of fluorescence exhibited by crystals of native green fluorescence protein (GFP). The crystals were generated by water dialysis of highly purified GFP obtained from the jellyfish Aequorea. We find that the fluorescence becomes six times brighter when the excitation, or emission, beam is polarized parallel (compared with perpendicular) to the crystal long axis. Thus, the major dipoles of the fluorophores must be oriented very nearly parallel to the crystal long axis. Observed in a polarizing microscope between parallel polars instead of either a polarizer or analyzer alone, the fluorescence polarization ratio rises to an unexpectedly high value of about 30:1, nearly the product of the fluorescence excitation and emission ratios, suggesting a sensitive method for measuring fluorophore orientations, even of a single fluorophore molecule. We have derived equations that accurately describe the relative fluorescence intensities of crystals oriented in various directions, with the polarizer and analyzer arranged in different configurations. The equations yield relative absorption and fluorescence coefficients for the four transition dipoles involved. Finally, we propose a model in which the elongated crystal is made of GFP molecules that are tilted 60° to align the fluorophores parallel to the crystal long axis. The unit layer in the model may well correspond to the arrangement of functional GFP molecules, to which resonant energy is efficiently transmitted from Ca2+-activated aequorin, in the jellyfish photophores.
Green fluorescence protein (GFP), initially extracted and purified from the luminescent jellyfish, Aequorea sp, converts the blue light (that would otherwise be emitted by the Ca2+-sensitive protein aequorin) into a brilliant green fluorescence, the “luminescence” emitted by this jellyfish (1–3). Both of these proteins are localized at high concentration in the photophores of Aequorea. Today, GFP and its genetically encoded variants are widely used as noninvasive fluorescent biosensors for protein expression, protein dynamics, and protein–protein interactions in living cells (4, 5).
According to x-ray crystallographic analyses (6, 7), GFP is a barrel-shaped molecule (28 kDa, made of 238-aa residues), about 24 Å in diameter and 42 Å in length. The outer cylinder of the barrel (the “β can”) is composed of 11 antiparallel β sheets capped with α-helical stretches of the molecule, part of which extends to the interior of the can and forms the fluorescent chromophore. The chromophore is situated near the center of the β can and lies at ≈60° to the long axis of the can. Interaction between the (posttranslationally modified) tripeptide chromophore and those of neighboring residues is shown to determine the exact fluorescence property of GFP and its related constructs (6–8); the chromophore is no longer fluorescent when isolated from the β can.
In this paper, we report on the anisotropic fluorescent properties of GFP as measured in crystals of native GFP (see Materials and Methods). Both the excitation and emission by the GFP crystals are found to show high polarization ratios. In addition, we show that the maximum-to-minimum fluorescence ratio observed between parallel polars is apparently governed by the product of the excitation and emission polarization ratios, reaching the high value of ≈30:1. These high values suggest that the chromophores in the crystals are arranged uniformly and that the crystals are effectively uniaxial.
These observations allow us to: (i) deduce the orientation of the chromophores, and in turn the packing arrangement of the GFP molecules, in the crystals of native GFP; and (ii) calculate the relative fluorescence polarization efficiencies of the absorbing chromophore dipoles and emitting dipoles. In addition, (iii) the exceptionally high fluorescence ratios observed between parallel polars are expected to provide a new method for dynamically observing, and quantifying, the changing orientation of fluorescent chromophores constituting (or attached to) functional molecular structures.
Materials and Methods
Preparation of GFP Crystals.
GFP extracted from the jellyfish Aequorea was separated from aequorin and purified by column chromatography on anion exchangers and size-exclusion gels (3) by using improved chromatographic media now available. Highly purified GFP (2 mg, A400 nm/A280 nm = 1.3) in 0.3 ml of 10 mM sodium phosphate buffer (pH 7.0) containing 0.1 M NaCl was placed in a 0.5-ml Slide-A-Lyzer cassette with a float (Pierce) and dialyzed overnight against 500 ml of deionized water contained in a borosilicate glass (Pyrex, Corning, NY) beaker at 4°C with slow stirring. The dialysis was continued with two more changes of fresh deionized water, without stirring. Crystals began to form in 2 days during dialysis with the last change of water. The contents of the cassette were transferred into a small plastic test tube with a syringe (18-gauge needle) and left standing at room temperature without cover to grow the crystals by spontaneous evaporation.
Determined by SDS/PAGE with Coomassie blue staining, both the mother liquor and the crystals contained >98% pure GFP of molecular mass 28 kDa (published as supporting information on the PNAS web site, www.pnas.org).
Preparation of GFP Crystals for Microscopy.
Crystals in 5–10 μl of water were sandwiched between two 0.17-mm-thick fused quartz coverslips and mounted on a stainless steel support slide. The preparation was sealed with Valap (a 1:1:1 heated mixture of Vaseline, lanolin, and paraffin). Glass slide and coverslips were not used, because they caused the crystals to dissolve from their ends in a few hours, presumably by leaching out of glass components into the unbuffered medium.
The Microscope Optical System.
Most of the observations were made in transmitted light fluorescence mode on a custom-built inverted polarizing microscope (9) in which light travels in a straight line between the virtual light source (output of the light-scrambling optical fiber) and the charge-coupled device (CCD) camera. Thus, the light path is free of any reflecting or beam-splitting components that can inadvertently affect the polarization state of the illuminating or imaging beam. The specimen, supported on a graduated very high-precision revolving stage, was illuminated by the output of a 100-W mercury arc lamp [Osram (Berlin) HBO-100]. Before reaching the specimen, the output of the lamp (made uniform in the aperture and field planes by passing through the fiber-optic light scrambler) was polarized through a Glan–Thompson polarizer [Karl Lambrecht (Chicago)] and filtered through a 450-nm low-pass filter [Corion (Holliston, MA) LS-450-F-K172-Corion]. The field diaphragm image was focused with a long-working-distance condenser [Nikon strain-free 0.52 numerical aperture (N.A.), 16-mm focal length] whose N.A. was set to 0.35. The specimen image was captured with an N.A. 0.4 objective lens (Leitz “32×/0.65 UMK,” equipped with an aperture diaphragm), followed by a Glan–Thompson analyzer, a 527 ± 15-nm barrier filter (Chroma Technology, Brattleboro, VT), and a zoom ocular (Nikon with c-mount) onto a research-grade digital CCD camera [Hamamatsu (Middlesex, NJ) Orca-1]. The strain-free condenser and objective lenses were used with moderately low numerical apertures to prevent the polarization aberrations that can be induced at high N.A. (10) and could interfere with our measurements.
The optical sections in Fig. 3C were acquired in the epifluorescence mode with the Orca-1 camera through a confocal unit [Yokogawa (Hachioji-shi, Tokyo) CSU-10 equipped with a 520 ± 6-nm barrier filter and illuminated by a 488-nm Argon ion laser] mounted on a Leica DMRA microscope equipped with a Plan Fluotar ×100/1.3 N.A. oil objective and controlled with an image acquisition and analysis computer [Universal Imaging (Media, PA) metamorph].
Figure 3.
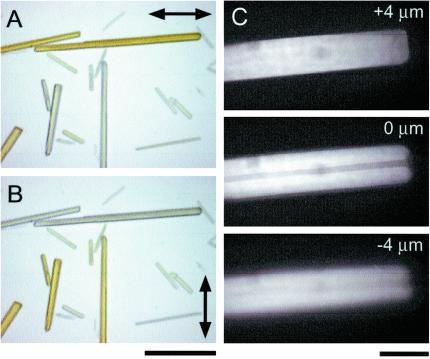
(A and B) Dichroism of GFP crystals observed with quartz halogen illuminator. Double-headed arrows: polarizer transmission axis. (Bar = 30 μm.) (C) Confocal epifluorescence optical sections taken 4 μm above, at, and 4 μm below the mid plane of GFP crystal with large hollow core. The asymmetry of the image above and below the midplane and the strong flare reflects the high refractive index of the crystal wall material. (Bar = 10 μm.)
The color images were acquired by transillumination with a Zeiss AxioCam charge-coupled device camera on a Zeiss Axio-Plan-2ie microscope equipped with a Plan Neofluar ×20/0.50 N.A. objective lens. For Fig. 1, a Zeiss filter cube that included a 510 ± 25-nm barrier filter was inserted above the objective lens, and the specimen was illuminated with a quartz halogen lamp through a polarizing filter and a 450-nm low pass filter. For Fig. 3A, the excitation and barrier filters were removed and only the polarizing filter was used.
Figure 1.
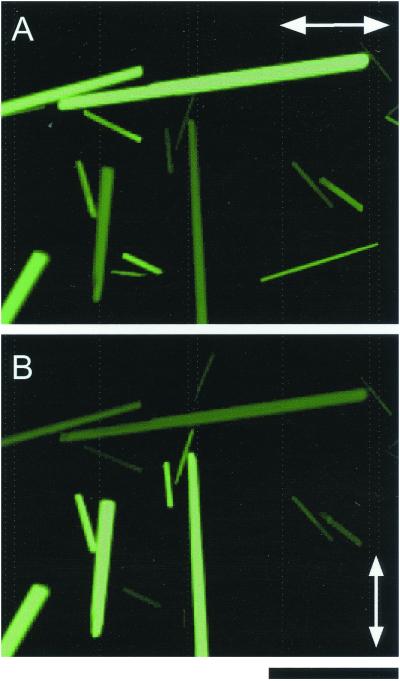
(A and B) Fluorescence of GFP crystals illuminated with <460-nm wavelength plane polarized light and observed through 527 ± 15-nm barrier filter. The transmission axis of the polarizer is oriented horizontally in A and vertically in B. No analyzer was present. (Bar = 30 μm.)
Image Analysis.
Fluorescence and background intensities were measured directly from the 12-bit digital images captured by the Orca-1 camera into metamorph. Exposures, which were kept constant for any series of experiments, were chosen to keep the maximum and minimum pixel intensity values lying well within the linear range of the system, and with the minimum fluorescence intensity still significantly higher than the background. The average pixel values, of a circular region of interest narrower than the width of the crystals, were measured for the crystal fluorescence and corrected by subtracting the adjoining background pixel values for the same size area.
Results
Fluorescence Polarization of Native GFP Crystals.
When column-purified GFP from Aequorea are repeatedly dialyzed against distilled water in a borosilicate glass beaker, numerous needle- to rod-shaped crystals appear in 2 days (see Materials and Methods). As pellets in a plastic centrifuge tube, they show a bright green fluorescence that can be seen by the naked eye even in room light. The crystals range from less than 1 μm to many micrometers in width and up to several hundred micrometers in length (3). Many of the crystals showed a thin hollow core, as described later. Nevertheless, x-ray diffraction analysis showed these to be true three-dimensional crystals (see supporting information II on the PNAS web site).
We examined the fluorescence of these crystals with a transilluminating polarizing microscope (see Materials and Methods) under several polarization conditions. When the ratio of maximum-to-minimum fluorescence intensities were measured under polarized illumination (i.e., the polarizer is present but the analyzer is absent), the intensity is maximum when the crystal long axis lies exactly parallel to the transmission axis of the polarizer and minimum when the two are perpendicular to each other. The ratio of fluorescence intensities was ≈6:1 (Fig. 1 A and B, Table 1). In other words, the anisotropic excitation ratio, or the dichroic absorption ratio, of the GFP crystals amounts to ≈6:1. This is a remarkably high number, for example, compared with the 4:1 dichroic ratio between 240- and 390-nm wavelength for B-form DNA, in which the UV-absorbing nucleotide bases are all aligned at nearly 90° to the fiber axis (11, 12).
Table 1.
Anisotropy of fluorescence observed in crystals of native GFP
| Presence of polarizer | Presence of analyzer | Max-to-min fluorescence ratio
|
||
|---|---|---|---|---|
| Mean ± SD | n | Minimum, median, maximum | ||
| Yes | No | 6.22 ± 1.00 | 16 | 4.73, 5.96, 8.11 |
| No | Yes | 5.93 ± 1.06 | 23 | 4.31, 5.78, 7.79 |
| Yes | Yes* | 29.61 ± 6.69 | 28 | 18.94, 29.57, 44.82 |
| Yes | Yes† | 1.23 ± 0.01 | 3 | 1.23, 1.23, 1.24‡ |
Polarizer transmission axis is parallel to analyzer transmission axis.
Polarizer transmission axis is perpendicular to analyzer transmission axis.
Ratio of minimum1-to-minimum2 [see supporting information III(iv) on the PNAS web site].
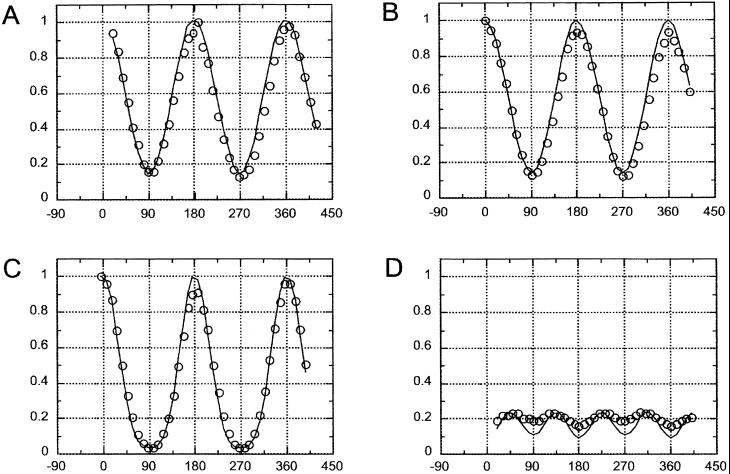
Fig. 2A plots the fluorescence intensity changes under polarized illumination, with the crystal long axis oriented in various directions relative to the stationary polarizer transmission axis. The measured points [Fig. 2A (open circles)] fit closely with the fractional absorption expected of a dipole absorber oriented at different angles (φ) to the polarized excitation light, i.e., they follow a cos2 φ relationship [Eq. 1]. Therefore, the chromophores that absorb the polarized excitation in the native GFP crystals act as though they were dipoles that are all oriented nearly parallel to the crystal long axis.
Figure 2.
Normalized fluorescence intensities of crystal versus orientation angle of microscope stage. (A) Open circles: intensity measurements with polarizer present, analyzer absent. Polarizer transmission axis is at 0°. Solid line: plot of I/I0 = 14.05 × cos2φ + 2.65 (from Eq. 1). (B) Open circles: intensity measurements with analyzer present but no polarizer. Analyzer transmission axis is at 0°. Solid line: Plot of I/I0 = 14.55 × cos2φ + 2.40 (from Eq. 2). (C) Open circles: Intensity measurements with both polarizer and analyzer present with their axes parallel to each other and oriented at 0°. Solid line: plot of I/I0 = 22.5 × cos4φ + 2.8 × cos2φ + 1 (from Eq. 3). (D) Open circles: intensity measurements with both polarizer and analyzer present with their axes crossed. Solid line: plot of I/I0 = −11.25 × cos4φ + 11.0 × cos2φ + 2.15 (from Eq. 4). The data points in each graph are for an individual crystal with the microscope stage turned every 10°. The points are shown in measured sequence from left to right, with their maxima normalized to 1.0. Except in B and D, the graphs were derived from separate crystals. The intensities in B and D were both normalized by using the peak value in B.
When the crystals are illuminated with nonpolarized light, and the emitted fluorescence is examined through an analyzer (i.e., the polarizer is absent, the analyzer is present), the maximum-to-minimum brightness ratio is again ≈6:1 (Table 1). Furthermore, the fluorescence intensity is again maximum when the analyzer transmission axis is oriented parallel to the crystal long axis and minimum when the two are perpendicular. In other words, the fluorescence-emitting chromophores also behave as an array of dipole emitters whose major transition moments are oriented parallel to the long axis of the crystal of native GFP.
Fig. 2B (open circles) plots the fluorescence changes observed through the analyzer in the absence of a polarizer, with the crystal axis turned to various orientations relative to the analyzer transmission axis. Again the measured points fit a cos2 φ relationship (Eq. 2).
Fig. 2C plots the relative fluorescence emitted by a crystal of native GFP observed between parallel polars, i.e., polarizer and analyzer are both present, with their transmission axes oriented parallel to each other. Again, as the stage is turned and the crystal is rotated around the axis of the microscope, the fluorescence becomes maximum when the crystal long axis comes to lie parallel to the transmission axes of the polars and minimum when the crystal lies perpendicular to the transmission axes of the polars. However, the ratio of fluorescence intensities rose to an extremely high value of ≈30:1 (Fig. 2C; Table 1). We were surprised to find such a high value that approximates the product of the fluorescence excitation and emission polarization ratios.
So far as we are aware, these are the first observations that suggest that the maximum-to-minimum ratio of polarized fluorescence emitted by a chromophore (not moving randomly as in a solution) is, in fact, the product of the dichroism for absorption of the excitation light multiplied by the maximum-to-minimum ratio of polarized fluorescence emission that would be excited by nonpolarized light.
It is also interesting to note how the use of parallel polars dramatically increases the polarization ratio, in fact, by several-fold compared with using a single polarizer or analyzer alone. The use of optical systems using parallel polars would, therefore, be expected to significantly improve the sensitivity for measuring the orientation of fluorescent chromophores in general, not limited to those in GFP.
Equations for Relative Fluorescence Intensities.
Although the fluorescence polarization ratio measured between parallel polars appears to reflect the product of the excitation and emission polarization ratios, the curve in Fig. 2C does not quite fit a cos4φ {= (cos2φ)2} function. In fact, the measured points fit a curve partway between a cos4φ and a cos2φ curve.
To further explore the underlying events, one of us (M.S.) analyzed the quantitative relationships between the events that should be taking place in the dipoles undergoing polarized absorption and emission, making the first-order assumption that the major dipole axes are oriented parallel to the crystal long axis.
As detailed in supporting information III(i) (on the PNAS web site), with the polarizer present, but in the absence of an analyzer (Fig. 2A), the equation becomes:
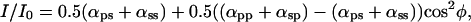 |
1 |
where the αs are proportionately coefficients for the intensity vectors of the fluorescence oriented parallel (αpp, αps) and perpendicular (αsp, αss) to the crystal long axis, relative to the excitation by blue light of unit intensity oriented parallel (αpp, αsp) or perpendicular (αps, αss) to the crystal long axis. φ is the angle between the crystal long axis (major dipole axis) and the transmission axes of the polarizer or analyzer as appropriate.
When the analyzer is present but the polarizer is absent [Fig. 2B, open circles; supporting information III(ii) on the PNAS web site], the equation becomes:
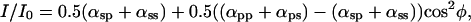 |
2 |
again, proportional to cos2φ plus an offset as in Eq. 1.
For parallel polars, the fluorescence ratio becomes a function that is the sum of a cos4(φ) and a cos2(φ) term plus a small offset, as expressed by the following equation:
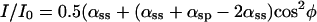 |
3 |
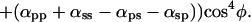 |
The curve for Eq. 3 [supporting information III(iii) on the PNAS web site], using the four coefficients calculated from the measured fluorescence polarization ratios, is plotted in Fig. 2C together with the observed points (open circles). The calculated curve indeed matches the observations very closely.
When the polarizer and analyzer are crossed with each other [Fig. 2D (open circles); supporting information III(iv) on the PNAS web site], the equation becomes:
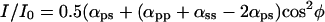 |
4 |
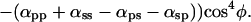 |
As predicted by Eq. 4 (where αps is very nearly equal to αsp), we find that the fluorescence intensity varies only slightly as the crystal orientation is changed between crossed polars. Furthermore, as predicted from the equation, the fluorescence intensity rises and falls four times during a 360° rotation of the crystal axis rather than twice, as was the case for the previous three conditions of measurement. The fluorescence intensity is maximum when the crystal long axis is oriented at approximately 45, 135, 225, and 315° rather than twice at 0 and 180°. Finally, between crossed polars, the fluorescence intensity minimum does not become as low as the minimum for the case of parallel polars (compare Fig. 2 D with C).
Solving these equations, we arrive at the four relative coefficients as: αpp = 29.6 ± 6.7, αps = 4.3 ± 0.5, αsp = 3.8 ± 0.8, and αss = 1, with αss chosen as 1 to normalize these coefficients (see supporting information III on the PNAS web site).
Other Optical Properties of the Crystals.
When observed with a polarizing microscope, illuminated through a 546 ± 25-nm interference filter that removes the fluorescence excitation, the GFP crystals showed a weak birefringence, with the refractive index very slightly larger across the crystal. The coefficient of birefringence was −1.6 × 10−3 (nanometer retardance per micrometer thickness, measured at 546-nm illumination). The weak negative birefringence suggests that the β cans of GFP (in which the β sheets run diagonally at an angle somewhat smaller than 45° to the major axis of the β can) are oriented with a large tilt angle relative to the length of the rod-shaped GFP crystal.
Observed in white light, the crystals also display a discernable visible light dichroism (Fig. 3 A and B). The crystals appeared in a yellowish straw color when the crystal long axis lies parallel to the polarizer or analyzer transmission axis and very pale blue in the perpendicular orientation. This dichroism undoubtedly reflects the orientation of the GFP chromophores whose major blue-absorbing dipoles lie oriented along the length of the crystal.
We noticed that many of the crystals in exact focus showed a weaker fluorescence along the axis of the crystal. At high magnification of a confocal microscope, several of the crystals showed a distinct hollow core (Fig. 3C). Despite this hollow cylindrical morphology, x-ray diffraction patterns of our crystals showed an unambiguous three-dimensional lattice (see supporting information II on the PNAS web site).
Discussion
Utility of the Polarizing Microscope.
As demonstrated, a transilluminating polarizing microscope (to which appropriate excitation and barrier filters are added) provides a major advantage for measuring anisotropy of fluorescence. The distribution of specimen fluorescence, in turn, can be rapidly recorded and measured with the aide of a wide dynamic range modern charge-coupled device camera.
With a transilluminating polarizing microscope, one can: (i) rapidly switch between different modes of polarized excitation and emission; (ii) readily measure specimen fluorescence as a function of stage angles, i.e., at various specimen orientations; and (iii) avoid using beam splitters, birefringent elements, and other optical components that could inadvertently alter the polarization states of the illuminating or imaging beam.
From measurements of the relative fluorescence intensities made under different combinations of polarizer, analyzer, and crystal axis orientations, one can derive the coefficients for fluorescence excitation and emission parallel and perpendicular to the crystal axis. When the crystal contains well-aligned chromophores, those values, in turn, should reflect the fluorescence quantum efficiencies of the major and minor transition dipoles. Additionally, one could, e.g., establish the degree of coherence between the absorbed excitation and emitted fluorescence and the degree of ellipticity of the fluorescence emitted.
Compared with using a single polarizer or analyzer, the use of parallel polars gives rise to a major increase in fluorescence intensity ratios as a function of fluorophore orientation. This new approach should dramatically improve the signal-to-noise ratio and sensitivity for following dynamic changes in orientation, or energy transfer, of individual fluorophores, e.g., when angular orientation changes for portions of single molecules are to be measured by using the polarized emission of single fluorophores (e.g., see refs. 13–15).
Significance of Fluorescence Polarization Ratios.
Between parallel polars, the fluorescence of the native GFP crystal became maximum when the crystal long axis was oriented parallel to the transmission axes of the polarizer and analyzer and minimum at right angles to this direction (Fig. 2C). The ratio of maximum-to-minimum fluorescence was as high as 30:1 (Table 1). When the fluorescence is maximum, the excitation absorbing and fluorescence emitting dipoles must both be oriented very nearly parallel to the polarizer and the analyzer transmission directions. That we observe a fluorescence polarization ratio that approximates the product of the polarized absorption and emission coefficients suggests not only that the chromophores are well aligned parallel to the crystal axis, but also that there is little dissipative loss of energy between fluorescence excitation and emission.
Between crossed polars, a fluorescence minimum is observed when the crystal axis is oriented perpendicular to the polarizer or analyzer axis (open circles in Fig. 2D). These minimum values are, however, several times larger than the minimum observed when the polarizer and analyzer transmission axes are oriented parallel to each other (see Fig. 2C). In fact, the very weak fluorescence emitted by a crystal whose long axis is oriented perpendicular to the transmission axes of polars that are oriented parallel to each other increases in intensity several-fold when the analyzer is turned 90°. This somewhat counterintuitive rise in fluorescence is explained by the fact that the component of polarized emission that parallels the crystal axis is now fully transmitted by the analyzer.
The relative coefficients (α) that we derive are phenomenological values, i.e., they relate to the polarization components parallel and perpendicular to the long axis of the crystal. They cannot be directly ascribed to the anisotropies of the absorbing or emitting chromophores of GFP. Nevertheless, our observations suggest that the transition moment of the chromophore in GFP is not so much planar (as the arrangement of the tripeptide alone may suggest), but that it is nearly linear. They may be highly elongated prolate ellipsoids, oriented parallel to the length of the tubular crystal (Fig. 4C).
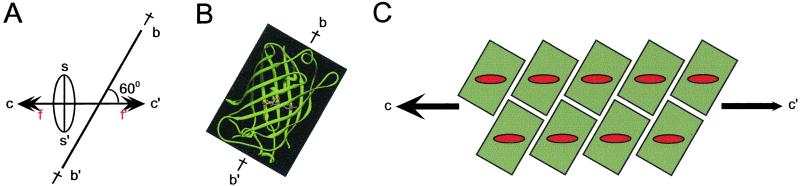
Figure 4.
Schematic of crystal structure. (A) Orientations relative to the long crystal axis (c–c′) of: major dipole for fluorophores (f–f′), slow birefringence axis (s–s′), and β can major axis (b–b′). (B) Published structure of GFP molecule based on x-ray crystallographic analyses (6, 7). (C) Schematic array of molecules fitting conditions shown in A and B. While maintaining the parallel alignment of the chromophore ellipsoids' major axes, this schematic could be modified with the backbone of the β cans rotated around the long crystal axis to occupy two or more discrete orientations.
In our analysis, we assumed that the major dipole axes of the GFP chromophores were all uniformly oriented parallel to the crystal long axis. If, instead, we assume that the dipoles, each of which we now assume to possess infinite polarization ratios, were to be oriented with some scatter angle relative to the crystal long axis, what would be the maximum angle they could deviate from the crystal long axis and still show the polarization ratios similar to what we observed? Model calculations (assuming two sets of incoherently illuminated dipoles that are oriented in a plane normal to the light path at various degrees from the crystal axis) show that the maximum angle by which such dipoles could deviate is approximately 22°. These numbers set a limit to the tilt angle that the chromophores could exhibit relative to the crystal long axis, assuming that each dipole exhibits an infinite polarization ratio for fluorescence excitation and emission.
Proposed Arrangement of the GFP Molecules Within the Crystal and Jellyfish Photophore.
We envision that the major absorbing and emitting dipoles of the fluorescent chromophores in our water-dialyzed crystals of native GFP are regularly aligned parallel to the crystal long axis (Fig. 4 A f–f′, C). Taking into account reported x-ray crystallographic data showing that the plane of the conjugated bonds of the fluorescent chromophores are oriented approximately 60° to the long axis of the β can (Fig. 4B), we come up with a schematic model for the arrangements of the GFP molecules in the crystals as shown in Fig. 4C and as expanded in the figure legend.
Within the light-emitting cells of the jellyfish Aequorea, GFP and aequorin molecules are tightly packed in small membrane-bound photophores (3). The intact photophores emit only green light characteristic of GFP fluorescence but no blue light from aequorin.
We speculate that within the photophores, sheets of GFP molecules, with chromophores oriented in a regular array as depicted in Fig. 4C, alternate with sheets of aequorin molecules so that the two chromophore groups are in close proximity and oriented parallel to each other. Blue energy from the aequorin layer, triggered by Ca2+ ions, would then nonradiatively excite the green fluorescence of GFP. Fingers or sheets of cell membrane, layered next to the aequorin layers, could efficiently control the Ca2+ environment of aequorin. We hope to test this model by checking the fluorescence anisotropy and ultrastructure of the intact Aequorea photophores.
The thin rod-shaped crystals that we used for our optical analyses were formed in ≈2 days by dialysis of highly concentrated GFP against deionized water. The same material, diluted by 3-fold to more slowly produce somewhat larger rod-shaped crystals (of up to 15 μm in width), was used to obtain the x-ray diffraction pattern (see supporting information II on the PNAS web site). By growing crystals with even better formed faces, we may be able to measure the fluorescence polarization ratios for light traveling along different axes of the crystal. The scatter of the data we report in Table 1 may then be explained as representing different ratios of the polarized fluorescence originating from chromophores viewed from different angles.
Supplementary Material
Acknowledgments
We thank Dr. Toshiya Senda of the Biological Information Research Center, National Institute of Advanced Industrial Science and Technology, Tokyo, for the x-ray diffraction analysis; Dr. Raymond E. Stephens of the Marine Biological Laboratory (MBL), Woods Hole, MA, for the protein analysis; and Drs. Kensal van Holde of Oregon State University, Carolyn Cohen of Brandeis University, Edward D. Salmon of the University of North Carolina, and Rudolf Oldenbourg of MBL, Woods Hole, for careful reading and comments on draft versions of the manuscript. We also thank Rudi Rottenfusser and Louis Kerr for help with the Zeiss microscope at the MBL Central Microscopy Facility, Bob Knudson for fabricating microscope parts as needed, and Jane MacNeil for typing and editing the paper. Loans or gifts of equipment were provided by Chroma Technology, Dage-MTI, Hamamatsu Photonics, Leica, Nikon, Olympus, Universal Imaging, Yokogawa Electric, and Carl Zeiss. We are also grateful for support provided by Dr. Yoshinori Fujiyoshi of Kyoto University; JEOL; a New Energy and Industrial Technology Development Organization Fellowship (to M.G.); a National Institutes of Health (NIH) Postdoctoral Fellowship (to P.T.T.), and Rudolf Oldenbourg's NIH grant (to M.S.).
Abbreviations
- GFP
green fluorescence protein
- N.A.
numerical aperture
References
- 1.Johnson F H, Shimomura O, Saga Y, Gershman L C, Reynolds G T, Waters J R. J Cell Comp Physiol. 1962;60:85–103. [Google Scholar]
- 2.Morin J G, Hastings J W. J Cell Physiol. 1971;77:313–318. doi: 10.1002/jcp.1040770305. [DOI] [PubMed] [Google Scholar]
- 3.Morise H, Shimomura O, Johnson F H, Winant J. Biochemistry. 1974;13:2656–2662. doi: 10.1021/bi00709a028. [DOI] [PubMed] [Google Scholar]
- 4.Chalfie M, Kain S, editors. Green Fluorescent Protein: Properties, Applications, and Protocols. New York: Wiley–Liss; 1998. [Google Scholar]
- 5.Sullivan K F, Kay S A, editors. Green Fluorescent Proteins, Methods in Cell Biology. San Diego: Academic; 1999. [DOI] [PubMed] [Google Scholar]
- 6.Ormö M, Cubitt A B, Kallio K, Gross L A, Tsien R Y, Remington S J. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Moss L G, Phillips G N., Jr Nat Biotechnol. 1996;14:1246–1251. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- 8.Ward W W. In: Green Fluorescent Protein: Properties, Applications, and Protocols. Chalfie M, Kain S, editors. New York: Wiley–Liss; 1998. pp. 45–75. [Google Scholar]
- 9.Inoué S, Spring K R. Video Microscopy–The Fundamentals. 2nd Ed. New York: Plenum; 1997. pp. 158–161. [Google Scholar]
- 10. Shribak, M., Inoué, S. & Oldenbourg, R. (2002) Opt. Eng.41, in press.
- 11.Seeds W E. Progr Biophys Biophys Chem. 1953;3:27–46. [Google Scholar]
- 12.Inoué S, Sato H. In: Molecular Architecture in Cell Physiology. Haysahi T, Szent-Györgyi A G, editors. New York: Prentice–Hall; 1966. pp. 209–248. [Google Scholar]
- 13.Corrie J E T, Brandmeier B D, Ferguson R E, Trentham D R, Kendrick-Jones J, Hopkins S C, van der Heide U A, Goldman Y E, Sabido-David C, Dale R E, et al. Nature (London) 1999;400:425–430. doi: 10.1038/22704. [DOI] [PubMed] [Google Scholar]
- 14.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Nature (London) 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 15.Warshaw D M, Hayes E, Gaffney D, Lauzon A, Wu J, Kennedy G, Trybus K, Lowey S, Berger C. Proc Natl Acad Sci USA. 1998;95:8034–8039. doi: 10.1073/pnas.95.14.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.