Abstract
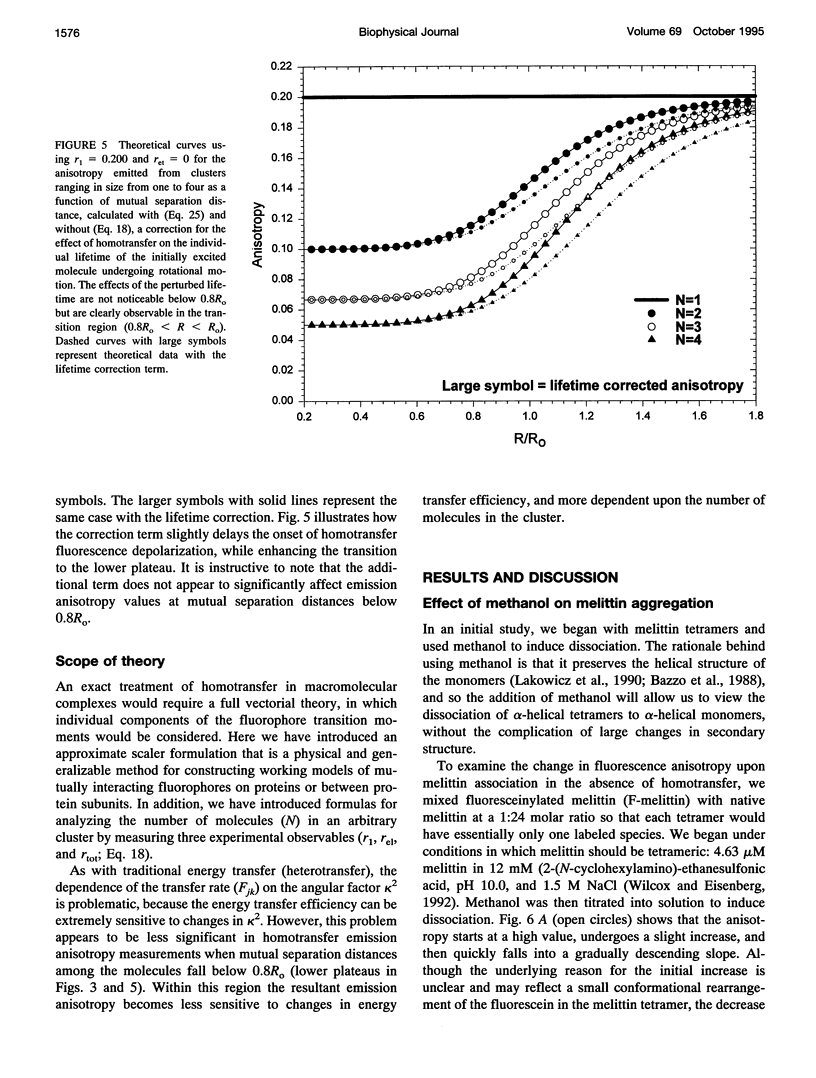
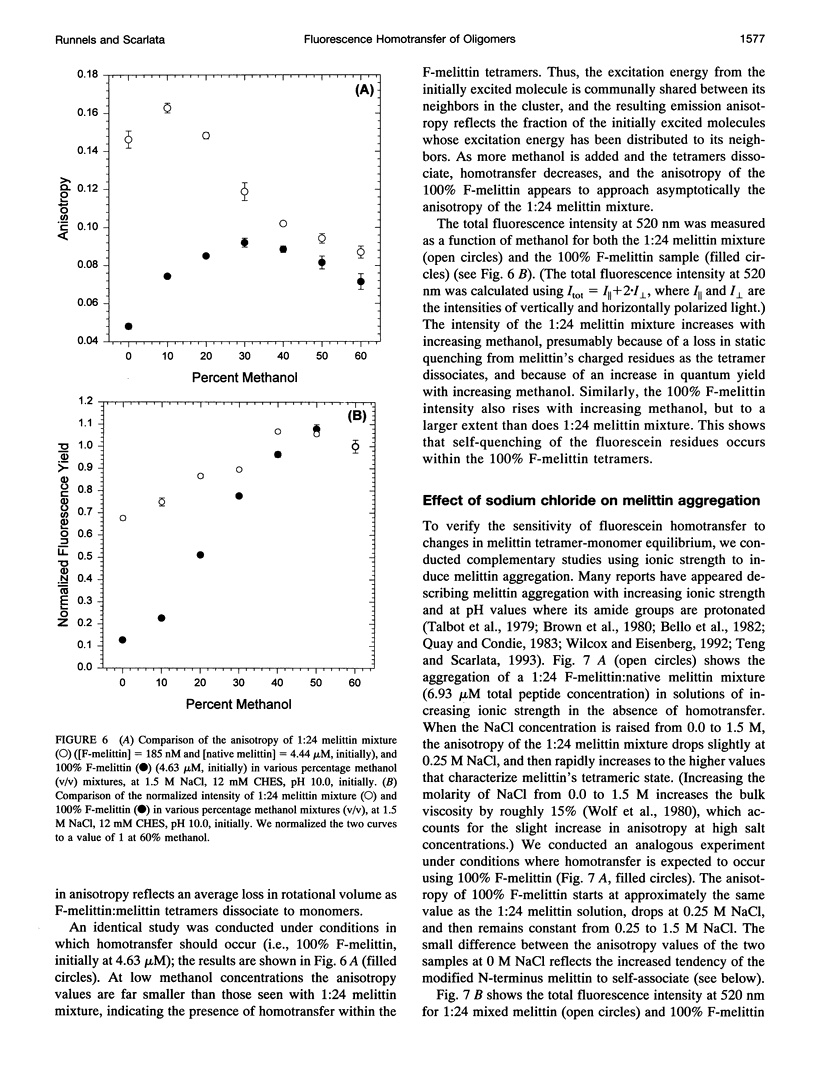
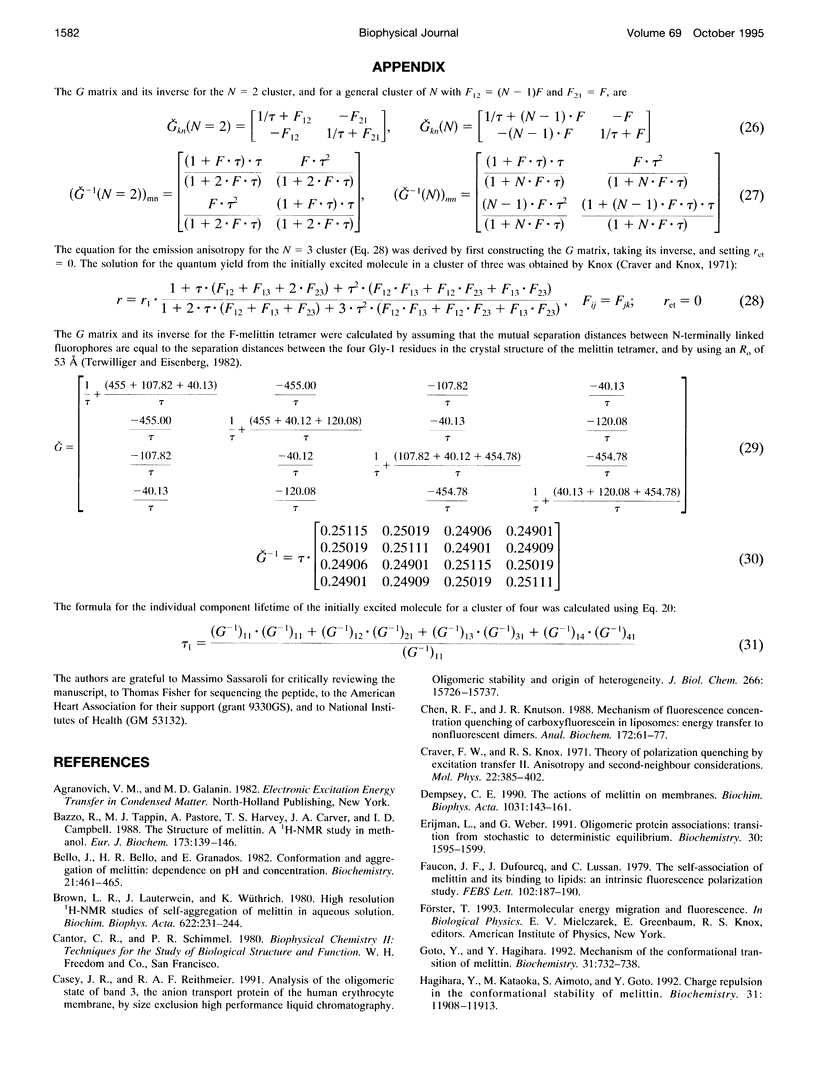
Fluorescence homotransfer (electronic energy transfer between identical fluorophores) has the potential to quantitate the number of subunits in membrane protein oligomers. Homotransfer strongly depolarizes fluorescence emission as a result of intermolecular excitation energy exchange between an initially excited, oriented molecule and a randomly oriented neighbor. We have theoretically treated fluorescein labeled subunits in an oligomer as a cluster of molecules that can exchange excitation energy back and forth among the subunits within that group. We find that the larger the number of subunits, the more depolarized is the emission. The general equations to calculate the expected anisotropy for complexes composed of varying numbers of labeled subunits are presented. Self-quenching of fluorophores, orientation, and changes in lifetime are also discussed and/or considered. To test this theory, we have specifically labeled melittin on its N-terminal with fluorescein and monitored its monomer to tetramer equilibrium both in solution and in lipid bilayers. The calculated anisotropies are close to the experimental values when non-fluorescent fluorescein dimers are taken into account. Our results show that homotransfer may be a promising method to study membrane-protein oligomerization.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzo R., Tappin M. J., Pastore A., Harvey T. S., Carver J. A., Campbell I. D. The structure of melittin. A 1H-NMR study in methanol. Eur J Biochem. 1988 Apr 5;173(1):139–146. doi: 10.1111/j.1432-1033.1988.tb13977.x. [DOI] [PubMed] [Google Scholar]
- Bello J., Bello H. R., Granados E. Conformation and aggregation of melittin: dependence on pH and concentration. Biochemistry. 1982 Feb 2;21(3):461–465. doi: 10.1021/bi00532a007. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Lauterwein J., Wüthrich K. High-resolution 1H-NMR studies of self-aggregation of melittin in aqueous solution. Biochim Biophys Acta. 1980 Apr 25;622(2):231–244. doi: 10.1016/0005-2795(80)90034-3. [DOI] [PubMed] [Google Scholar]
- Casey J. R., Reithmeier R. A. Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J Biol Chem. 1991 Aug 25;266(24):15726–15737. [PubMed] [Google Scholar]
- Chen R. F., Knutson J. R. Mechanism of fluorescence concentration quenching of carboxyfluorescein in liposomes: energy transfer to nonfluorescent dimers. Anal Biochem. 1988 Jul;172(1):61–77. doi: 10.1016/0003-2697(88)90412-5. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Erijman L., Weber G. Oligomeric protein associations: transition from stochastic to deterministic equilibrium. Biochemistry. 1991 Feb 12;30(6):1595–1599. doi: 10.1021/bi00220a022. [DOI] [PubMed] [Google Scholar]
- Faucon J. F., Dufourcq J., Lussan C. The self-association of melittin and its binding to lipids: an intrinsic fluorescence polarization study. FEBS Lett. 1979 Jun 1;102(1):187–190. doi: 10.1016/0014-5793(79)80956-4. [DOI] [PubMed] [Google Scholar]
- Goto Y., Hagihara Y. Mechanism of the conformational transition of melittin. Biochemistry. 1992 Jan 28;31(3):732–738. doi: 10.1021/bi00118a014. [DOI] [PubMed] [Google Scholar]
- Hagihara Y., Kataoka M., Aimoto S., Goto Y. Charge repulsion in the conformational stability of melittin. Biochemistry. 1992 Dec 1;31(47):11908–11914. doi: 10.1021/bi00162a033. [DOI] [PubMed] [Google Scholar]
- Hebert D. N., Carruthers A. Cholate-solubilized erythrocyte glucose transporters exist as a mixture of homodimers and homotetramers. Biochemistry. 1991 May 14;30(19):4654–4658. doi: 10.1021/bi00233a003. [DOI] [PubMed] [Google Scholar]
- Hebert D. N., Carruthers A. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J Biol Chem. 1992 Nov 25;267(33):23829–23838. [PubMed] [Google Scholar]
- John E., Jähnig F. Aggregation state of melittin in lipid vesicle membranes. Biophys J. 1991 Aug;60(2):319–328. doi: 10.1016/S0006-3495(91)82056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. S. On the theory of trapping of excitation in the photosynthetic unit. J Theor Biol. 1968 Nov;21(2):244–259. doi: 10.1016/0022-5193(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Gryczynski I., Wiczk W., Laczko G., Prendergast F. C., Johnson M. L. Conformational distributions of melittin in water/methanol mixtures from frequency-domain measurements of nonradiative energy transfer. Biophys Chem. 1990 Jul;36(2):99–115. doi: 10.1016/0301-4622(90)85014-w. [DOI] [PubMed] [Google Scholar]
- Lauterwein J., Brown L. R., Wüthrich K. High-resolution 1H-NMR studies of monomeric melittin in aqueous solution. Biochim Biophys Acta. 1980 Apr 25;622(2):219–230. doi: 10.1016/0005-2795(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Quay S. C., Condie C. C. Conformational studies of aqueous melittin: thermodynamic parameters of the monomer-tetramer self-association reaction. Biochemistry. 1983 Feb 1;22(3):695–700. doi: 10.1021/bi00272a026. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T. Kinetics of polymerization of a fluoresceinated derivative of complement protein C9 by the membrane-bound complex of complement proteins C5b-8. Biochemistry. 1984 Jul 3;23(14):3260–3267. doi: 10.1021/bi00309a021. [DOI] [PubMed] [Google Scholar]
- Steinberg I. Z. Long-range nonradiative transfer of electronic excitation energy in proteins and polypeptides. Annu Rev Biochem. 1971;40:83–114. doi: 10.1146/annurev.bi.40.070171.000503. [DOI] [PubMed] [Google Scholar]
- Talbot J. C., Dufourcq J., de Bony J., Faucon J. F., Lussan C. Conformational change and self association of monomeric melittin. FEBS Lett. 1979 Jun 1;102(1):191–193. doi: 10.1016/0014-5793(79)80957-6. [DOI] [PubMed] [Google Scholar]
- Teng Q., Scarlata S. Effect of high pressure on the association of melittin to membranes. J Biol Chem. 1993 Jun 15;268(17):12434–12442. [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. I. Structure determination and partial refinement. J Biol Chem. 1982 Jun 10;257(11):6010–6015. doi: 10.2210/pdb1mlt/pdb. [DOI] [PubMed] [Google Scholar]
- Timmins P. A., Zaccai G. Low resolution structures of biological complexes studied by neutron scattering. Eur Biophys J. 1988;15(5):257–268. doi: 10.1007/BF00256476. [DOI] [PubMed] [Google Scholar]
- Voss J., Jones L. R., Thomas D. D. The physical mechanism of calcium pump regulation in the heart. Biophys J. 1994 Jul;67(1):190–196. doi: 10.1016/S0006-3495(94)80469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Daniel E. Cooperative effects in binding by bovine serum albumin. II. The binding of 1-anilino-8-naphthalenesulfonate. Polarization of the ligand fluorescence and quenching of the protein fluorescence. Biochemistry. 1966 Jun;5(6):1900–1907. doi: 10.1021/bi00870a017. [DOI] [PubMed] [Google Scholar]
- Wilcox W., Eisenberg D. Thermodynamics of melittin tetramerization determined by circular dichroism and implications for protein folding. Protein Sci. 1992 May;1(5):641–653. doi: 10.1002/pro.5560010510. [DOI] [PMC free article] [PubMed] [Google Scholar]




