Abstract
This study aimed to investigate the ultrastructural anatomy of the developing ACL tibial enthesis. We hypothesized that enthesis architecture would progressively mature and remodel, eventually resembling that of the adult by the early postnatal stage. Five fresh-frozen human pediatric cadaveric knees aged 1–36 months underwent anatomical dissection to harvest the ACL insertion and underlying tibial chondroepiphysis. The samples were prepared for scanning electron microscopy (SEM) to examine the ultrastructural anatomy of the enthesis and underwent histological staining for circular polarized light (CPL) and light microscopy imaging. SEM analysis of the 1- and 8-month-old samples revealed a shallow interdigitation between the dense fibrous (ligamentous) tissue and unmineralized chondrogenic tissues, with a minimal transition zone. By 11-month, a more complex transition zone was present. By age 19- and 36-month-old, a progressively more complex and defined fibrocartilage zone was observed. CPL analysis revealed distinct collagen fiber continuity, alignment, and organization changes over time. By 19 and 36 months, the samples exhibited complex fiber arrangements and a progression toward uniform fiber orientation. Similarly, histological analysis demonstrated progressive remodeling of the enthesis with increasing age. Our results suggest that the ACL enthesis of the developing knee begins to mimic that of an adult as early as 19 months of age, as a more complex transition between ligamentous and chondro-epiphyseal tissue can be appreciated. We hypothesize that the observed changes are likely due to mechanical loading of the enthesis with the onset of weightbearing. Future investigations of ACL reconstruction and repair will benefit from improved understanding of the chondro-epiphyseal/ACL regions.
Keywords: ACL, enthesis, pediatric knee, SEM
1 ∣. INTRODUCTION
Anterior cruciate ligament injury occurs commonly in the pediatric and adult populations, with an increasing prevalence.1,2 For younger patients or active older individuals, surgical management with ligament reconstruction is considered standard of care.3 Numerous graft types have been described, including bone-patellar tendon-bone, quadrupled hamstring, quadriceps, and various allografts, each with advantages and disadvantages.4 Regardless of graft type, all grafts undergo a “ligamentization” process with gradual changes in microstructure and composition of the tissue and incorporation into the surrounding bone.5,6 Ultimate graft function is critically dependent upon secure healing of the tendon graft to bone.
The native enthesis has a unique structure, occurring through interdigitation of collagen fibers of the ligament with those of the adjacent bone, by way of a transition zone of unmineralized and mineralized fibrocartilage. This unique structure allows for graduated change in stiffness and favorable stress distribution properties, diminishing stress concentration at the transition between soft tissue (ligament) and hard tissue (bone).7 However, the complex microstructure and composition of the native enthesis is not regenerated following ACL reconstruction with a tendon graft; rather, healing occurs via formation of fibrovascular scar tissue.8,9
With an improved understanding of ACL biology, surgical technique, and techniques for biological augmentation, there is a growing emphasis on tissue regeneration. At the same time, there has recently been renewed interest in primary ACL repair following complete rupture in certain patient populations. To understand tissue healing and regeneration, it is important to understand development. While the ultrastructure of the native human ACL has been well studied in adults and animal models, the microscopic ultrastructure of the developing ACL enthesis has not been previously described.10-13
The purpose of this study was to investigate the ultrastructural anatomy of the developing human ACL tibial enthesis at the extremely early postnatal stage, which we defined as the period of 1–36 months of age. We hypothesize that enthesis architecture progressively matures and remodels, eventually resembling that of the adult, and that enthesis structure will be largely completed by the early postnatal stage.
2 ∣. METHODOLOGY
2.1 ∣. Cadaveric specimen acquisition
Cadaveric tissue used in this study did not include any patient identifying information; thus an Institutional Review Board exemption was obtained. In accordance with the waiver for IRB approval, prior consent to use this tissue for research purposes was obtained from families, there was no subsequent contact with families that made these tissue donations, and no genetic tests were performed. Five fresh-frozen human cadaveric knees (from mid-femur to mid-tibia) were obtained from AlloSource Inc, a tissue procurement facility, for anatomical dissection. Samples utilized in this study included human knees aged 1–36 months old, including two females, three males. No specimen had prior lower extremity surgery or history of lower extremity trauma.
2.2 ∣. Specimen dissection
Dissection was performed at the level of the knee joint, starting with an anterior approach, through a midline incision centered along the patella, extended proximally to the quadriceps tendon and distally to the patellar tendon. The extensor mechanism was then completely removed (Figure 1A). In a flexed position, the medial, lateral, and posterior borders of the ACL insertion were identified and incised sharply with a #10 blade, isolating the ACL insertion site along tibial chondroepiphysis (Figure 1B). The ACL was transected at the mid-substance level and the chondroepiphysis block including the ACL tibial foot-print was removed (Figure 1C). A suture was then placed along anterior-medial aspect of bone block to aid in specimen orientation (Figure 1D). Each harvested sample was then placed into individual 50 mL conical centrifuge tube containers (Falcon model 352070; Corning) and 30 mL of 70% ethanol (ETOH) was added to each container.
FIGURE 1.

Labeled images of the 1-month old tissue sample harvesting process including: (A) Removal of the extensor mechanism, (B) isolation of the tibial chondroepiphysis block including the anterior cruciate ligament (ACL) tibial insertion, (C) sectioning of the tissue block along the mid-section of the ACL enthesis, and (D) placement of a suture at the anteromedial aspect of the ACL insertion site on the tibia to aid in specimen orientation during scanning electron microscopy imaging.
2.3 ∣. Scanning electron microscopy imaging
Samples were transected in half using a #10 blade (Figure 2). One half section of the enthesis (Figure 3) was passed through a graded series of ethanol concentrations up to 100% ethanol. The specimen was then placed in a Denton Vacuum DCP-1 Critical Point Drying Apparatus (Denton Vacuum) under pressure to substitute ethanol in the tissues with liquid carbon dioxide. Once returned to atmosphere, the liquid carbon dioxide vaporized, rendering the tissue dry for imaging. Samples were imaged by a Zeiss Gemini 300 field emission scanning electron microscope (SEM; Zeiss) in secondary electron emission mode at 488 nm pixel resolution for observing tissue morphology.
FIGURE 2.

Labeled image of the transected chondroepiphysis block. Surrounded by a dashed outline is the anterior cruciate ligament enthesis zone.
FIGURE 3.

After the chondroepiphysis block including the anterior cruciate ligament tibial foot-print was transected in half, the tissue samples were split into two groups including one for scanning electron microscopy, and the other for histology.
2.4 ∣. Histology and circular polarized light microscopy
The other half of each specimen was prepared for histology. The tissues were processed through graded ethanol substitution to 100% ethanol, infiltrated with methylmethacrylate monomer (Fisher Scientific Company) and polymerized by exposure to ultraviolet light. The polymerized blocks were ground to a uniform 1200 grit finish using a Buehler Metaserv 250 Grinder-Polisher (Buehler). The ground surface was mounted to an EXAKT methylmethacrylate histology slide (EXAKT Technologies, Inc.) with cyanoacrylate adhesive, sectioned through with the Exakt 300 CP Band System, and ground to ca. 110 μm thickness with the Exakt 400CS Grinding System. The sections (and adjacent blocks) were then polished using diamond suspension on the Buehler Metaserv to a ~1 μm surface finish and approximately 100 μm final section thickness.
Histological sections were first imaged by circularly polarized light (CPL) microscopy using a Leica-Leitz DMRX/E Universal Microscope (Leica Microsystems) employing a Leica HC PL Fluotar 10/0.3 objective lens.14 Images of the entire enthesis were acquired at 1.29 μm/pixel with a QIClick color video camera (QImaging) and automated Marzhauser stage (Märzhäuser Wetzlar) using Objective Imaging Surveyor montaging software (Objective Imaging). Histological sections were then surface-stained with Stevenel's Blue and Van Giesen (chemicals from: Alfa Aesar) and imaged by conventional bright field microscopy.15
2.5 ∣. Image analysis
For each sample the enthesis transition zone between the soft and hard tissues (soft tissue defined as the ligamentous midsubstance or fibrocartilage, and hard tissue defined as bone or epiphyseal cartilage before the completion of secondary ossification) was assessed by the first author (S.H.P.) who is experienced in SEM and histological analysis and by two additional investigators (T. J. U., C. E. K.) experienced in both techniques. Each sample was classified on a 6-point scale with zero representing no transition zone, 3 representing a transition zone, defined as interdigitation of tissues, but no “new” tissue morphology and five representing a distinct transition zone that qualitatively had a distinct ultrastructural difference compared to the ligamentous and chondro-epiphyseal tissue (i.e., fibrocartilage zone). Intraclass correlation coefficients (ICC) were determined for transition zone measurements between reviewers using SPSS Software version 28.0 (IBM Corp.). ICC was also determined for the first author between the initial measurements, and measurements repeated 8 weeks later.
3 ∣. RESULTS
SEM analysis provided details of the arrangement and organization of the ultrastructural components within the developing entheses. Analysis 1- and 8-month-old samples revealed a shallow interdigitation between the dense fibrous (ligamentous) and unmineralized chondrogenic tissues, with minimal transition zone (Figure 4A,B). In the 11-month-old, a more complex transition zone between the soft and hard tissues can be appreciated (Figure 4C). While the depth of interdigitation remains shallow, a progressively more complex and defined fibrocartilage zone is observed in the 19- and 36-month-old samples (Figure 4D,E).
FIGURE 4.

Labeled field emission scanning electron microscopy images of the anterior cruciate ligament tibial enthesis of the included study samples. Scale bar is 100 μm in all cases (A–E). (A) The 1-month old sample shows a shallow interdigitation between the ligamentous and chondro-epiphyseal tissue, arrows depict the ligamentous fiber orientation and the dotted line depicts the abrupt transition from ligamentous and chondro-epiphyseal tissues. (B) The 8-month old sample shows a minimal transition zone between ligamentous and chondro-epiphyseal tissue, arrows depict the ligamentous fiber orientation and the dotted line depicts the abrupt transition from ligamentous and chondro-epiphyseal tissues. (C) The 11-month old sample begins to show a more complex transition zone between the soft and hard tissues, outlined in the brackets. (D) The 19-month old sample shows a defined transition zone between ligamentous and chondro-epiphyseal tissue. A fibrocartilage zone, outlined by brackets, can begin to be appreciated. (E) The 36-month old sample shows a complex transition zone between ligamentous and chondro-epiphyseal tissue. A fibrocartilage zone, outlined by brackets, can be appreciated.
CPL imaging provided insights into the collagen fiber continuity, alignment, and organization across different developmental stages of the cadaveric knee ACL entheses samples, revealing distinctive patterns of progressive remodeling with increasing age. Analysis of the 1-month-old sample revealed an absence of collagen fiber penetration into the chondro-epiphyseal tissue, characterized by an abrupt transition without interdigitation of collagen fibers between the dense fibrous (ligamentous) and unmineralized chondrogenic tissues (Figure 5A). A clear demarcation between tissue types at this early stage can be observed. In the 8-month-old sample, uniform, linear collagen fiber interdigitation across the soft and hard tissues can be observed. This stage begins to display a minimal yet discernible transition zone, suggesting early enthesis remodeling and development (Figure 5B). In the 11-month-old sample, a more complex transition zone with more extensive and deeper collagen fiber interdigitation can be appreciated. Notably, this sample exhibits a slight loss of fiber alignment compared to the 8-month-old sample, indicating dynamic changes in tissue architecture. Additionally, please note that the black zones visualized in the lower left corner of the image are unfortunately secondary to damage the sample obtained before imaging. Given that the enthesis was still visualized, the image was included in our results (Figure 5C). The 19- and 36-month old samples show a progressive elaboration of the fibrocartilage zone. The 19-month-old sample presents a complex organization of collagen fibers, while by 36 months, a return to a more uniform fiber orientation is observed, suggesting a maturation of the enthesis (Figure 5D,E).
FIGURE 5.

Labeled Circularly polarized light microscopy images of the anterior cruciate ligament tibial enthesis of the included study samples. Scale bar is 100 μm in all cases (A–E). (A) The 1-month-old sample exhibits an abrupt transition between ligamentous and chondro-epiphyseal tissues with no collagen fiber penetration into the chondro-epiphyseal tissue, outlined by the dotted line. (B) The 8-month-old sample shows a minimal transition zone, outline by the dotted line, with some uniform collagen fiber penetration observed between ligamentous and chondro-epiphyseal tissues, outlined by the arrows. (C) The 11-month-old sample displays a more complex transition zone with disorganized collagen fiber penetration between the soft and hard tissues, outlined by brackets. Please note that the black zones depicted in the lower left corner of the image are secondary to damage the sample obtained before being imaged. (D) The 19-month-old sample reveals a defined transition zone with complex collagen fiber organization, where a fibrocartilage zone begins to be evident, outlined by brackets. (E) The 36-month-old sample demonstrates a complex transition zone and a distinct fibrocartilage zone with uniform fiber orientation, outlined by brackets.
Similarly, histological examination revealed insights into the composition of the extracellular matrix of the samples, which showed gradual restructuring of the enthesis as age advanced. While the 1-month-old sample has an abrupt transition between the dense fibrous (ligamentous) and unmineralized chondrogenic tissues, there is an increased interdigitation of tissues in samples 8- and 11-month old (Figure 6A-C). By 11 months old, a discrete transition zone is appreciated, with the appearance of a more complex interdigitation of ligamentous and chondroepiphyseal tissues. However, the staining pattern remains consistent with the younger samples, indicating no significant changes in tissue morphology (Figure 6C). By ages 19- and 36-month a fibrocartilage transition zone composed of distinctly different cartilage matrix macromolecules can be appreciated, as indicated by the blue staining within the bracketed fibrocartilage zone. This staining represents the presence of new tissue morphology, in particular an increase of proteoglycans and type II collagen which are typically associated with fibrocartilage development (Figure 6D,E).
FIGURE 6.
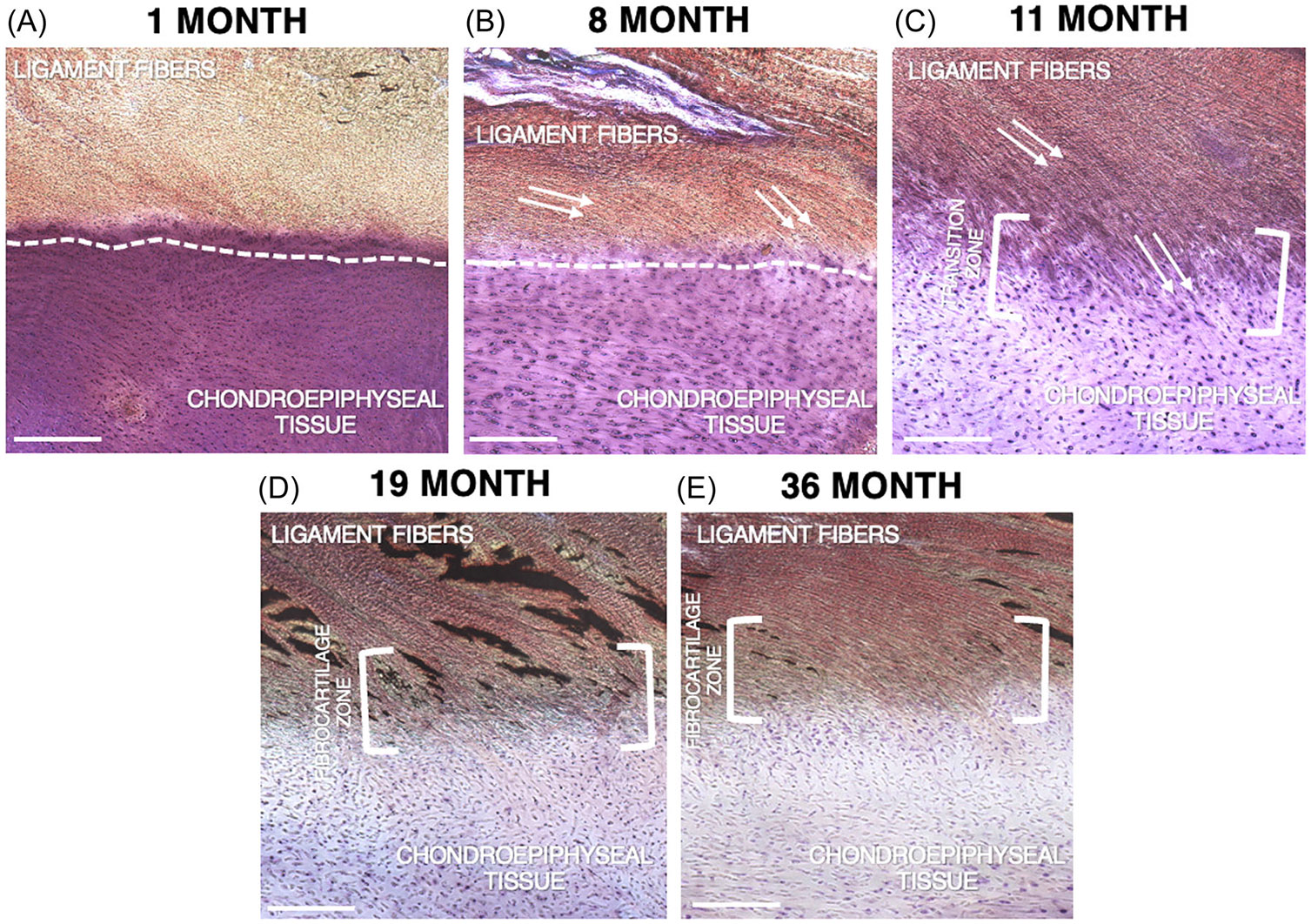
Labeled Light microscopy images of the histological analysis of the anterior cruciate ligament tibial enthesis of the included study samples. Scale bar is 100 μm in all cases (A–E). Samples were stained using Stevenel's Blue and Van Giesen. (A) The 1-month-old sample shows an abrupt transition and shallow interdigitation between the ligamentous and chondro-epiphyseal tissue, outlined by the dotted line. (B) The 8-month-old sample shows a minimal transition zone between ligamentous and chondro-epiphyseal tissue, outline by the dotted line. Ligament fiber orientation can be appreciated, as indicated by the arrows. (C) The 11-month old sample begins to show a more complex transition zone between the soft and hard tissues, outlined by the brackets. (D) The 19-month old sample shows a defined transition zone between ligamentous and chondro-epiphyseal tissue. A fibrocartilage zone composed of a new tissue morphology as seen by the distinct and novel blue staining can begin to be appreciated in the bracketed zone. (E) The 36-month old sample shows a complex transition zone between ligamentous and chondro-epiphyseal tissue. A fibrocartilage zone composed of distinctly different cartilage matrix macromolecules can be appreciated, as indicated by the blue staining within the bracketed fibrocartilage zone.
Excellent inter-rater reliability of transition zone classification was found with ICC for SEM of 0.990 and 1.000, for circularly polarized luminescence of 0.995, and for histology of 1.000. Excellent intra-rater reliability of transition zone classification was also found with ICC for SEM of 1.000 and 0.947, for circularly polarized luminescence of 1.000, and for histology of 0.989.
4 ∣. DISCUSSION
This study investigated the development of the ultrastructural anatomy of the developing human ACL enthesis in the early postnatal stage. Our results suggest continued enthesis ultrastructural maturation in the early postnatal period, as the ACL ultrastructure begins to differ as early as 19 months when compared to that of the 1-month-old. These findings are consistent with our hypothesis that enthesis architecture progressively matures and remodels in the early postnatal period. While the early events in embryonic tissue development and patterning are likely due to intrinsic cells and associated molecular signals, mechanical loading likely plays a critically important role after the onset of limb motion and eventual weight-bearing postnatally. As the focus upon surgical repair continues to advance, a more complete understanding of ACL development may guide further clinical and basic science research in this area.
Due to the limited availability of these human specimens, the literature on the developing human ACL enthesis is scarce. There is also a paucity of studies describing the anatomical ultrastructure of the ACL enthesis. In a 1970 study, Cooper and Misol examined the insertion of the patellar tendon and medial collateral ligament (MCL) in adult dogs using SEM. They described that the attachment of the skeletally mature MCL to the femur and tibia occurred via the interdigitation of ligamentous collagen fibers and the adjacent bone, and proposed a transitional structure that had four separate zones; (i) ligament, (ii) unmineralized fibrocartilage, (iii) mineralized fibrocartilage, and (iv) bone.11 In 1983, Arnoczky analyzed the microanatomy of the adult human ACL and, like Cooper and Misol, described a fibrocartilage transition zone that mediated the ligament-to-bone attachment.10 Recently, studies conducted by Zhao and colleagues characterized the porcine ACL and adult human ACL more extensively.12,13 These authors reported that the ACL tibial enthesis was a specialized micro- to nano-scale structural continuum consisting of at least three distinct transition zones that appeared to be adapted to serve joint function at the macroscopic level. Moreover, they determined that the anteromedial (AM) bundle insertion differed significantly from the posterolateral (PL) bundle. While the AM bundle displayed a complex deep interdigitation into bone, the PL bundle interdigitation and merging was shallow and more focal.13 These investigators later reported similar findings in the human ACL, with the AM bundle insertion displaying stronger interdigitation while there was a more gradual merging of the soft tissue and bone in the PL bundle.12
In this investigation, SEM analysis of the 1- and 8-month-old postnatal ACL enthesis structure revealed shallow interdigitation between the dense fibrous (ligamentous) and unmineralized chondrogenic tissues, with minimal transition zone. The ligamentous tissue did not penetrate too deeply into the chondro-epiphyseal tissue. In the 11-month-old, a more complex transition zone between the soft and hard tissues can be appreciated. While the depth of interdigitation remains low, there seemed to be distinct transition zones between the ligamentous tissue and chondro-epiphysis. A progressively more complex and defined fibrocartilage zone between the ligamentous and chondro-epiphyseal tissue was observed in the 19- and 36-month-old samples, which began to resemble the mature enthesis structure. CPL analysis revealed distinct changes in collagen fiber continuity, alignment, and organization over time. Younger samples revealed minimal fiber penetration and abrupt transitions between tissue types, which evolved into a more organized and interdigitated structure by 8 months, suggesting initiation of enthesis maturation. By 19 and 36 months, the samples not only exhibit complex fiber arrangements but also a progression toward uniform fiber orientation, indicative of mature entheseal structures. Histological analysis demonstrated a similar pattern of remodeling with increasing age. While the younger samples demonstrated a rudimentary junction lacking extensive mechanical interlocking between soft and hard tissues, by ages 19 and 36 months a more sophisticated and structured interface composed of a complex and distinct fibrocartilage zone featuring distinctly different cartilage matrix macromolecules is appreciated. The distinct blue staining pattern observed in the fibrocartilage zone indicates an increase of proteoglycans and type II collagen, both of which are typically associated with fibrocartilage development. Thus, starting at age 19 months, ACL ultrastructure begins to resemble that of the adult, but is still undergoing maturation.
We speculate that the maturation and remodeling of the developing enthesis is due to the effects of mechanical loading. It is well-established that cells in the embryonic limb are mechanosensitive.16-19 Moreover, secreted signaling molecules such as growth and differentiation factors-(GDF) 5, 6, and 7 play an important role in embryogenesis and joint development.20 Studies have shown that following ectopic implantation, GDF-5, 6, and 7 have the capacity to induce neotendon/ligament differentiation in in vitro and in vivo animal models.21-23 Concomitantly, it has been demonstrated that mechanical loading of the knee joint has profound effects on ligaments, tendon, cartilage, and bone at the molecular and cellular level.24,25 It is likely that there is a complex interaction between cell-intrinsic molecular signals and extrinsic mechanical loads. The appearance of a nascent fibrocartilage zone between ligament and bone is consistent with a changing and more complex mechanical loading environment. Prior studies have demonstrated that tensile loading promotes development and maintenance of a dense fibrous connective tissue phenotype, such as ligament and tendon, while compressive loading favors a chondrogenic phenotype.26-28 Our findings in this limited analysis support the notion that mechanical loading promotes development of cartilage and bone, resulting in the fibrocartilage zone between the ligamentous and chondro-epiphyseal tissues. This intervening fibrocartilage zone has material properties that are intermediate between ligament and bone, thus serving to diminish and modulate stress concentration at the junction of soft tissue and bone.29
An important limitation of contemporary ACL reconstruction techniques is that the ultrastructural anatomy of the native enthesis is not reproduced, with lack of formation of the fibrocartilage zone of a normal enthesis. The resultant attachment site has inferior material properties, predisposing to micromotion at the tendon graft-to-bone interface, and possible recurrent knee laxity. It is clear that future surgical strategies should aim to replicate the native biological, structural, and mechanical features of the ligament-to-bone attachment site, and support development of the fibrocartilage transition zone.30 Improved understanding of the cellular and molecular mechanisms of enthesis development may inform innovative strategies to replicate the enthesis and to create tissue-engineered scaffolds or new surgical techniques that incorporate grafts that will mimic the native structure and function of the enthesis.
This study has several limitations that must be acknowledged. Due to the rarity of these samples, only five specimens at differing stages of development could be obtained for study. Future studies should aim to evaluate a larger range of ages to gain a better understanding of the effect of mechanical loading on the development and maturation of the ultrastructure of the ACL enthesis. Moreover, a limited set of analyses were performed. Additional histological staining could have provided further insights into other tissue- and cellular-level metrics. Transcriptional profiling to examine gene expression in the cells in the developing enthesis would add important insight into the underlying molecular signals, and how the transcriptome evolves with time and with the onset of mechanical loading. Single cell RNA sequencing would allow deeper understanding of underlying molecular mechanisms. New techniques of spatial transcriptomics would also allow understanding of gene expression patterns in specific tissue zones. Lastly, further insight may be gained by trying to identify and distinguish the AM and PL bundles of the ACL, as their appearance may further reflect the effects of mechanical stimulation of the developing ACL.
5 ∣. CONCLUSION
In this study we have analyzed the ultrastructure of the human ACL during the early post-natal period. Our results suggest that the ACL enthesis of the developing knee begins to mimic that of an adult as early as 19 months of age, as a more complex transition between ligamentous and chondro-epiphyseal tissue becomes present. While further analysis is needed, changes in structure and composition of the developing ACL are likely due to mechanical loading of the enthesis with the onset of weightbearing, or other biological processes during development. Improved understanding of the cellular and molecular events in ligament development may inform strategies to improve healing of current ACL graft reconstruction techniques as well as suggest innovative tissue-engineering approaches.
ACKNOWLEDGMENTS
The authors are grateful for funding for this study provided by the Rudin Foundation Resident Research Grant and the Hospital for Special Surgery Pediatric Service Research Award. The Zeiss Gemini 300 field emission scanning electron microscopy was provided courtesy of the National Institutes of Health S10 Shared Instrumentation Program, grant number 1S10Od026989-01. We would also like to gratefully acknowledge Dr. Lionel E. Lazaro, MD and Dr. Stephen Doty, PhD for their assistance with the study methodology, and AlloSource (Centennial, CO, USA) for providing the tissue specimens utilized for this research study. S. H. P., T. J. U., K. M. L., C. E. K., and T. G. B. have nothing to disclose. K. G. S. owns stock options from nView, Inc and Sario, Inc. D. W. G. is a consultant for Arthrex, Inc., receives royalties from Arthrex, Inc. and Orthopediatrics and is a faculty speaker for Synthes. S. A. R. is a consultant for Novartis and Advance Medical, owns stock options from ORTHO RTI and receives research support from OREF, NIH, and Angiocrine Biosciences, Inc.
REFERENCES
- 1.Beck NA, Lawrence JTR, Nordin JD, DeFor TA, Tompkins M. ACL tears in school-aged children and adolescents over 20 years. Pediatrics. 2017;139(3):1–9. [DOI] [PubMed] [Google Scholar]
- 2.Herzog MM, Marshall SW, Lund JL, Pate V, Mack CD, Spang JT. Trends in incidence of ACL reconstruction and concomitant procedures among commercially insured individuals in the United States, 2002-2014. Sports Health. 2018;10(6):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diermeier T, Rothrauff BB, Engebretsen L, et al. Treatment after anterior cruciate ligament injury: panther symposium ACL treatment consensus group. Orthop J Sports Med. 2020;8(6):2325967120931097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin KM, Boyle C, Marom N, Marx RG. Graft selection in anterior cruciate ligament reconstruction. Sports Med Arthrosc. 2020;28(2):41–48. [DOI] [PubMed] [Google Scholar]
- 5.Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “Ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162–172. [DOI] [PubMed] [Google Scholar]
- 6.Lin K, Rodeo S. Tendon and ligament healing. In: Aaron RK, ed. Orthopaedic Basic Science: Foundations of Clinical Practice. 5th ed. American Academy of Orthopaedic Surrgeons; 2019. [Google Scholar]
- 7.Noyes FR, DeLucas JL, Torvik PJ. Biomechanics of anterior cruciate ligament failure: an analysis of strain rate sensitivity and mechanisms of failure in primates. J Bone Jt Surg. 1974;56(2):236–253. [PubMed] [Google Scholar]
- 8.Deng XH, Lebaschi A, Camp CL, et al. Expression of signaling molecules involved in embryonic development of the insertion site is inadequate for reformation of the native enthesis: evaluation in a novel murine ACL reconstruction model. J Bone Jt Surg. 2018;100(15):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galatz LM, Gerstenfeld L, Heber-Katz E, Rodeo SA. Tendon regeneration and scar formation: The concept of scarless healing. J Orthop Res. 2015;33:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnoczky SP. Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res. 1983;172:19–25. [PubMed] [Google Scholar]
- 11.Cooper RR, Misol S, Stimmel P. Tendon and ligament insertion. A light and electron microscopic study. J Bone Jt Surg. 1970;52(1):1–170. [PubMed] [Google Scholar]
- 12.Zhao L, Lee PVS, Ackland DC, Broom ND, Thambyah A. Microstructure variations in the soft-hard tissue junction of the human anterior cruciate ligament. Anat Rec. 2017;300(9):1547–1559. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Thambyah A, Broom ND. A multi-scale structural study of the porcine anterior cruciate ligament tibial enthesis. J Anat. 2014;224(6):624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromage TG, Goldman HM, McFarlin SC, Warshaw J, Boyde A, Riggs CM. Circularly polarized light standards for investigations of collagen fiber orientation in bone. Anat Rec B New Anat. 2003;274B(1):157–168. [DOI] [PubMed] [Google Scholar]
- 15.Del Cerro M, Cogen J, Del Cerro C. Stevenel's Blue, an excellent stain for optical microscopical study of plastic embedded tissues. Microsc Acta. 1980;83(2):117–121. [PubMed] [Google Scholar]
- 16.Drachman DB, Sokoloff L. The role of movement in embryonic joint development. Dev Biol. 1966;14(3):401–420. [Google Scholar]
- 17.Ell BF, Society R, Fellow MR, Canti RG. Experiments on the development in vitro of the avian knee-joint. Proc R Soc Lond B Biol Sci. 1934;116(799):316–351. [Google Scholar]
- 18.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000;37(2):127–133. [PubMed] [Google Scholar]
- 19.Mikic B, Wong M, Chiquet M, Hunziker EB. Mechanical modulation of tenascin-C and collagen-XII expression during avian synovial joint formation. J Orthop Res. 2000;18(3):406–415. [DOI] [PubMed] [Google Scholar]
- 20.Wang RN, Green J, Wang Z, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haddad-Weber M, Prager P, Kunz M, et al. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 2010;12(4):505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helm GA, Li JZ, Alden TD, et al. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg. 2001;95(2):298–307. [DOI] [PubMed] [Google Scholar]
- 23.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100(2):321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatib N, Parisi C, Nowlan N. Differential effect of frequency and duration of mechanical loading on fetal chick cartilage and bone development. Eur Cell Mater. 2021;41:531–545. [DOI] [PubMed] [Google Scholar]
- 25.Monaco G, el Haj AJ, Alini M, Stoddart MJ. Sodium hyaluronate supplemented culture media as a new hMSC chondrogenic differentiation media-model for in vitro/ex vivo screening of potential cartilage repair therapies. Front Bioeng Biotechnol. 2020;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evanko SP, Vogel KG. Proteoglycan synthesis in fetal tendon is differentially regulated by cyclic compression in vitro. Arch Biochem Biophys. 1993;307(1):153–164. [DOI] [PubMed] [Google Scholar]
- 27.Malaviya P, Bultler DL, Boivin GP, et al. An in vivo model for load-modulated remodeling in the rabbit flexor tendon. J Orthop Res. 2000;18(1):116–125. [DOI] [PubMed] [Google Scholar]
- 28.Vogel KG. The effect of compressive loading on proteoglycan turnover in cultured fetal tendon. Connect Tissue Res. 1996;34(3):227–237. [DOI] [PubMed] [Google Scholar]
- 29.Messner K. Postnatal development of the cruciate ligament insertions in the rat knee. Cells Tissues Organs. 1997;160(4):261–268. [DOI] [PubMed] [Google Scholar]
- 30.Locke RC, Abraham AC, Killian ML. Orthopedic interface repair strategies based on native structural and mechanical features of the multiscale enthesis. ACS Biomater Sci Eng. 2017;3(11):2633–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]


