Abstract
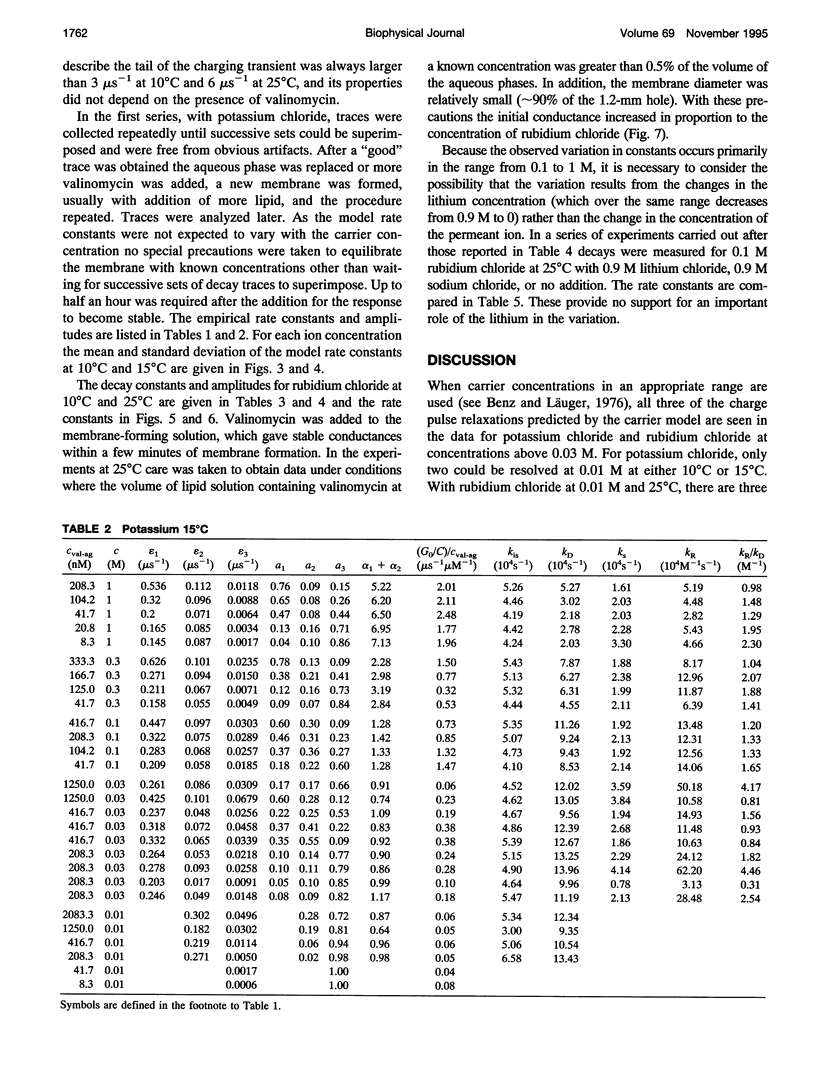
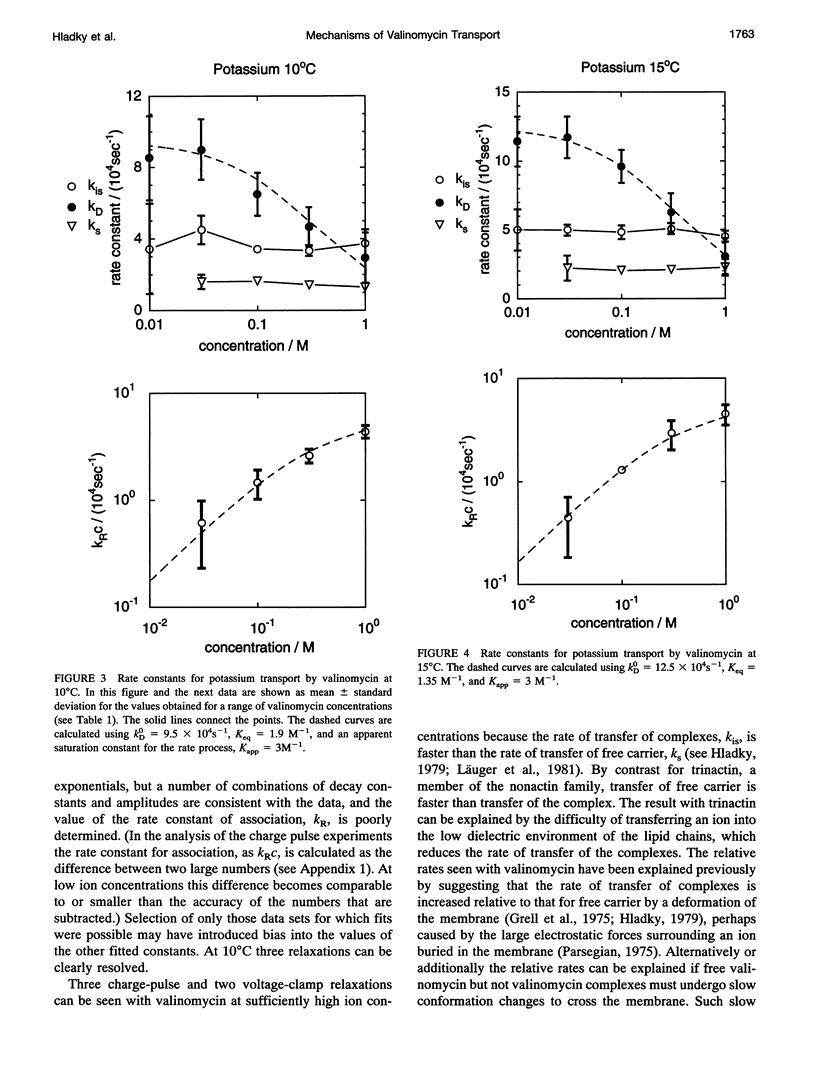
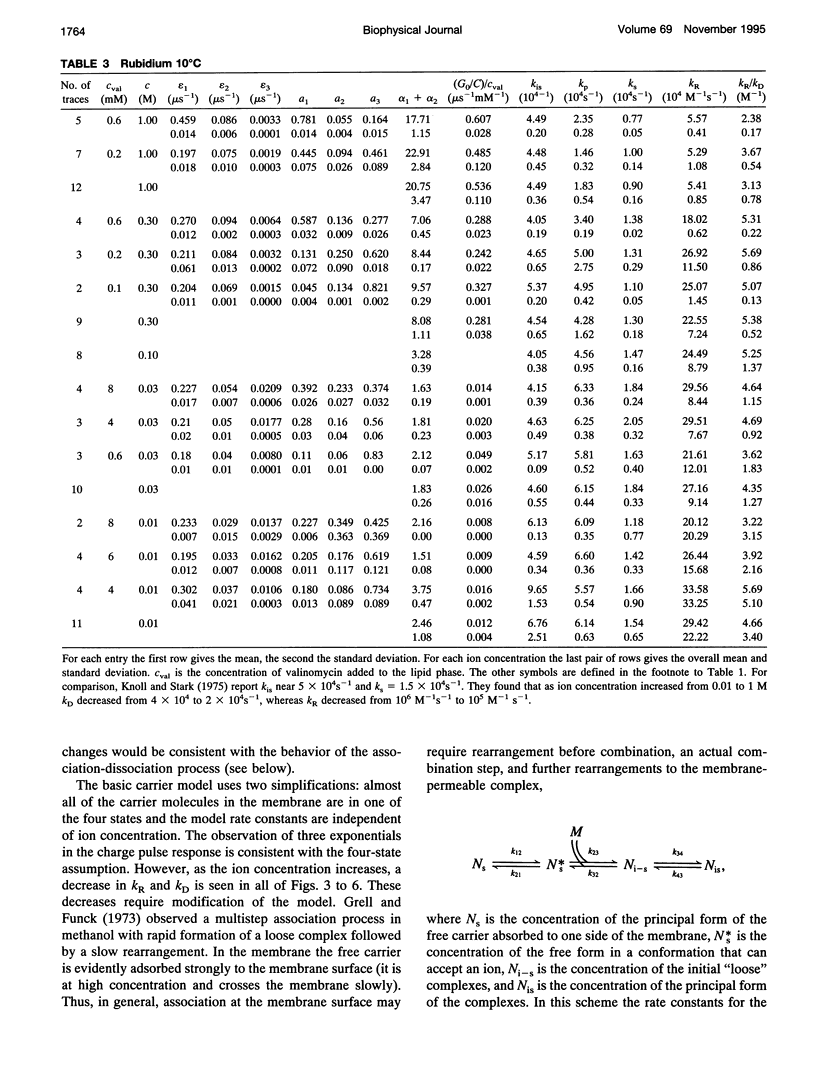
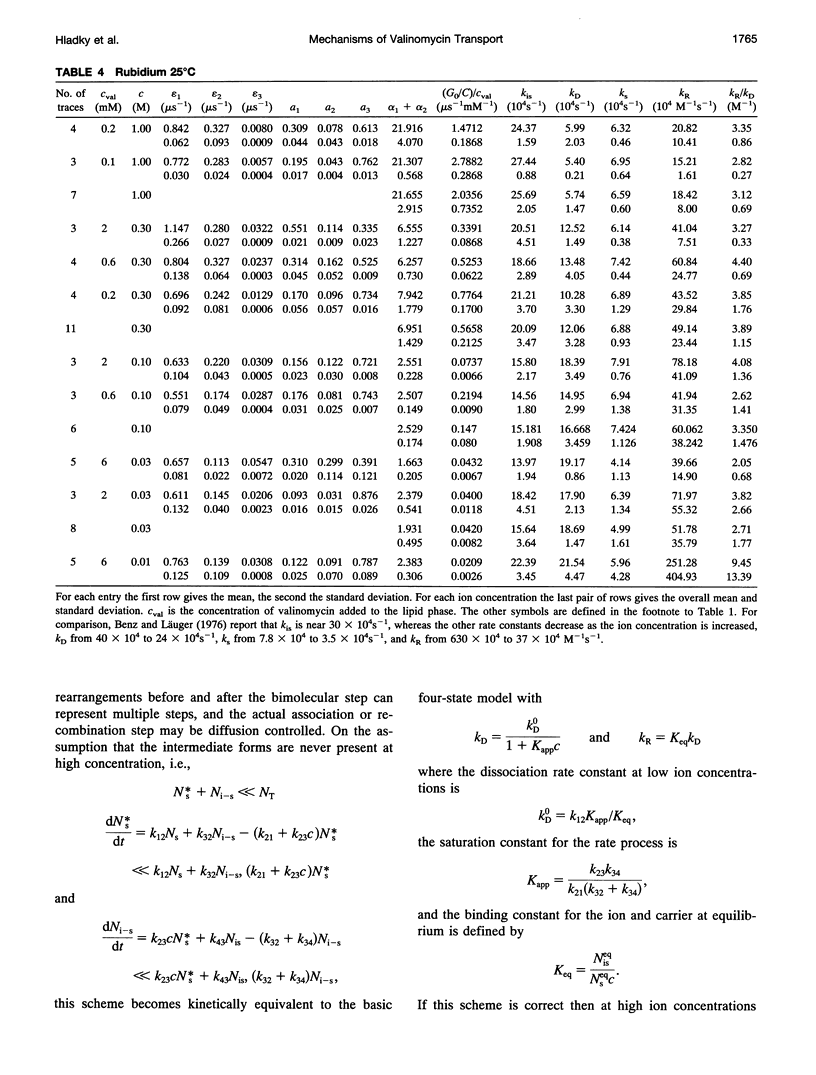
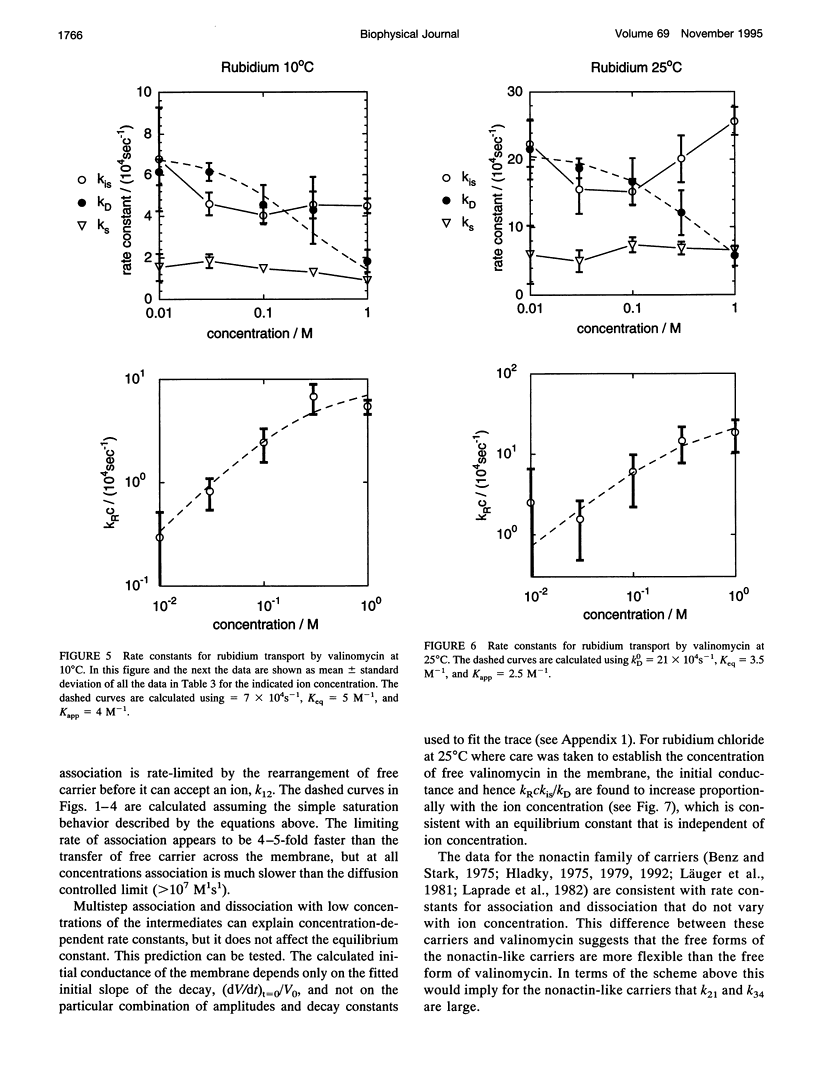
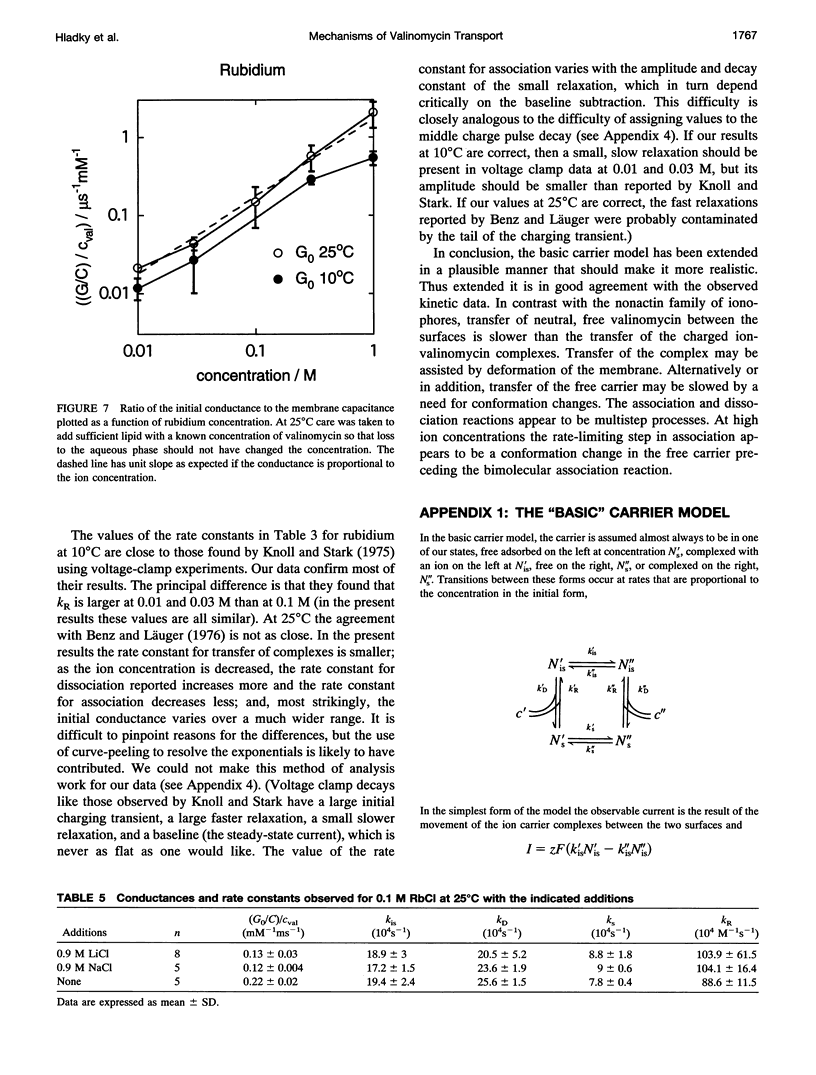
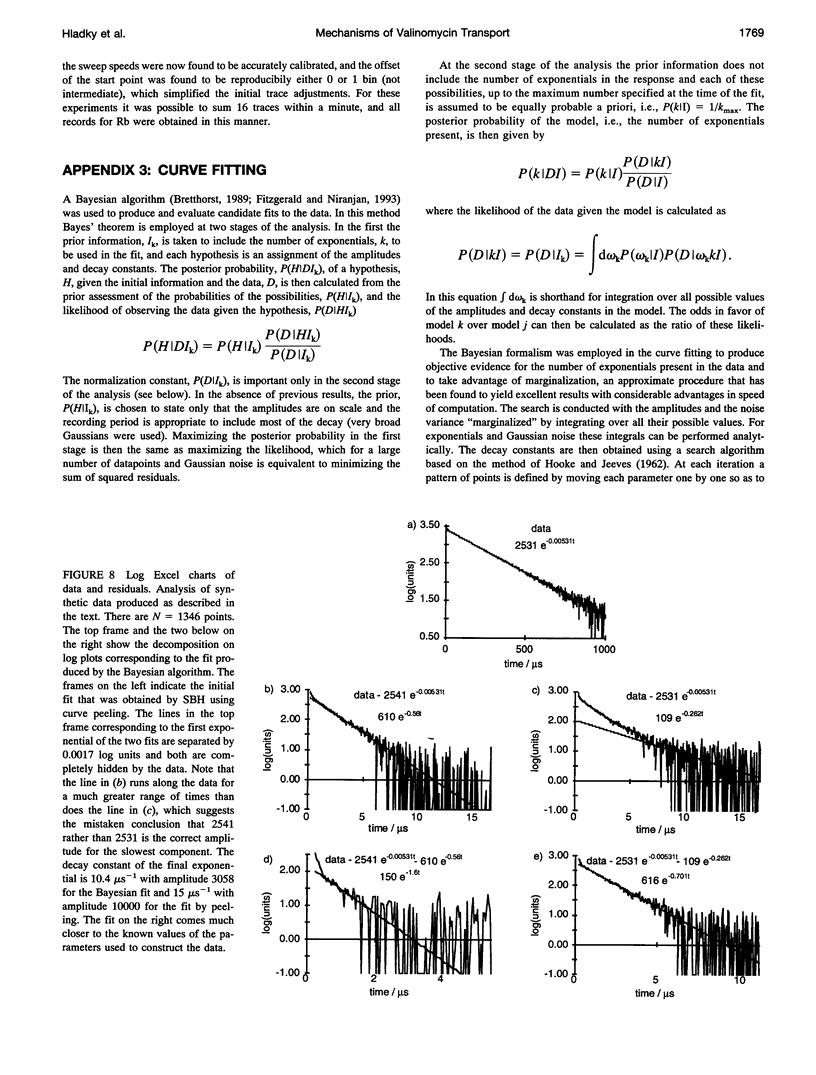
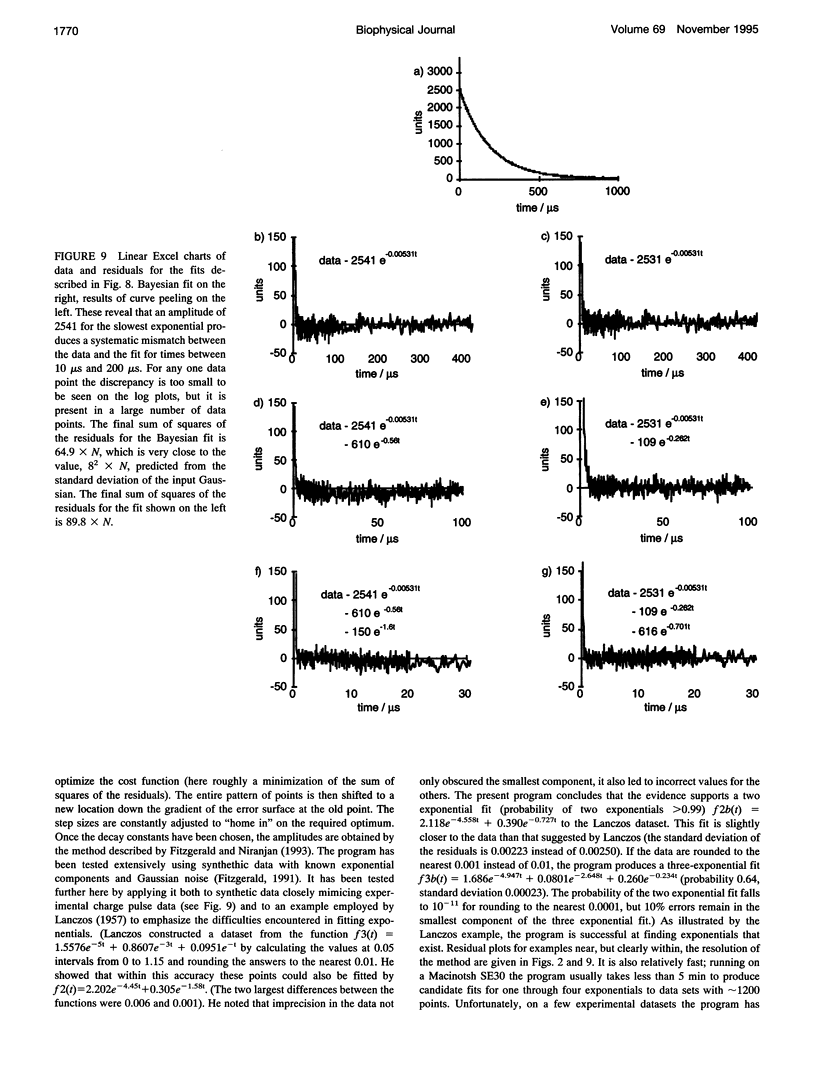
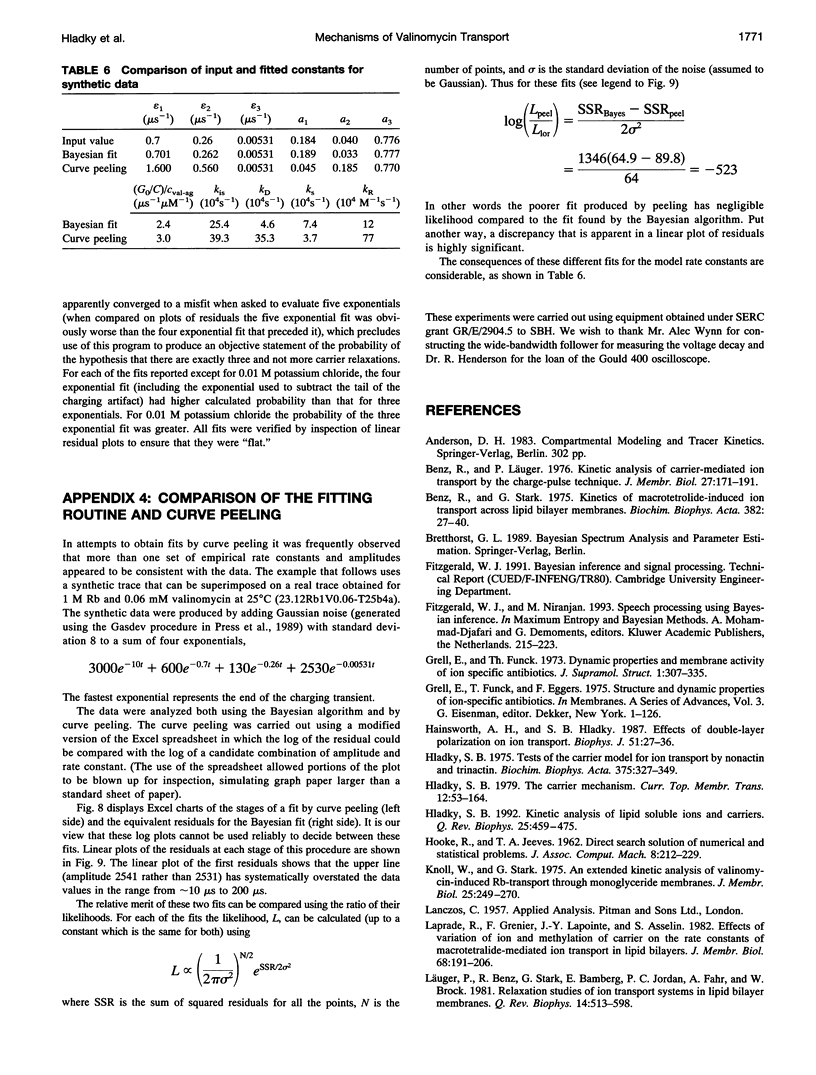
Even though valinomycin has been employed and studied extensively for over 30 years, the attempts to explain its mechanism have not been entirely successful. The basic carrier model uses four rate constants that describe association of an ion and carrier, transfer of the complex across the membrane, dissociation of the complex, and transfer of the free carrier back across the membrane. If the basic model is correct all of these constants are independent of ion concentration. In previous work with rubidium the rate constants for transfer of free carrier, transfer of complexes, and dissociation were independent of the concentration, but the rate constant for association varied markedly. No satisfactory explanation for these observations was proposed. In this study current relaxations after charge pulses have been analyzed using digital data acquisition, a Bayesian algorithm, and inspection of linear plots of residuals. In agreement with previous results the relaxations for sufficiently high rubidium or potassium concentrations contain three exponential components, but the rate constants for association and dissociation decrease to similar extents as ion concentration increases. A simple extension of the carrier model to allow a more realistic description of association and dissociation is in good agreement with the rate constants fitted in the present study but not those for low ion concentrations found in previous work. At high ion concentrations the rate-limiting step in association appears to be a change in the conformation of the free carrier preceding the bimolecular association reaction. Transfer of neutral, free valinomycin between the surfaces is slower than the transfer of the charged ion-valinomycin complexes. Transfer of the complex may be hastened by deformation of the membrane, or transfer of the free carrier may be slowed by a need for conformation changes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Läuger P. Kinetic analysis of carrier-mediated ion transport by the charge-pulse technique. J Membr Biol. 1976 Jun 9;27(1-2):171–191. doi: 10.1007/BF01869135. [DOI] [PubMed] [Google Scholar]
- Benz R., Stark G. Kinetics of macrotetrolide-induced ion transport across lipid bilayer membranes. Biochim Biophys Acta. 1975 Feb 28;382(1):27–40. doi: 10.1016/0005-2736(75)90369-7. [DOI] [PubMed] [Google Scholar]
- Grell E., Funck T. Dynamic properties and membrane activity of ion specific antibiotics. J Supramol Struct. 1973;1(4):307–335. doi: 10.1002/jss.400010408. [DOI] [PubMed] [Google Scholar]
- Grell E., Funck T., Eggers F. Structure and dynamic properties of ion-specific antibiotics. Membranes. 1975;3:1–126. [PubMed] [Google Scholar]
- Hainsworth A. H., Hladky S. B. Effects of double-layer polarization on ion transport. Biophys J. 1987 Jan;51(1):27–36. doi: 10.1016/S0006-3495(87)83308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B. Kinetic analysis of lipid soluble ions and carriers. Q Rev Biophys. 1992 Nov;25(4):459–475. doi: 10.1017/s0033583500004376. [DOI] [PubMed] [Google Scholar]
- Hladky S. B. Tests of the carrier model for ion transport by nonactin and trinactin. Biochim Biophys Acta. 1975 Feb 14;375(3):327–349. doi: 10.1016/0005-2736(75)90351-x. [DOI] [PubMed] [Google Scholar]
- Knoll W., Stark G. An extended kinetic analysis of valinomycin-induced Rb-transport through monoglyceride membranes. J Membr Biol. 1975;25(3-4):249–270. doi: 10.1007/BF01868578. [DOI] [PubMed] [Google Scholar]
- Laprade R., Grenier F., Lapointe J. Y., Asselin S. Effects of variation of ion and methylation of carrier on the rate constants of macrotetralide-mediated ion transport in lipid bilayers. J Membr Biol. 1982;68(3):191–206. doi: 10.1007/BF01872264. [DOI] [PubMed] [Google Scholar]
- Läuger P., Benz R., Stark G., Bamberg E., Jordan P. C., Fahr A., Brock W. Relaxation studies of ion transport systems in lipid bilayer membranes. Q Rev Biophys. 1981 Nov;14(4):513–598. doi: 10.1017/s003358350000247x. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A. Ion-membrane interactions as structural forces. Ann N Y Acad Sci. 1975 Dec 30;264:161–171. doi: 10.1111/j.1749-6632.1975.tb31481.x. [DOI] [PubMed] [Google Scholar]
- Stark G., Ketterer B., Benz R., Läuger P. The rate constants of valinomycin-mediated ion transport through thin lipid membranes. Biophys J. 1971 Dec;11(12):981–994. doi: 10.1016/S0006-3495(71)86272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDAS W. F. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J Physiol. 1952 Sep;118(1):23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]