Abstract
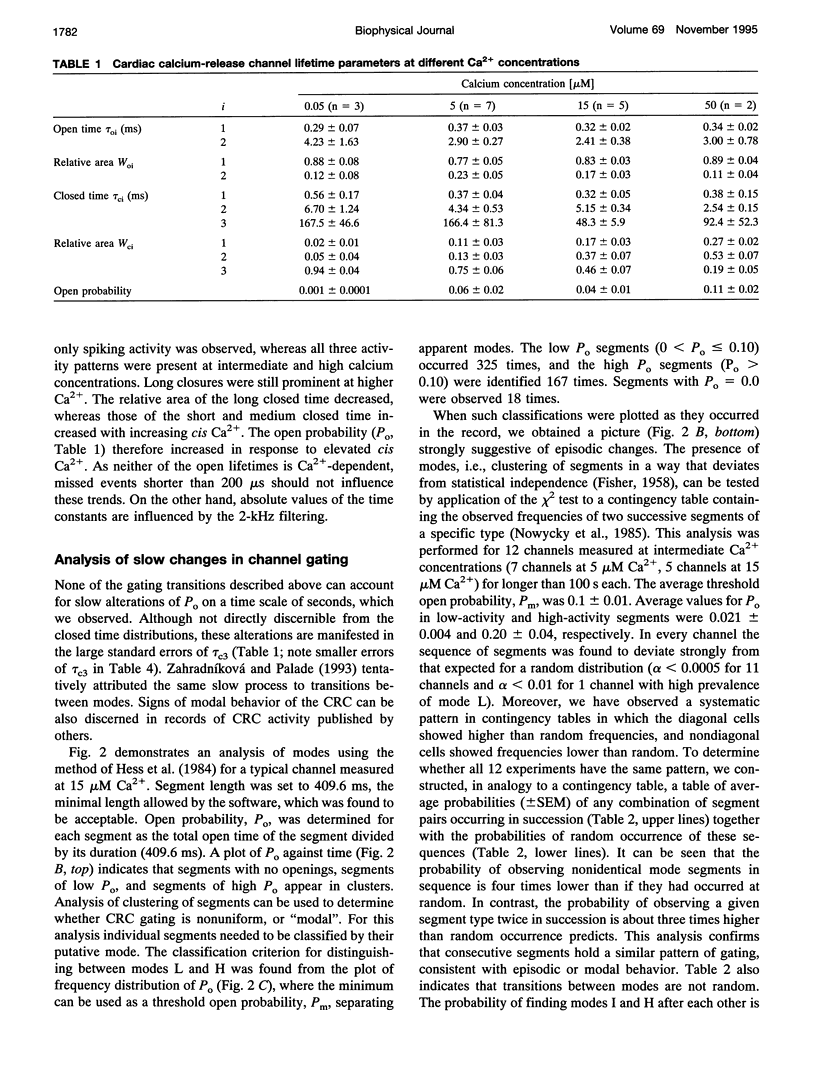
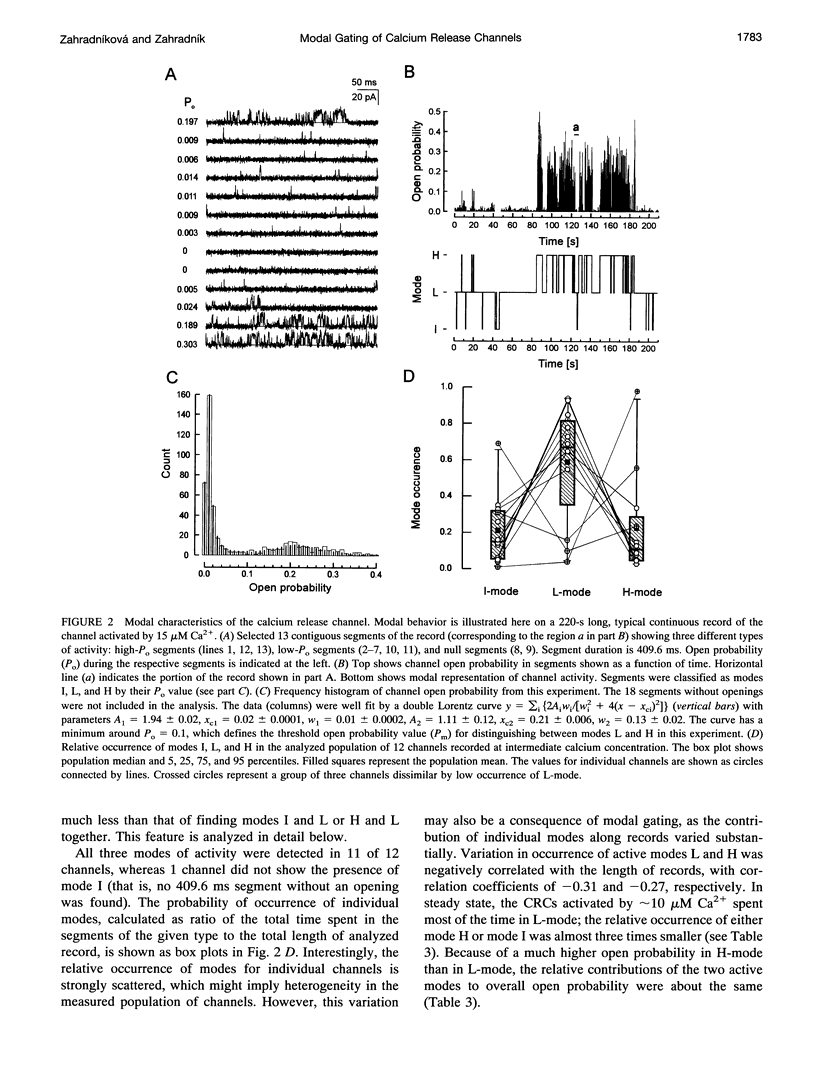
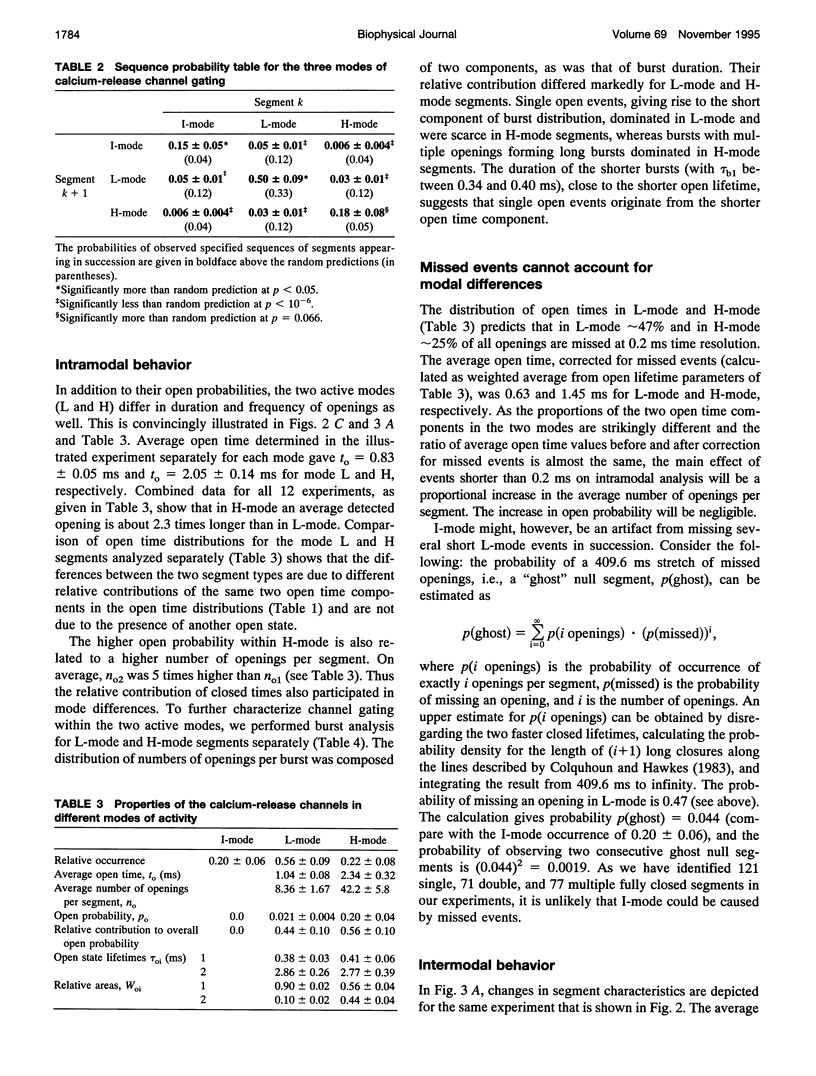
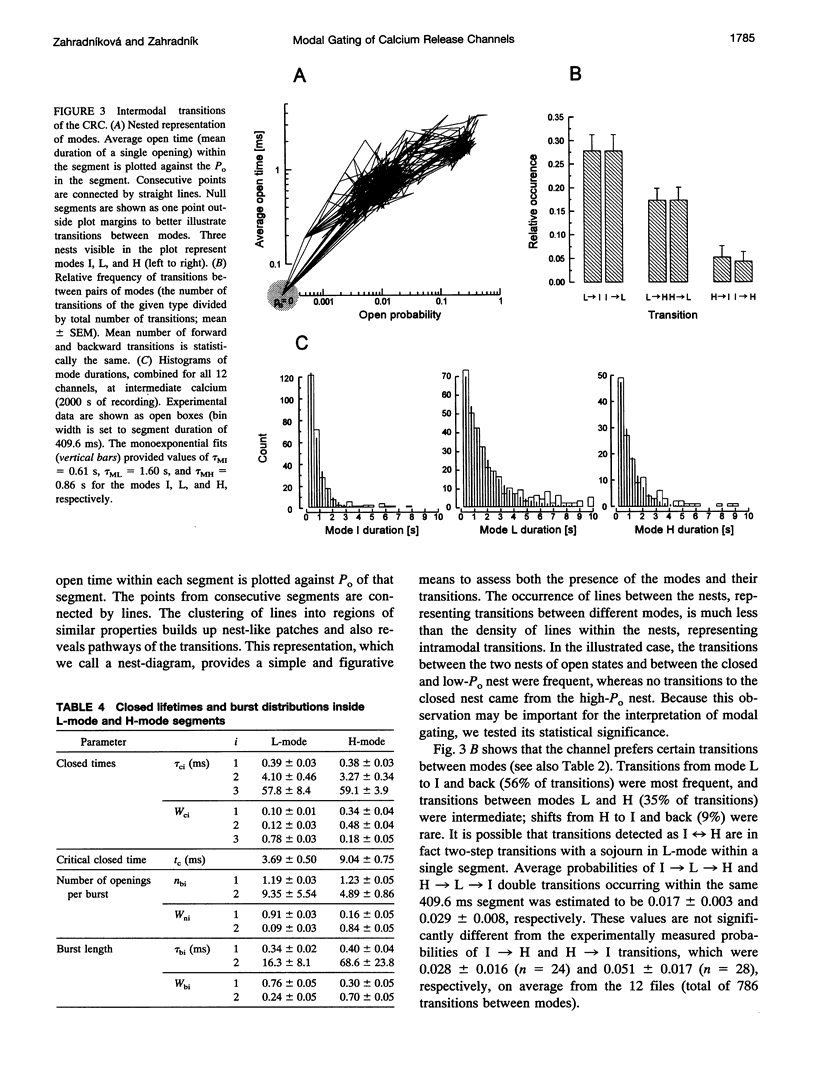
Single channel activity of the cardiac ryanodine-sensitive calcium-release channel in planar lipid membranes was studied in order to elucidate the calcium-dependent mechanism of its steady-state behavior. The single channel kinetics, observed with Cs+ as the charge carrier at different activating (cis) Ca2+ concentrations in the absence of ATP and Mg2+, were similar to earlier reports and were extended by analysis of channel modal behavior. The channel displayed three episodic levels of open probability defining three gating modes: H (high activity), L (low activity), and I (no activity). The large difference in open probabilities between the two active modes resulted from different bursting patterns and different proportions of two distinct channel open states. I-mode was without openings and can be regarded as the inactivated mode of the channel; L-mode was composed of short and sparse openings; and H-mode openings were longer and grouped into bursts. Modal gating may explain calcium-release channel adaptation (as transient prevalence of H-mode after Ca2+ binding) and the inhibitory effects of drugs (as stabilization of mode I), and it provides a basis for understanding the regulation of calcium release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. H., Williams A. J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J Gen Physiol. 1990 May;95(5):981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Chu A., Fill M., Stefani E., Entman M. L. Cytoplasmic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca(2+)-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. J Membr Biol. 1993 Jul;135(1):49–59. doi: 10.1007/BF00234651. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol. 1991 Jan;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Györke S., Vélez P., Suárez-Isla B., Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+. Biophys J. 1994 Jun;66(6):1879–1886. doi: 10.1016/S0006-3495(94)80981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. L., Alvarez R. M., Fill M., Hawkes M. J., Brush K. L., Schilling W. P., Stefani E. [3H]PN200-110 and [3H]ryanodine binding and reconstitution of ion channel activity with skeletal muscle membranes. Anal Biochem. 1989 Nov 15;183(1):31–41. doi: 10.1016/0003-2697(89)90167-x. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994 Jun;12(6):1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Ma J. Desensitization of the skeletal muscle ryanodine receptor: evidence for heterogeneity of calcium release channels. Biophys J. 1995 Mar;68(3):893–899. doi: 10.1016/S0006-3495(95)80265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol. 1989 Feb;256(2 Pt 2):H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- Shomer N. H., Louis C. F., Fill M., Litterer L. A., Mickelson J. R. Reconstitution of abnormalities in the malignant hyperthermia-susceptible pig ryanodine receptor. Am J Physiol. 1993 Jan;264(1 Pt 1):C125–C135. doi: 10.1152/ajpcell.1993.264.1.C125. [DOI] [PubMed] [Google Scholar]
- Sipido K. R., Wier W. G. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991 Apr;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Gating of the native and purified cardiac SR Ca(2+)-release channel with monovalent cations as permeant species. Biophys J. 1994 Oct;67(4):1484–1494. doi: 10.1016/S0006-3495(94)80622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H. H., Kaplan J. H., Ellis-Davies G. C., Lederer W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995 Mar 31;267(5206):1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Bak J., Mészáros L. G. Heterogeneity of the cardiac calcium release channel as assessed by its response to ADP-ribose. Biochem Biophys Res Commun. 1995 May 16;210(2):457–463. doi: 10.1006/bbrc.1995.1682. [DOI] [PubMed] [Google Scholar]
- Zahradníková A., Palade P. Procaine effects on single sarcoplasmic reticulum Ca2+ release channels. Biophys J. 1993 Apr;64(4):991–1003. doi: 10.1016/S0006-3495(93)81465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


