Abstract
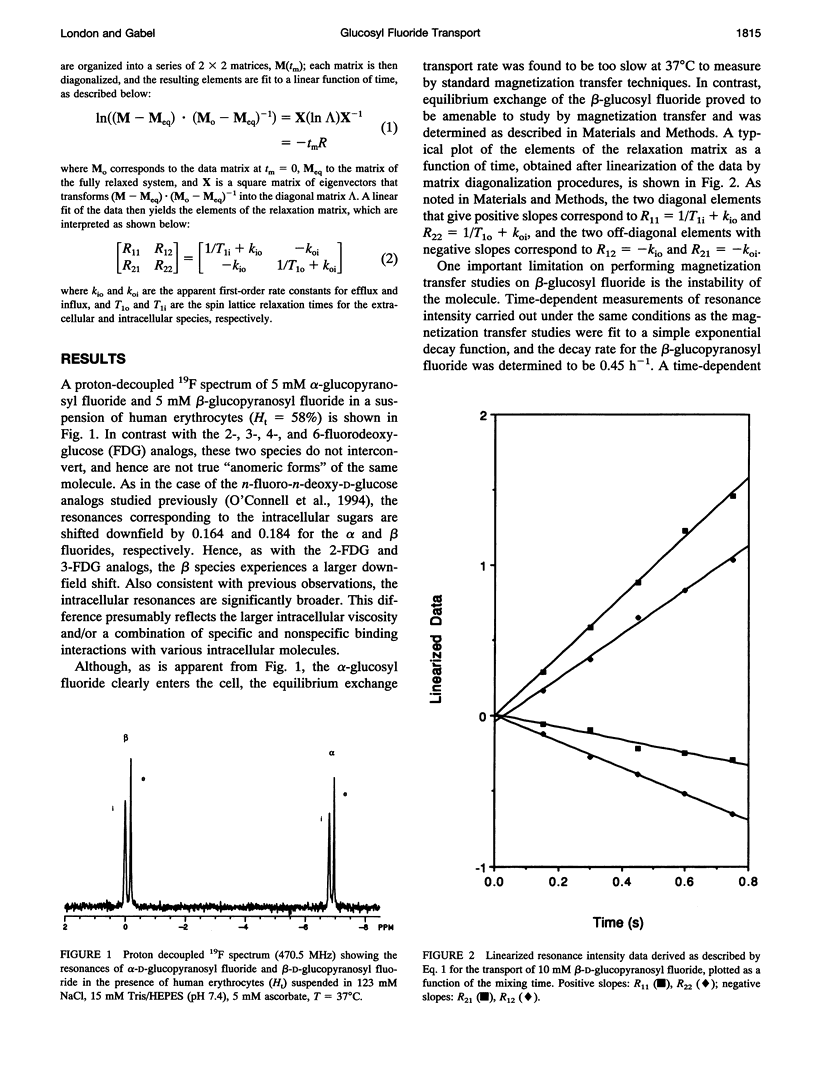
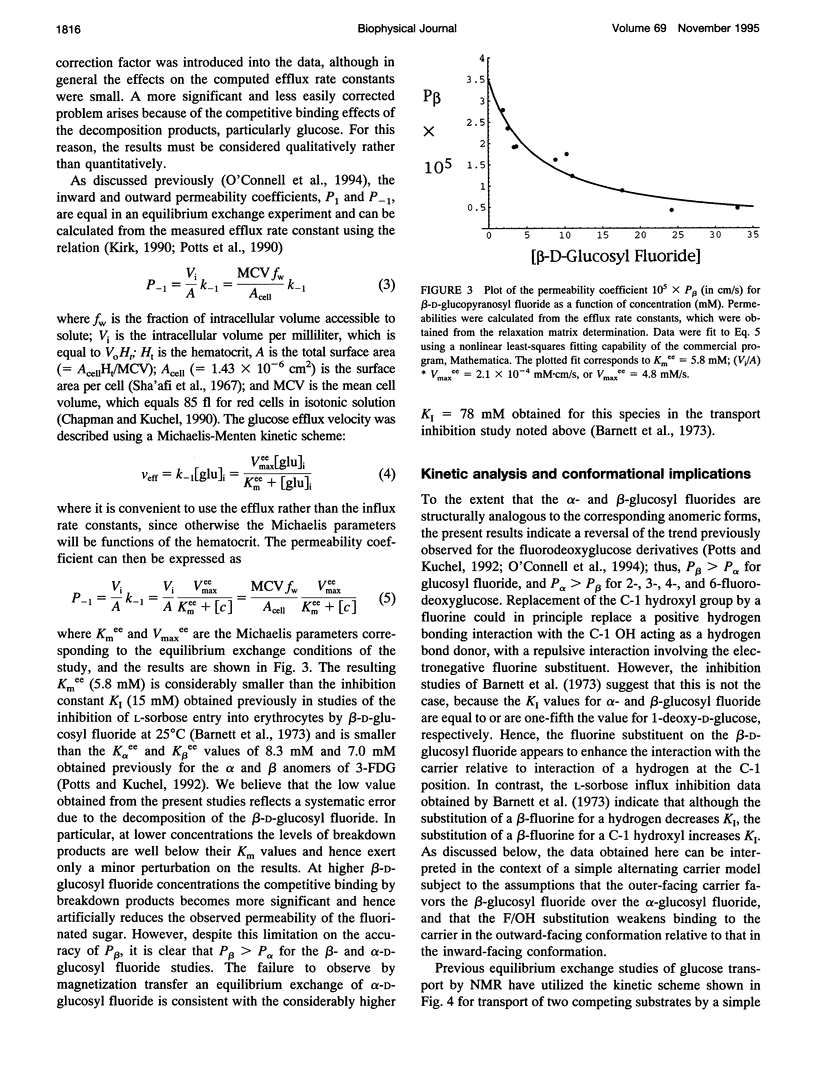
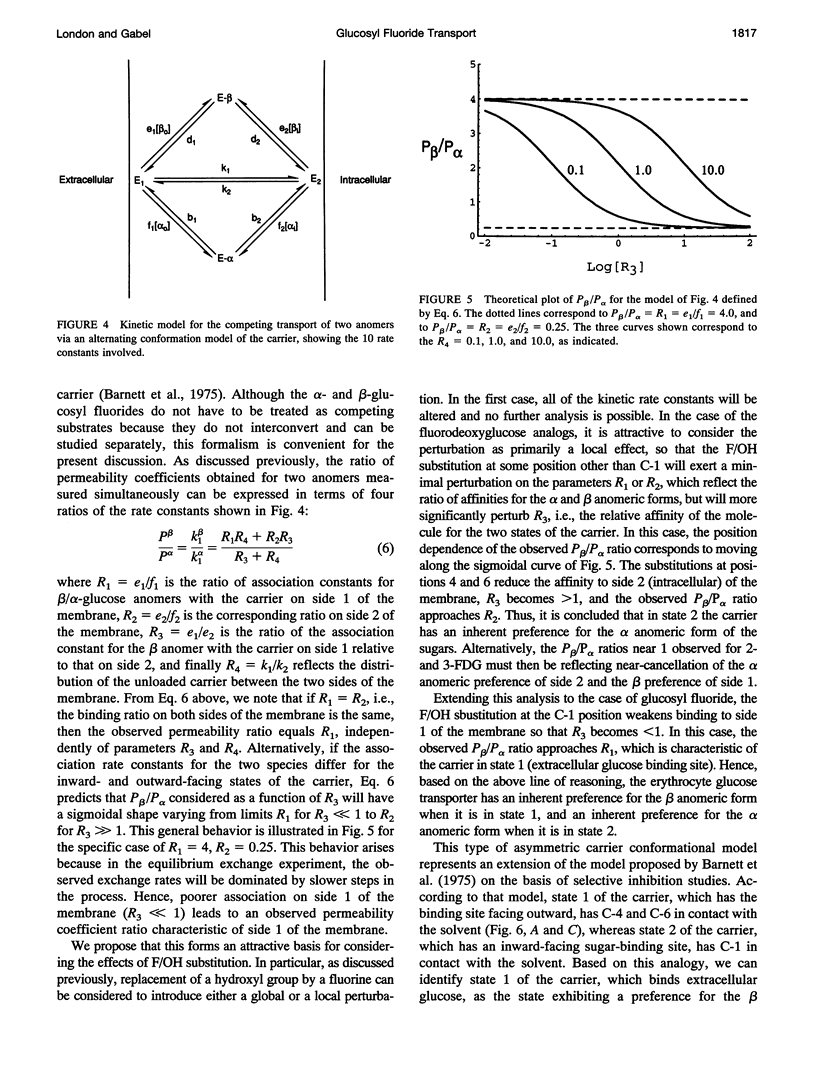
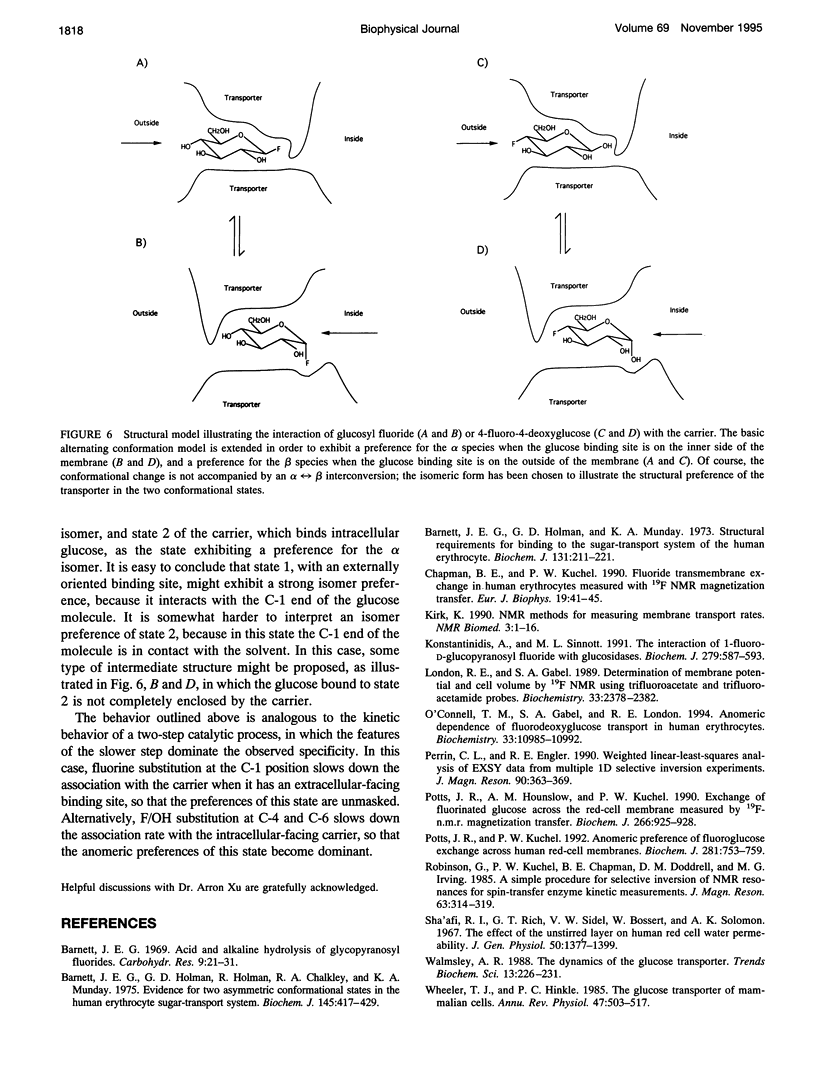
Fluorine-19 magnetization transfer studies have been used to measure the transport rate of glucopyranosyl fluorides under equilibrium exchange conditions. Although rate constants and permeabilities could be determined for beta-D-glucopyranosyl fluoride, the exchange rate for alpha-D-glucopyranosyl fluoride was found to be too slow for determination using this method. The time-dependent decomposition of the beta-glucopyranosyl fluoride also limits the accuracy of the numerical results for this species; however, it is clear that the permeabilities of the alpha and beta forms differ significantly, i.e., P beta > P alpha. This observation is in contrast to recent observations for n-fluoro-n-deoxyglucose, for which P alpha > P beta for n = 2, 3, 4, or 6. The difference can be explained in terms of a simple alternating conformation model in which one of the conformations (with an external sugar-binding site) exhibits a preference for the beta form of the molecule, while the second conformation (with an internal sugar binding site) exhibits a preference for the alpha form. Fluorine/hydroxyl substitutions unmask these preferences by selectively reducing the binding to one of the conformations, depending on the specific site of fluorination.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Holman G. D., Chalkley R. A., Munday K. A. Evidence for two asymmetric conformational states in the human erythrocyte sugar-transport system. Biochem J. 1975 Mar;145(3):417–429. doi: 10.1042/bj1450417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Holman G. D., Munday K. A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973 Feb;131(2):211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B. E., Kuchel P. W. Fluoride transmembrane exchange in human erythrocytes measured with 19F NMR magnetization transfer. Eur Biophys J. 1990;19(1):41–45. doi: 10.1007/BF00223572. [DOI] [PubMed] [Google Scholar]
- Kirk K. NMR methods for measuring membrane transport rates. NMR Biomed. 1990 Feb;3(1):1–16. doi: 10.1002/nbm.1940030102. [DOI] [PubMed] [Google Scholar]
- Konstantinidis A., Sinnott M. L. The interaction of 1-fluoro-D-glucopyranosyl fluoride with glucosidases. Biochem J. 1991 Oct 15;279(Pt 2):587–593. doi: 10.1042/bj2790587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London R. E., Gabel S. A. Determination of membrane potential and cell volume by 19F NMR using trifluoroacetate and trifluoroacetamide probes. Biochemistry. 1989 Mar 21;28(6):2378–2382. doi: 10.1021/bi00432a006. [DOI] [PubMed] [Google Scholar]
- O'Connell T. M., Gabel S. A., London R. E. Anomeric dependence of fluorodeoxyglucose transport in human erythrocytes. Biochemistry. 1994 Sep 13;33(36):10985–10992. doi: 10.1021/bi00202a018. [DOI] [PubMed] [Google Scholar]
- Potts J. R., Hounslow A. M., Kuchel P. W. Exchange of fluorinated glucose across the red-cell membrane measured by 19F-n.m.r. magnetization transfer. Biochem J. 1990 Mar 15;266(3):925–928. [PMC free article] [PubMed] [Google Scholar]
- Potts J. R., Kuchel P. W. Anomeric preference of fluoroglucose exchange across human red-cell membranes. 19F-n.m.r. studies. Biochem J. 1992 Feb 1;281(Pt 3):753–759. doi: 10.1042/bj2810753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Rich G. T., Sidel V. W., Bossert W., Solomon A. K. The effect of the unstirred layer on human red cell water permeability. J Gen Physiol. 1967 May;50(5):1377–1399. doi: 10.1085/jgp.50.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley A. R. The dynamics of the glucose transporter. Trends Biochem Sci. 1988 Jun;13(6):226–231. doi: 10.1016/0968-0004(88)90089-8. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]


