Abstract
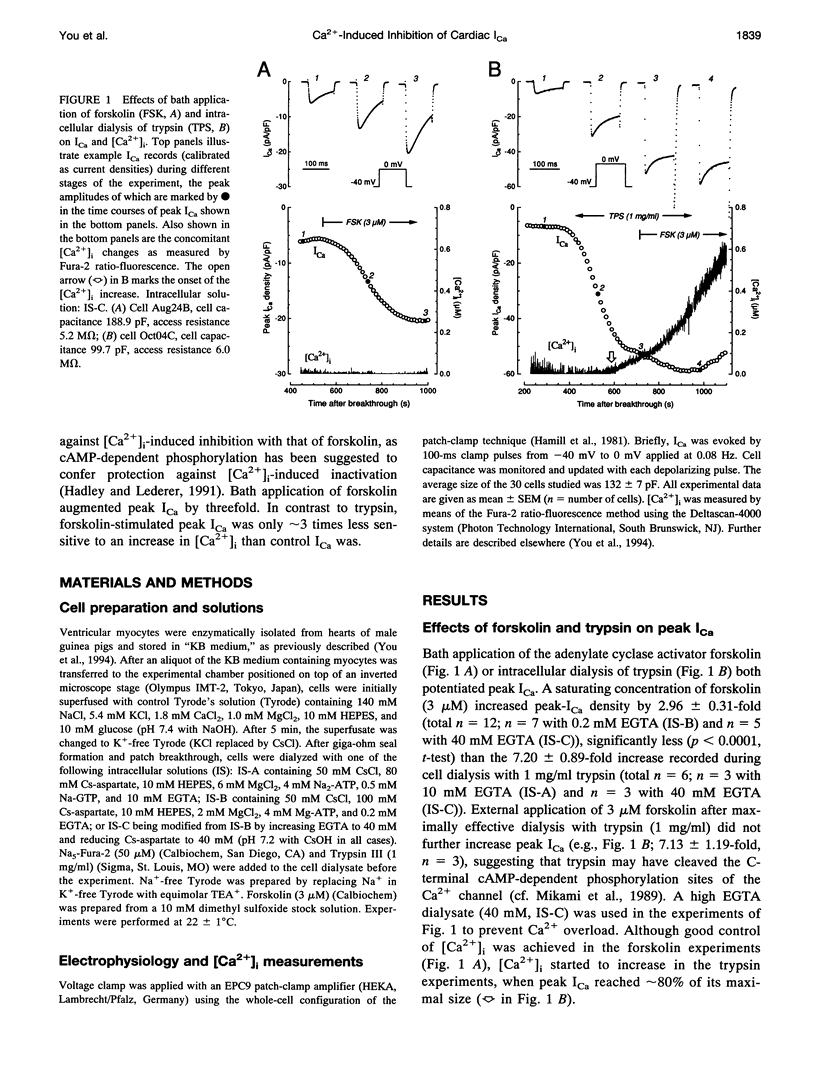
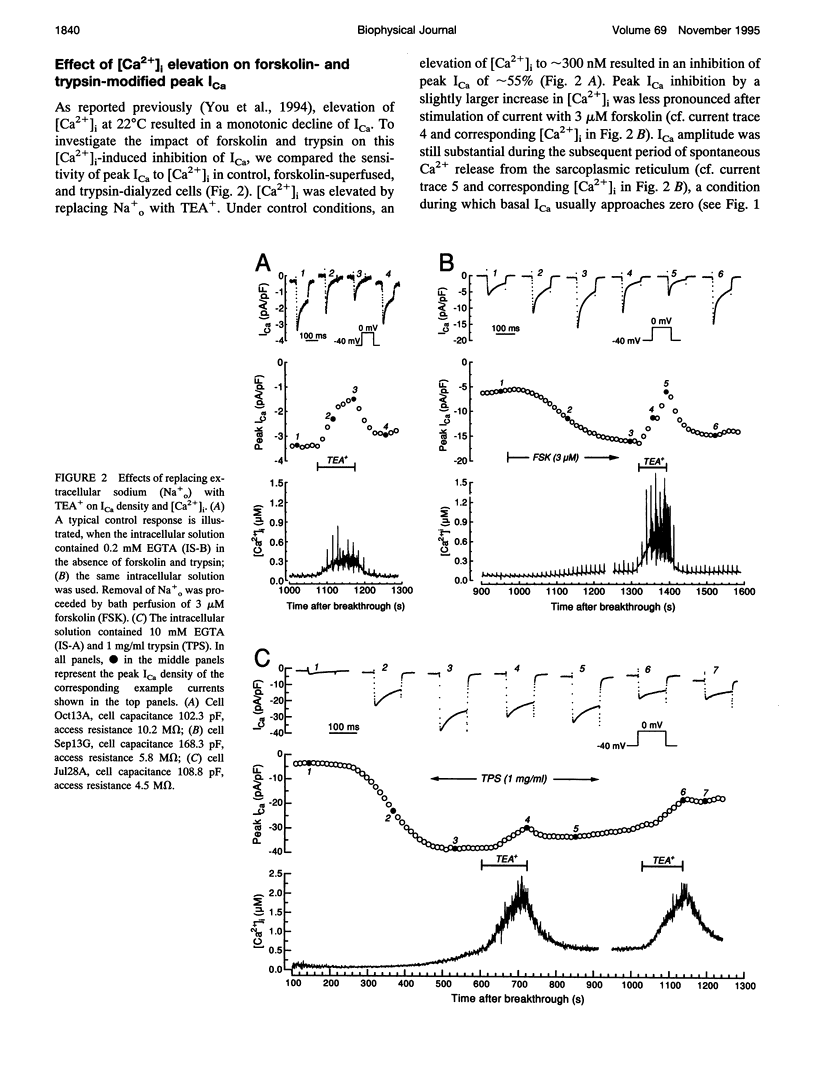
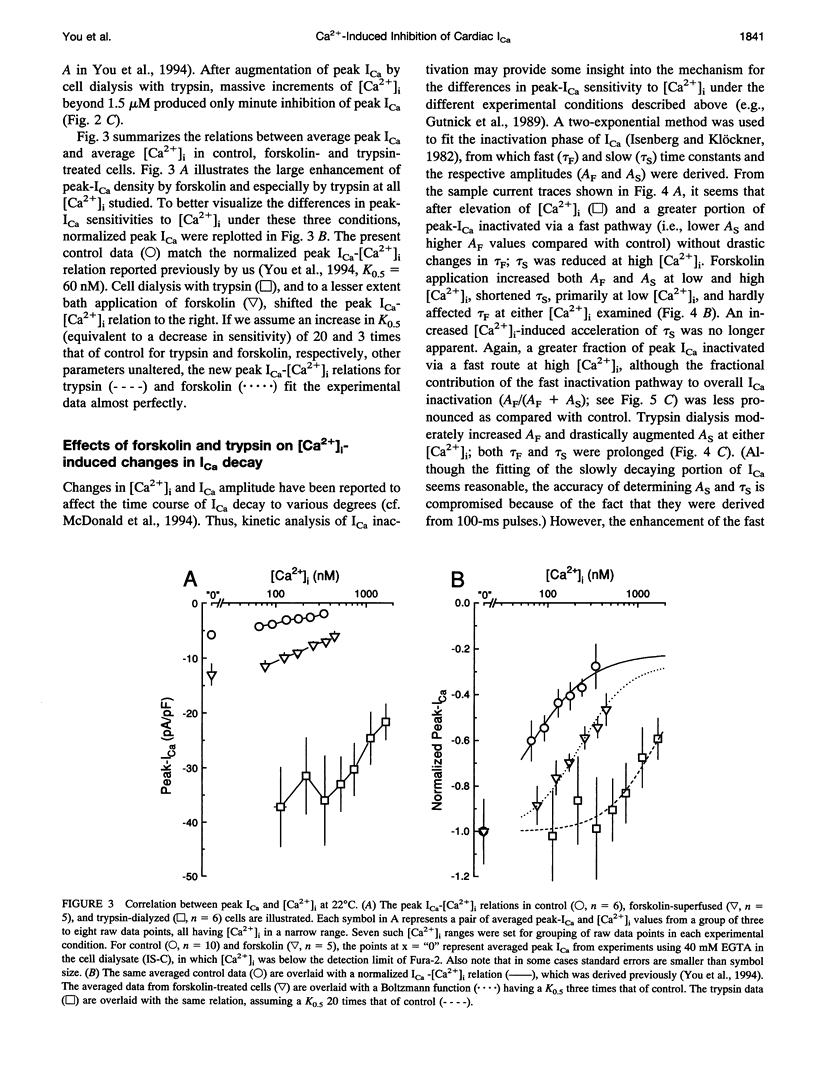
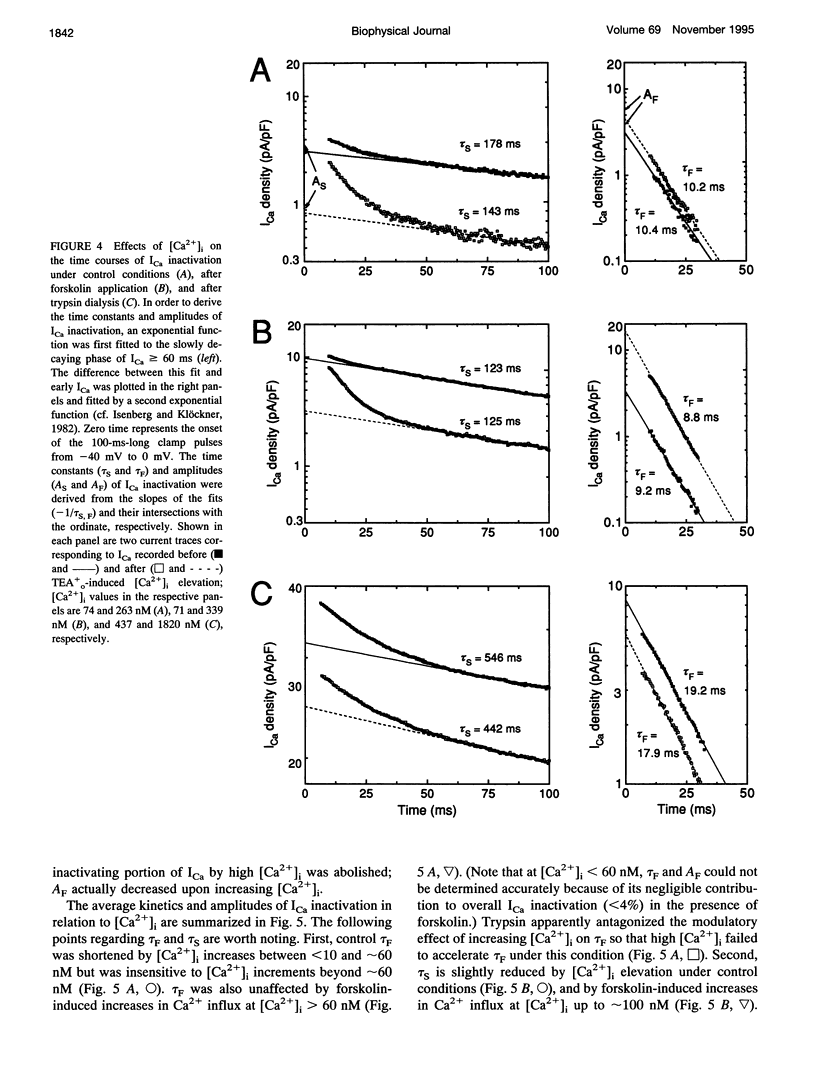
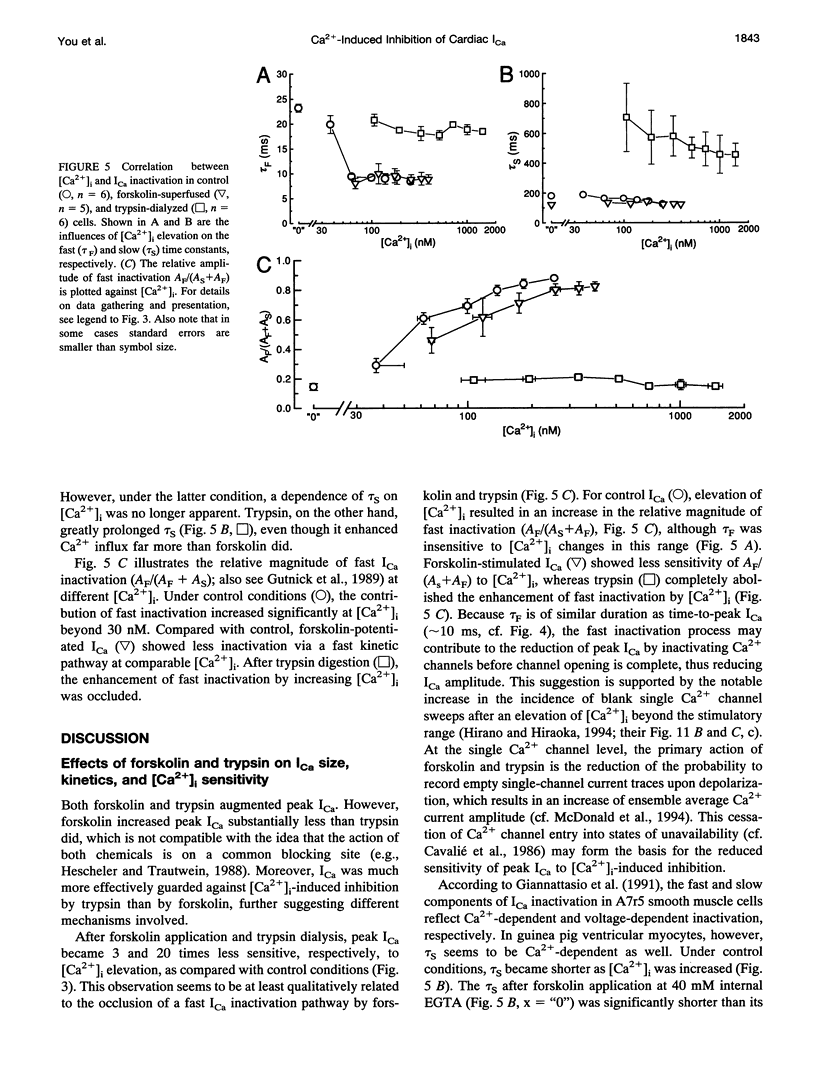
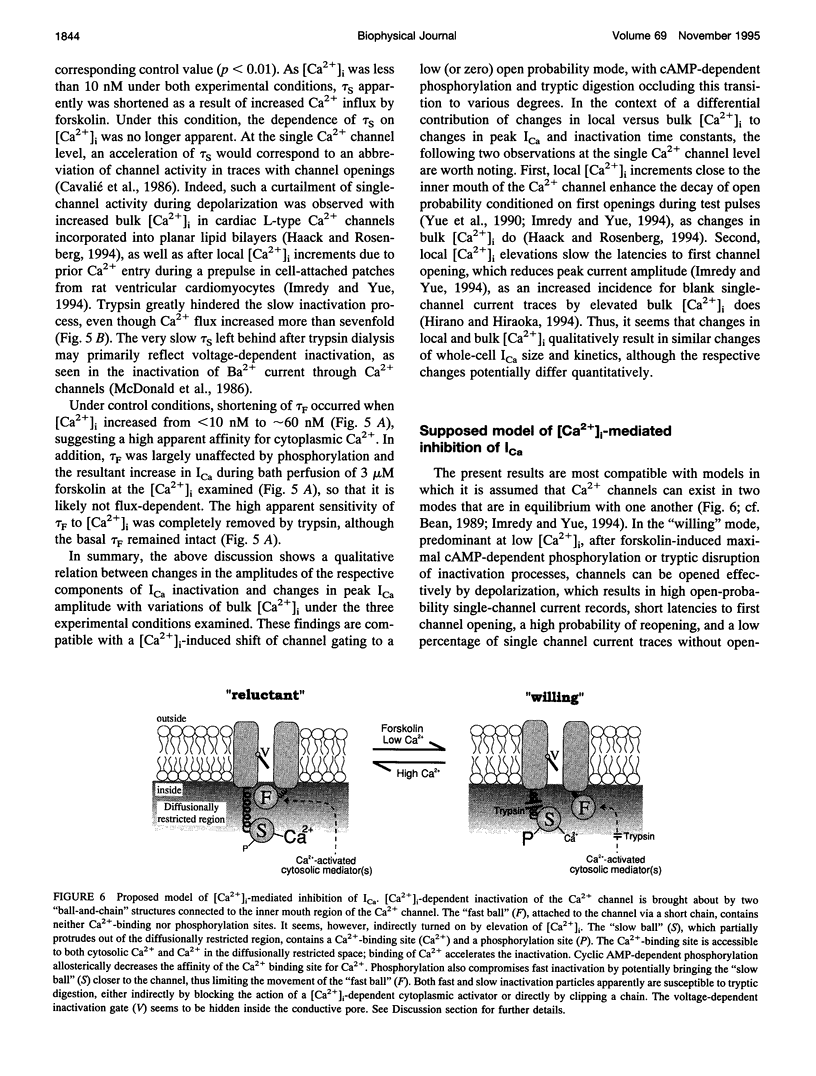
A key feature of trypsin action on ionic membrane currents including L-type Ca2+ current (ICa) is the removal of inactivation upon intracellular application. Here we report that trypsin also occludes the resting cytoplasmic free Ca2+ ([Ca2+]i)-induced inhibition of peak ICa in isolated guinea pig ventricular cardiomyocytes, using the whole-cell patch clamp in combination with the Fura-2 ratio-fluorescence technique. The effectiveness of trypsin to guard ICa against [Ca2+]i-induced inhibition was compared with that of forskolin, as cAMP-dependent phosphorylation had been suggested to confer protection against [Ca2+]i-induced inactivation. Intracellular dialysis of trypsin (1 mg/ml) augmented ICa by 7.2-fold, significantly larger than the threefold increase induced by forskolin (3 microM). Forskolin application after trypsin dialysis did not further enhance ICa. An increase in [Ca2+]i from resting levels (varied by 0.2, 10, and 40 mM EGTA dialysis) to submicromolar concentrations after replacement of external Na+ (Na(o)+) with tetraethylammonium (TEA+) resulted in monotonic inhibition of control ICa, elicited from a holding potential of -40 mV at 22 degrees C. AFter trypsin dialysis, however, ICa became less sensitive to submicromolar [Ca2+]i; the [Ca2+]i of half-maximal inhibition (K0.5, normally around 60 nM) increased by approximately 20-fold. Forskolin also increased the K0.5 by approximately threefold. These and accompanying kinetic data on ICa decay are compatible with a model in which it is assumed that Ca2+ channels can exist in two modes (a high open probability "willing" and a low open probability "reluctant" mode) that are in equilibrium with one another. An increase in [Ca2+]i places a larger fraction of channels in the reluctant mode. This interconversion is hindered by cAMP-dependent phosphorylation and becomes nearly impossible after tryptic digestion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Adams B. A., Niidome T., Numa S., Tanabe T. Function of a truncated dihydropyridine receptor as both voltage sensor and calcium channel. Nature. 1992 Nov 12;360(6400):169–171. doi: 10.1038/360169a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- De Jongh K. S., Warner C., Colvin A. A., Catterall W. A. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio B., Jones S. W., Scarpa A. Calcium currents in the A7r5 smooth muscle-derived cell line. Calcium-dependent and voltage-dependent inactivation. J Gen Physiol. 1991 Nov;98(5):987–1003. doi: 10.1085/jgp.98.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick M. J., Lux H. D., Swandulla D., Zucker H. Voltage-dependent and calcium-dependent inactivation of calcium channel current in identified snail neurones. J Physiol. 1989 May;412:197–220. doi: 10.1113/jphysiol.1989.sp017611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack J. A., Rosenberg R. L. Calcium-dependent inactivation of L-type calcium channels in planar lipid bilayers. Biophys J. 1994 Apr;66(4):1051–1060. doi: 10.1016/S0006-3495(94)80886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991 Dec;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Trautwein W. Modification of L-type calcium current by intracellularly applied trypsin in guinea-pig ventricular myocytes. J Physiol. 1988 Oct;404:259–274. doi: 10.1113/jphysiol.1988.sp017289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Hiraoka M. Dual modulation of unitary L-type Ca2+ channel currents by [Ca2+]i in fura-2-loaded guinea-pig ventricular myocytes. J Physiol. 1994 Nov 1;480(Pt 3):449–463. doi: 10.1113/jphysiol.1994.sp020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994 Jun;12(6):1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Brown A. M. Trypsin activation of atrial muscarinic K+ channels. Am J Physiol. 1989 Jul;257(1 Pt 2):H334–H338. doi: 10.1152/ajpheart.1989.257.1.H334. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Cavalié A., Trautwein W., Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflugers Arch. 1986 May;406(5):437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer S., Trautwein W., Pelzer D. J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994 Apr;74(2):365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Obejero-Paz C. A., Jones S. W., Scarpa A. Calcium currents in the A7r5 smooth muscle-derived cell line. Increase in current and selective removal of voltage-dependent inactivation by intracellular trypsin. J Gen Physiol. 1991 Dec;98(6):1127–1140. doi: 10.1085/jgp.98.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido K. R., Callewaert G., Carmeliet E. Inhibition and rapid recovery of Ca2+ current during Ca2+ release from sarcoplasmic reticulum in guinea pig ventricular myocytes. Circ Res. 1995 Jan;76(1):102–109. doi: 10.1161/01.res.76.1.102. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Mikami A., Numa S., Beam K. G. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990 Mar 29;344(6265):451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- You Y., Pelzer D. J., Pelzer S. Modulation of calcium current density by intracellular calcium in isolated guinea pig ventricular cardiomyocytes. Biochem Biophys Res Commun. 1994 Oct 28;204(2):732–740. doi: 10.1006/bbrc.1994.2520. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]