Abstract
The effect of pH and cholesterol on the dimyristoylphosphatidic acid (DMPA) model membrane system has been investigated by solid state 2H- and 31P-NMR. It has been shown that each of the three protonation states of the DMPA molecule corresponds to a 31P-NMR powder pattern with characteristic delta sigma values; this implies additionally that the proton exchange on the membrane surface is slow on the NMR time scale (millisecond range). Under these conditions, the 2H-labeled lipid chains sense only one magnetic environment, indicating that the three spectra detected by 31P-NMR are related to charge-dependent local dynamics or orientations of the phosphate headgroup or both. Chain ordering in the fluid phase is also found to depend weakly on the charge at the interface. In addition, it has also been found that the first pK of the DMPA membrane is modified by changes in the lipid lateral packing (gel or fluid phases or in the presence of cholesterol) in contrast to the second pK. The incorporation of 30 mol% cholesterol affects the phosphatidic acid bilayer in a way similar to what has been reported for phosphatidylcholine/cholesterol membranes, but to an extent comparable to 10-20 mol % sterol in phosphatidylcholines. However, the orientation and molecular order parameter of cholesterol in DMPA are similar to those found in dimyristoylphosphatidylcholine.
Full text
PDF

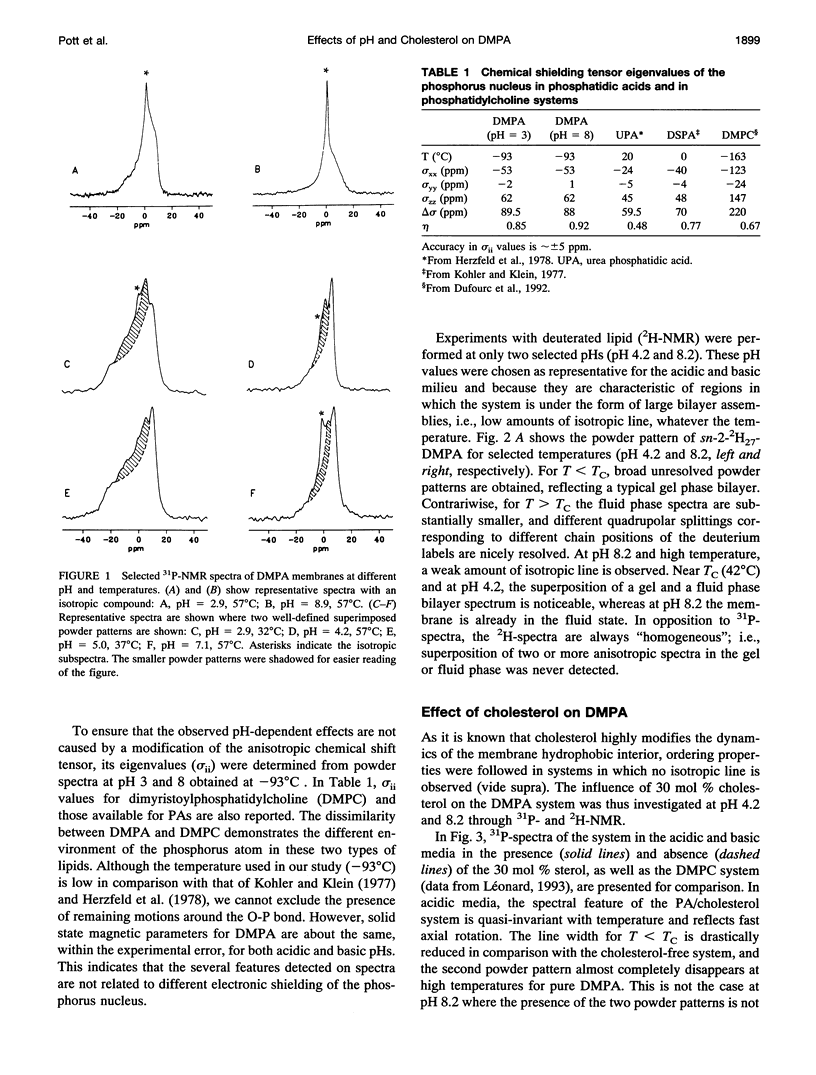
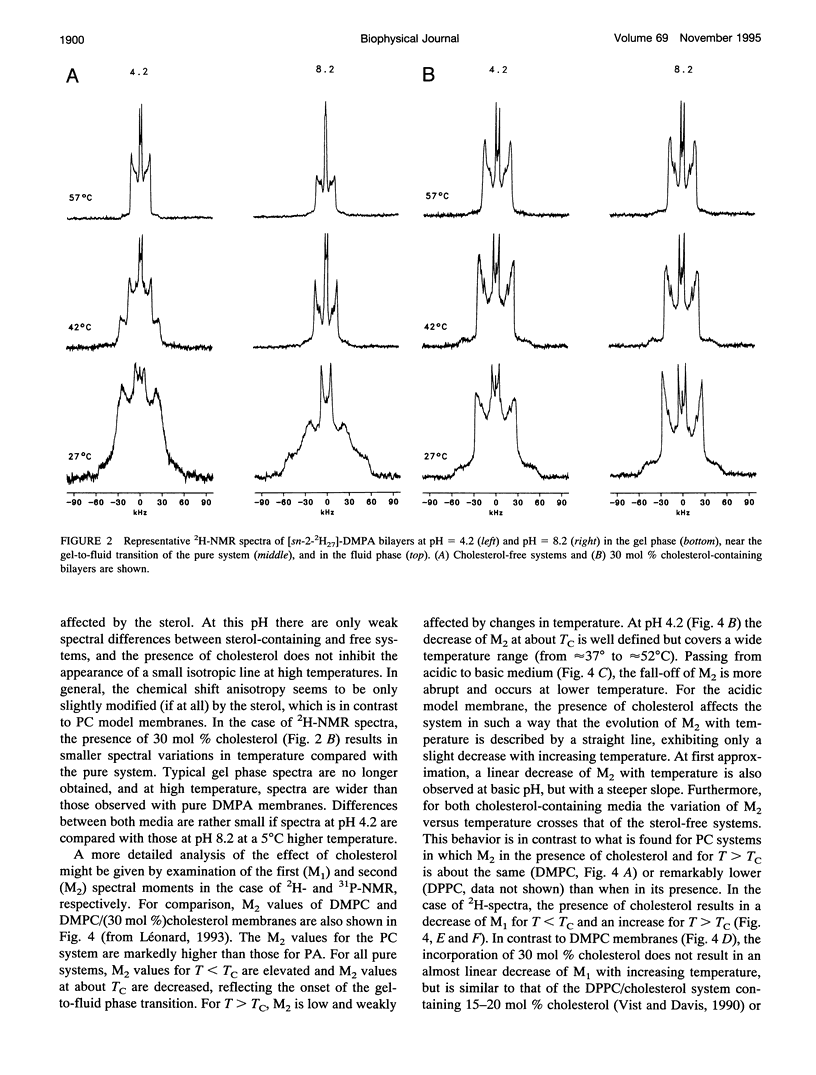
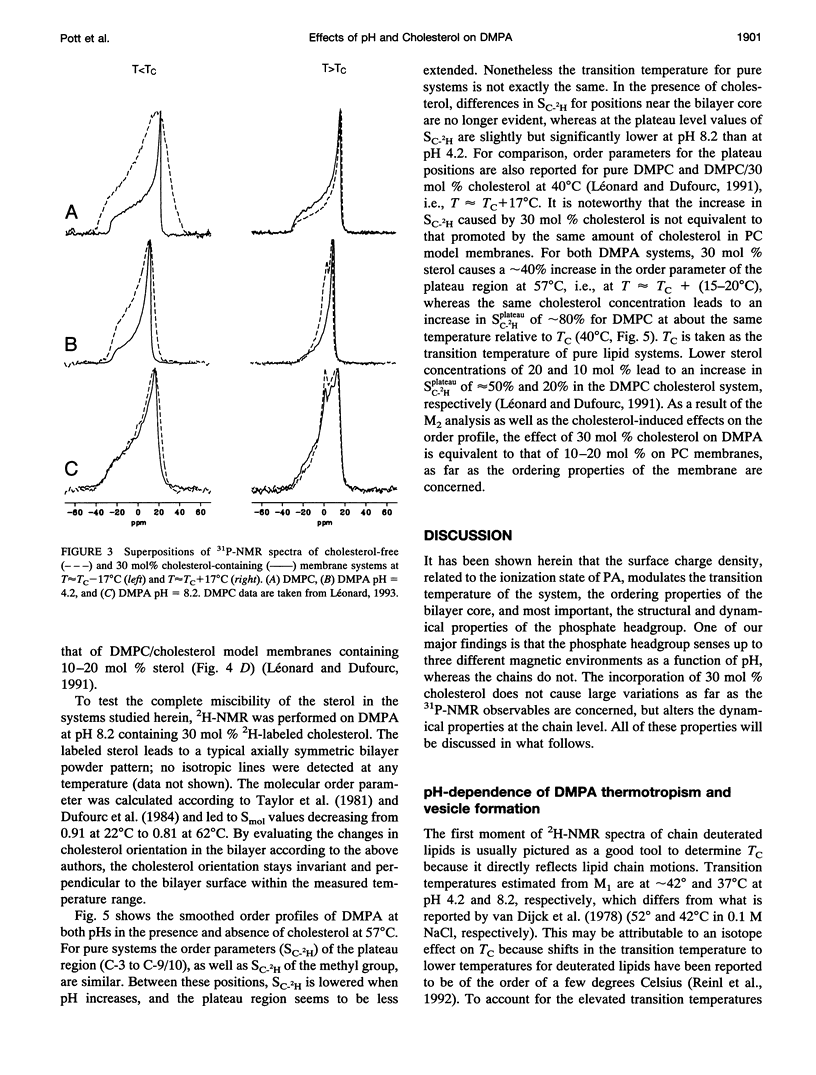
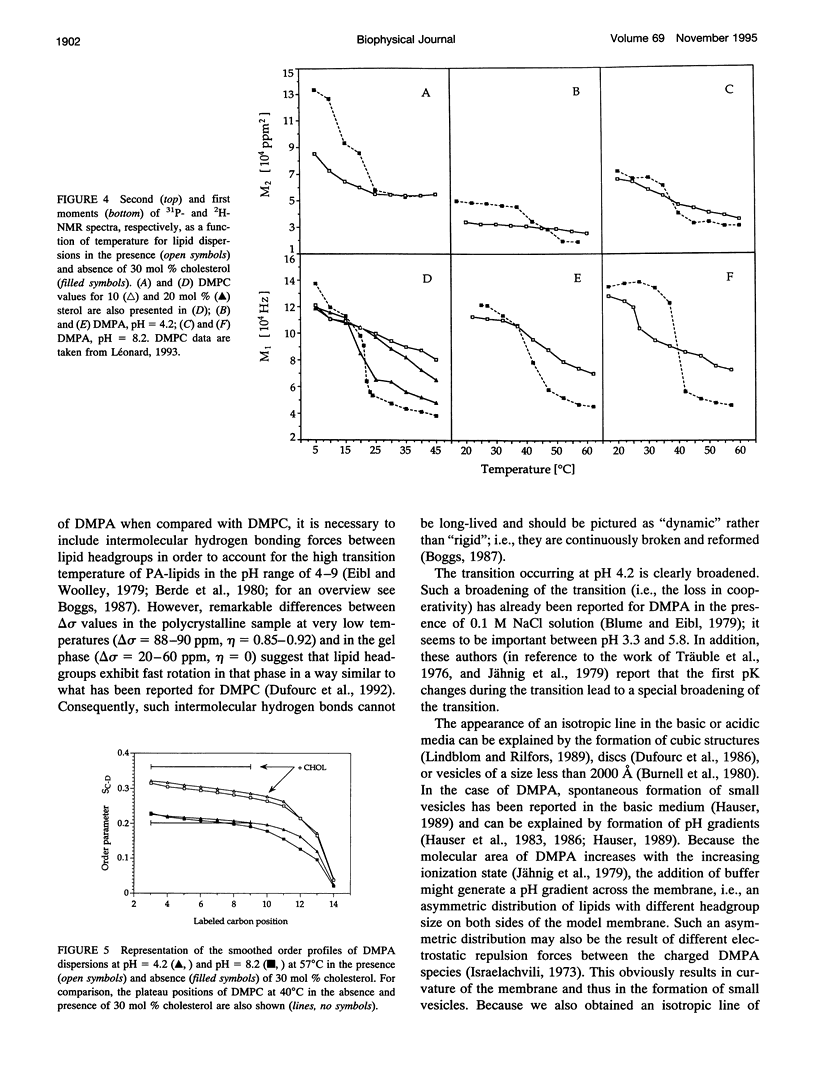
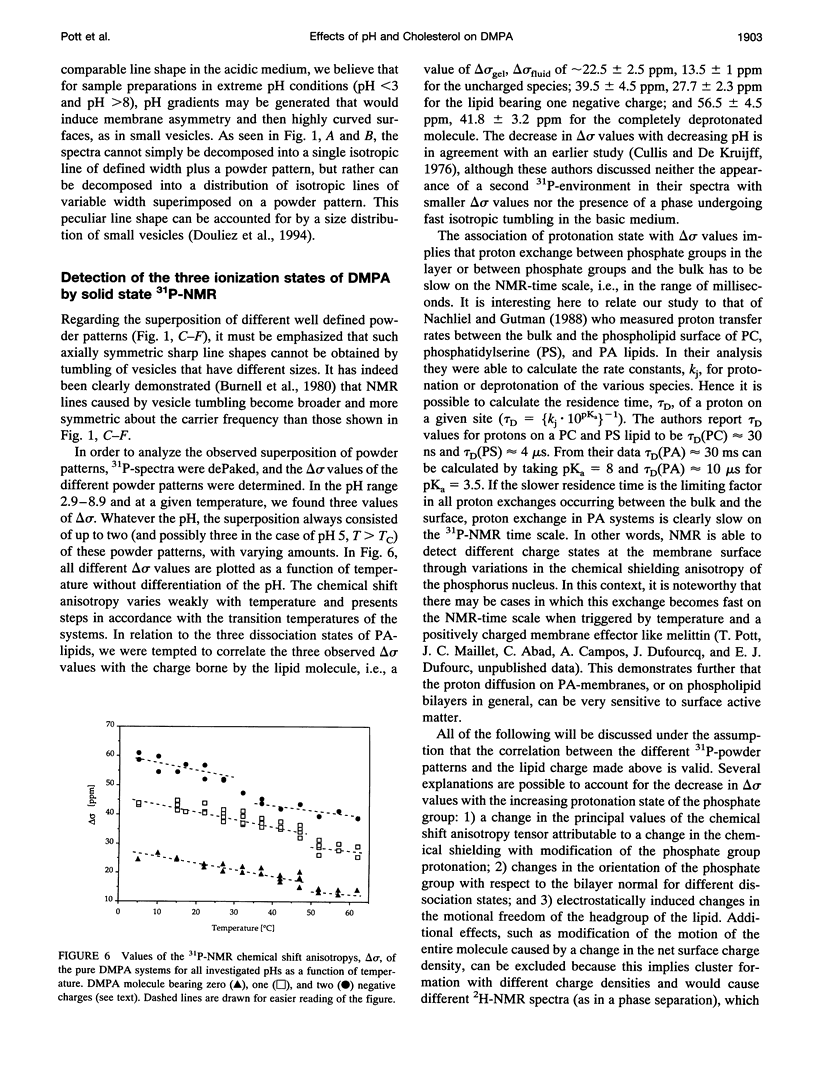
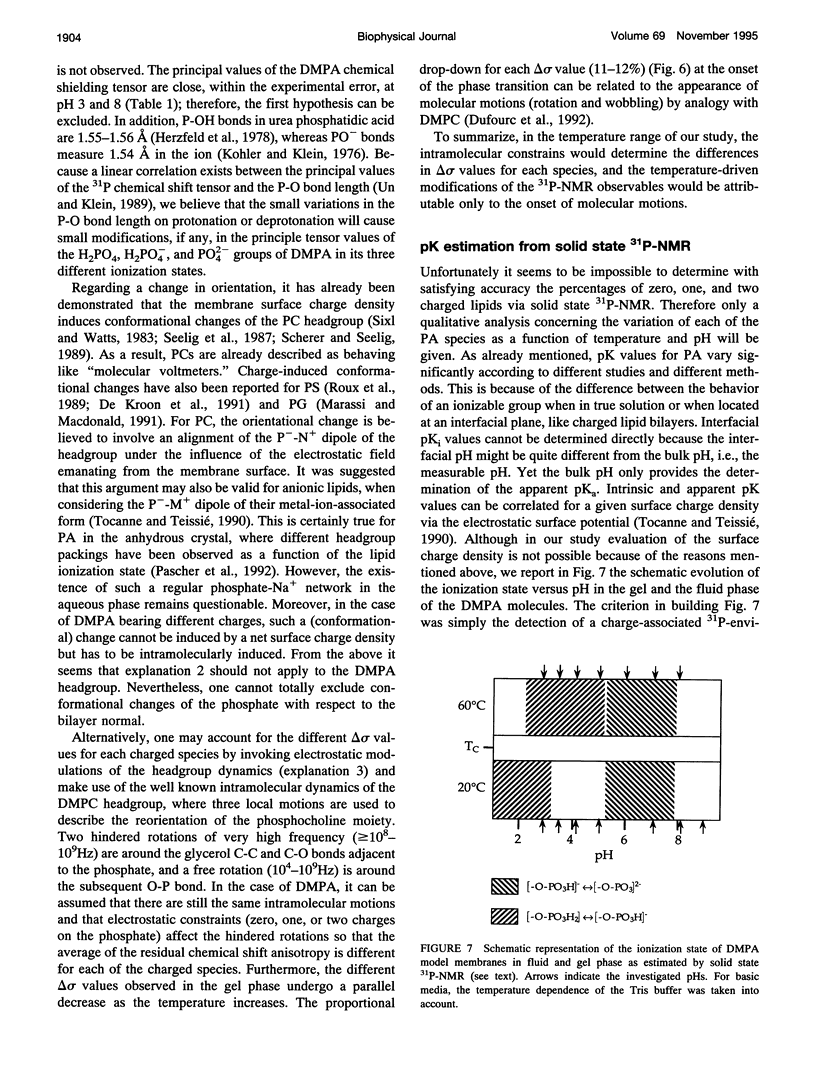

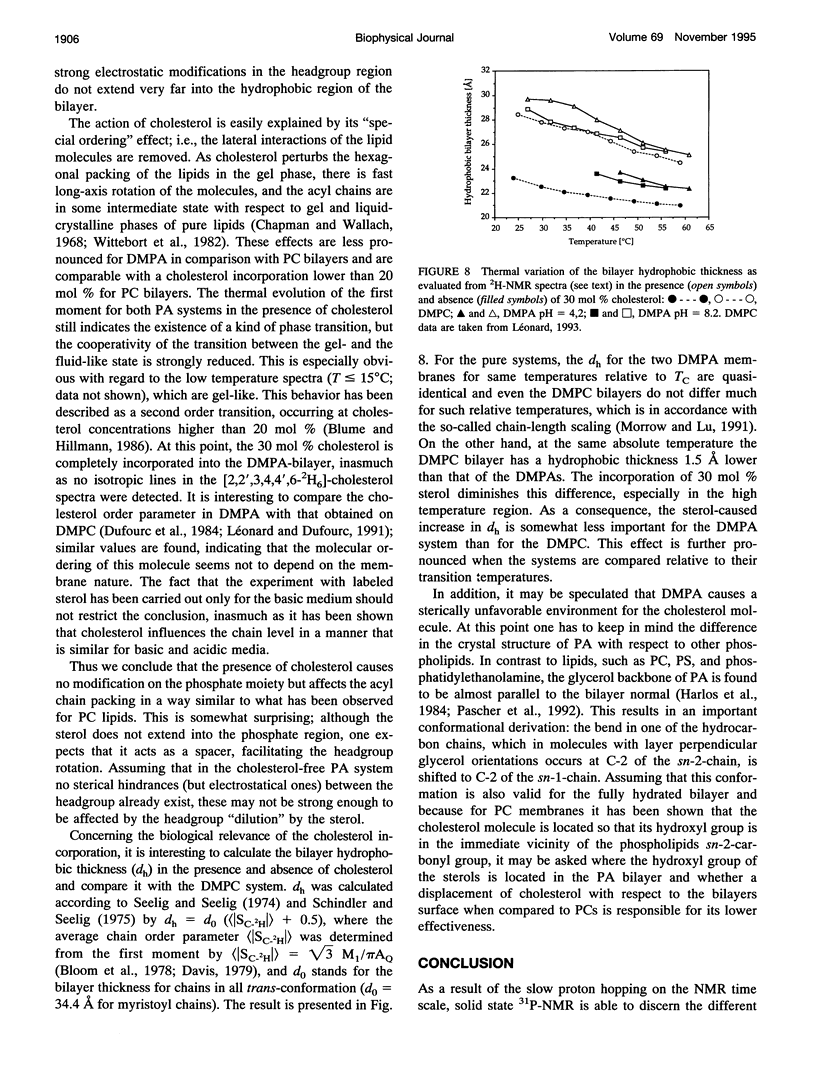


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berde C. B., Andersen H. C., Hudson B. S. A theory of the effects of head-group structure and chain unsaturation on the chain melting transition of phospholipid dispersions. Biochemistry. 1980 Sep 2;19(18):4279–4293. doi: 10.1021/bi00559a021. [DOI] [PubMed] [Google Scholar]
- Blume A., Eibl H. The influence of charge on bilayer membranes. Calorimetric investigations of phosphatidic acid bilayers. Biochim Biophys Acta. 1979 Nov 16;558(1):13–21. doi: 10.1016/0005-2736(79)90311-0. [DOI] [PubMed] [Google Scholar]
- Blume A., Hillmann M. Dimyristoylphosphatidic acid/cholesterol bilayers. Thermodynamic properties and kinetics of the phase transition as studied by the pressure jump relaxation technique. Eur Biophys J. 1986;13(6):343–353. doi: 10.1007/BF00265670. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Burnell E. E., Cullis P. R., de Kruijff B. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P-NMR lineshapes. Biochim Biophys Acta. 1980 Dec 2;603(1):63–69. doi: 10.1016/0005-2736(80)90391-0. [DOI] [PubMed] [Google Scholar]
- Copeland B. R., Andersen H. C. A theory of effect of protons and divalent cations on phase equilibria in charged bilayer membranes: comparison with experiment. Biochemistry. 1982 Jun 8;21(12):2811–2820. doi: 10.1021/bi00541a001. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruyff B. 31P NMR studies of unsonicated aqueous dispersions of neutral and acidic phospholipids. Effects of phase transitions, p2H and divalent cations on the motion in the phosphate region of the polar headgroup. Biochim Biophys Acta. 1976 Jul 1;436(3):523–540. doi: 10.1016/0005-2736(76)90438-7. [DOI] [PubMed] [Google Scholar]
- Davis J. H. Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys J. 1979 Sep;27(3):339–358. doi: 10.1016/S0006-3495(79)85222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Mayer C., Stohrer J., Althoff G., Kothe G. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys J. 1992 Jan;61(1):42–57. doi: 10.1016/S0006-3495(92)81814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourc E. J., Smith I. C., Dufourcq J. Molecular details of melittin-induced lysis of phospholipid membranes as revealed by deuterium and phosphorus NMR. Biochemistry. 1986 Oct 21;25(21):6448–6455. doi: 10.1021/bi00369a016. [DOI] [PubMed] [Google Scholar]
- Eibl H., Blume A. The influence of charge on phosphatidic acid bilayer membranes. Biochim Biophys Acta. 1979 Jun 2;553(3):476–488. doi: 10.1016/0005-2736(79)90303-1. [DOI] [PubMed] [Google Scholar]
- Eibl H., Woolley P. Electrostatic interactions at charged lipid membranes. Hydrogen bonds in lipid membrane surfaces. Biophys Chem. 1979 Nov;10(3-4):261–271. doi: 10.1016/0301-4622(79)85015-2. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Virtanen J. A., Vuori K., Patrikainen J., Kinnunen P. K. Role of the polar head group stereoconfiguration in the cation-induced aggregation of dimyristoylphosphatidylglycerol vesicles. Biochemistry. 1987 Dec 1;26(24):7542–7545. doi: 10.1021/bi00398a002. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gains N., Eibl H. J., Müller M., Wehrli E. Spontaneous vesiculation of aqueous lipid dispersions. Biochemistry. 1986 Apr 22;25(8):2126–2134. doi: 10.1021/bi00356a042. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gains N., Müller M. Vesiculation of unsonicated phospholipid dispersions containing phosphatidic acid by pH adjustment: physicochemical properties of the resulting unilamellar vesicles. Biochemistry. 1983 Sep 27;22(20):4775–4781. doi: 10.1021/bi00289a025. [DOI] [PubMed] [Google Scholar]
- Hauser H. Mechanism of spontaneous vesiculation. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5351–5355. doi: 10.1073/pnas.86.14.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld J., Griffin R. G., Haberkorn R. A. Phosphorus-31 chemical-shift tensors in barium diethyl phosphate and urea-phosphoric acid: model compounds for phospholipid head-group studies. Biochemistry. 1978 Jul 11;17(14):2711–2718. doi: 10.1021/bi00607a003. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N. Theoretical considerations on the asymmetric distribution of charged phospholipid molecules on the inner and outer layers of curved bilayer membranes. Biochim Biophys Acta. 1973 Nov 16;323(4):659–663. doi: 10.1016/0005-2736(73)90179-x. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975 Jan 14;14(1):152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- Jähnig F., Harlos K., Vogel H., Eibl H. Electrostatic interactions at charged lipid membranes. Electrostatically induced tilt. Biochemistry. 1979 Apr 17;18(8):1459–1468. doi: 10.1021/bi00575a012. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A. Local dielectric properties around polar region of lipid bilayer membranes. J Membr Biol. 1985;85(3):225–231. doi: 10.1007/BF01871517. [DOI] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. 31P nuclear magnetic resonance chemical shielding tensors of phosphorylethanolamine, lecithin, and related compounds: Applications to head-group motion in model membranes. Biochemistry. 1976 Mar 9;15(5):967–974. doi: 10.1021/bi00650a004. [DOI] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. Orientation and dynamics of phospholipid head groups in bilayers and membranes determined from 31P nuclear magnetic resonance chemical shielding tensors. Biochemistry. 1977 Feb 8;16(3):519–526. doi: 10.1021/bi00622a028. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Cullis P. R., Bloom M. Modulation of the orientational order profile of the lipid acyl chain in the L alpha phase. Eur Biophys J. 1990;19(2):55–62. doi: 10.1007/BF00185086. [DOI] [PubMed] [Google Scholar]
- Laroche G., Dufourc E. J., Dufourcq J., Pézolet M. Structure and dynamics of dimyristoylphosphatidic acid/calcium complexes by 2H NMR, infrared, spectroscopies and small-angle x-ray diffraction. Biochemistry. 1991 Mar 26;30(12):3105–3114. doi: 10.1021/bi00226a018. [DOI] [PubMed] [Google Scholar]
- Laroche G., Dufourc E. J., Pézolet M., Dufourcq J. Coupled changes between lipid order and polypeptide conformation at the membrane surface. A 2H NMR and Raman study of polylysine-phosphatidic acid systems. Biochemistry. 1990 Jul 10;29(27):6460–6465. doi: 10.1021/bi00479a018. [DOI] [PubMed] [Google Scholar]
- Léonard A., Dufourc E. J. Interactions of cholesterol with the membrane lipid matrix. A solid state NMR approach. Biochimie. 1991 Oct;73(10):1295–1302. doi: 10.1016/0300-9084(91)90092-f. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Marassi F. M., Macdonald P. M. Response of the headgroup of phosphatidylglycerol to membrane surface charge as studied by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1991 Oct 29;30(43):10558–10566. doi: 10.1021/bi00107a027. [DOI] [PubMed] [Google Scholar]
- Pascher I., Lundmark M., Nyholm P. G., Sundell S. Crystal structures of membrane lipids. Biochim Biophys Acta. 1992 Dec 11;1113(3-4):339–373. doi: 10.1016/0304-4157(92)90006-v. [DOI] [PubMed] [Google Scholar]
- Reinl H., Brumm T., Bayerl T. M. Changes of the physical properties of the liquid-ordered phase with temperature in binary mixtures of DPPC with cholesterol: A H-NMR, FT-IR, DSC, and neutron scattering study. Biophys J. 1992 Apr;61(4):1025–1035. doi: 10.1016/S0006-3495(92)81910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M., Neumann J. M., Hodges R. S., Devaux P. F., Bloom M. Conformational changes of phospholipid headgroups induced by a cationic integral membrane peptide as seen by deuterium magnetic resonance. Biochemistry. 1989 Mar 7;28(5):2313–2321. doi: 10.1021/bi00431a050. [DOI] [PubMed] [Google Scholar]
- Sacré M. M., Tocanne J. F. Importance of glycerol and fatty acid residues on the ionic properties of phosphatidylglycerols at the air-water interface. Chem Phys Lipids. 1977 Apr;18(3-4):334–354. doi: 10.1016/0009-3084(77)90019-6. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 1990 Nov 27;29(47):10676–10684. doi: 10.1021/bi00499a015. [DOI] [PubMed] [Google Scholar]
- Scherer P. G., Seelig J. Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry. 1989 Sep 19;28(19):7720–7728. doi: 10.1021/bi00445a030. [DOI] [PubMed] [Google Scholar]
- Schindler H., Seelig J. Deuterium order parameters in relation to thermodynamic properties of a phospholiped bilayer. A statistical mechanical interpretation. Biochemistry. 1975 Jun 3;14(11):2283–2287. doi: 10.1021/bi00682a001. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Seelig J., Macdonald P. M., Scherer P. G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987 Dec 1;26(24):7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- Sixl F., Watts A. Headgroup interactions in mixed phospholipid bilayers. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1613–1615. doi: 10.1073/pnas.80.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne J. F., Teissié J. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim Biophys Acta. 1990 Feb 28;1031(1):111–142. doi: 10.1016/0304-4157(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Nisksch A., Jähnig F. Electrostatic interactions at charged lipid membranes. Measurement of surface pH with fluorescent lipoid pH indicators. Eur J Biochem. 1978 Feb 1;83(1):299–305. doi: 10.1111/j.1432-1033.1978.tb12094.x. [DOI] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990 Jan 16;29(2):451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Weisz K., Gröbner G., Mayer C., Stohrer J., Kothe G. Deuteron nuclear magnetic resonance study of the dynamic organization of phospholipid/cholesterol bilayer membranes: molecular properties and viscoelastic behavior. Biochemistry. 1992 Feb 4;31(4):1100–1112. doi: 10.1021/bi00119a019. [DOI] [PubMed] [Google Scholar]
- Wittebort R. J., Blume A., Huang T. H., Das Gupta S. K., Griffin R. G. Carbon-13 nuclear magnetic resonance investigations of phase transitions and phase equilibria in pure and mixed phospholipid bilayers. Biochemistry. 1982 Jul 6;21(14):3487–3502. doi: 10.1021/bi00257a036. [DOI] [PubMed] [Google Scholar]
- de Kroon A. I., Killian J. A., de Gier J., de Kruijff B. The membrane interaction of amphiphilic model peptides affects phosphatidylserine headgroup and acyl chain order and dynamics. Application of the "phospholipid headgroup electrometer" concept to phosphatidylserine. Biochemistry. 1991 Jan 29;30(4):1155–1162. doi: 10.1021/bi00218a038. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]


