Abstract
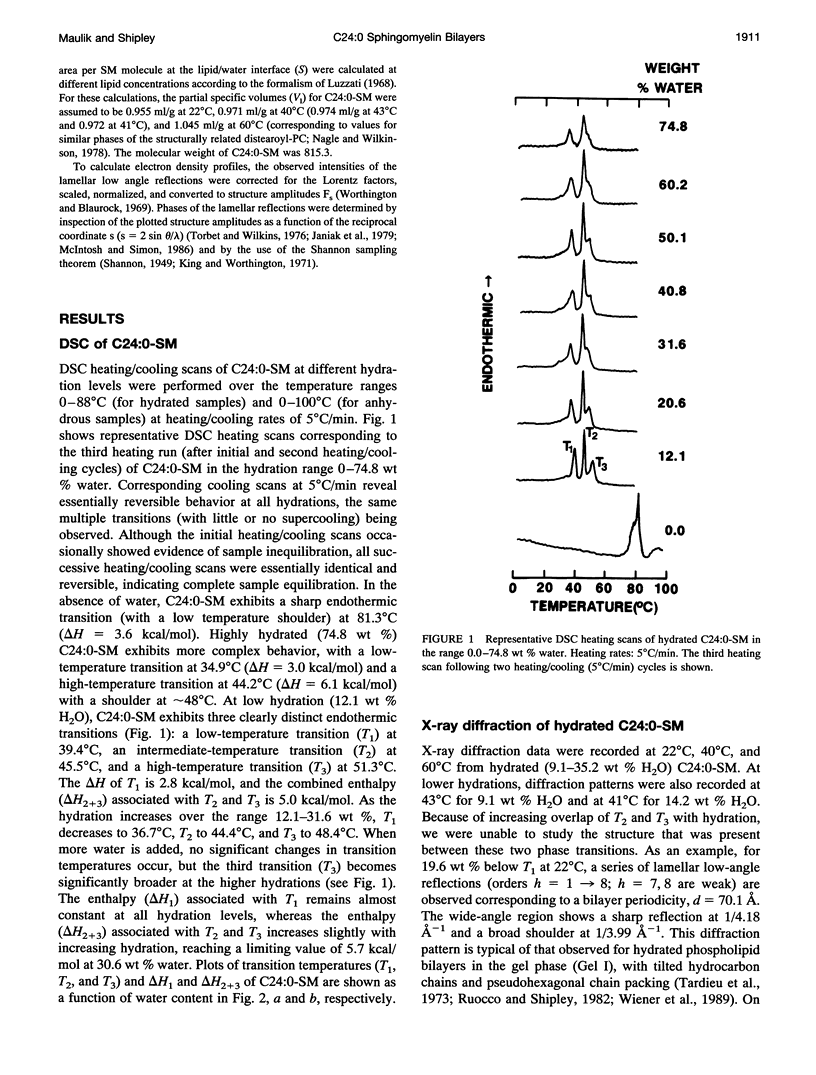
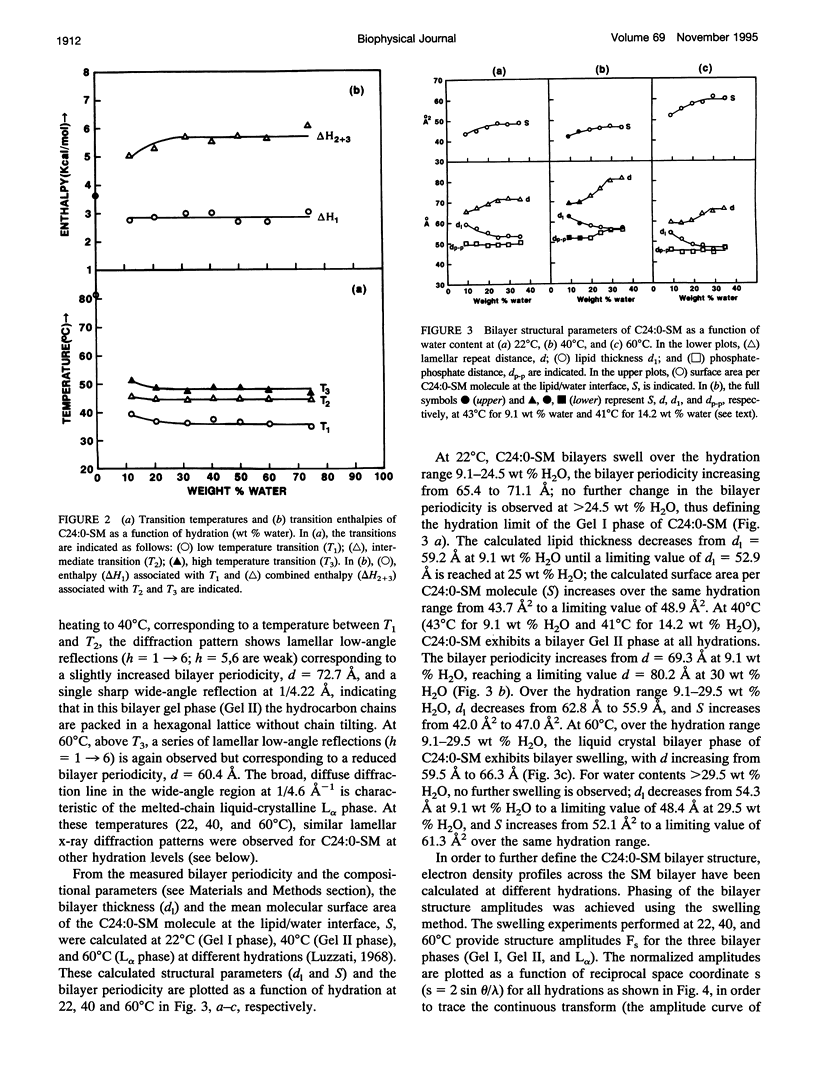
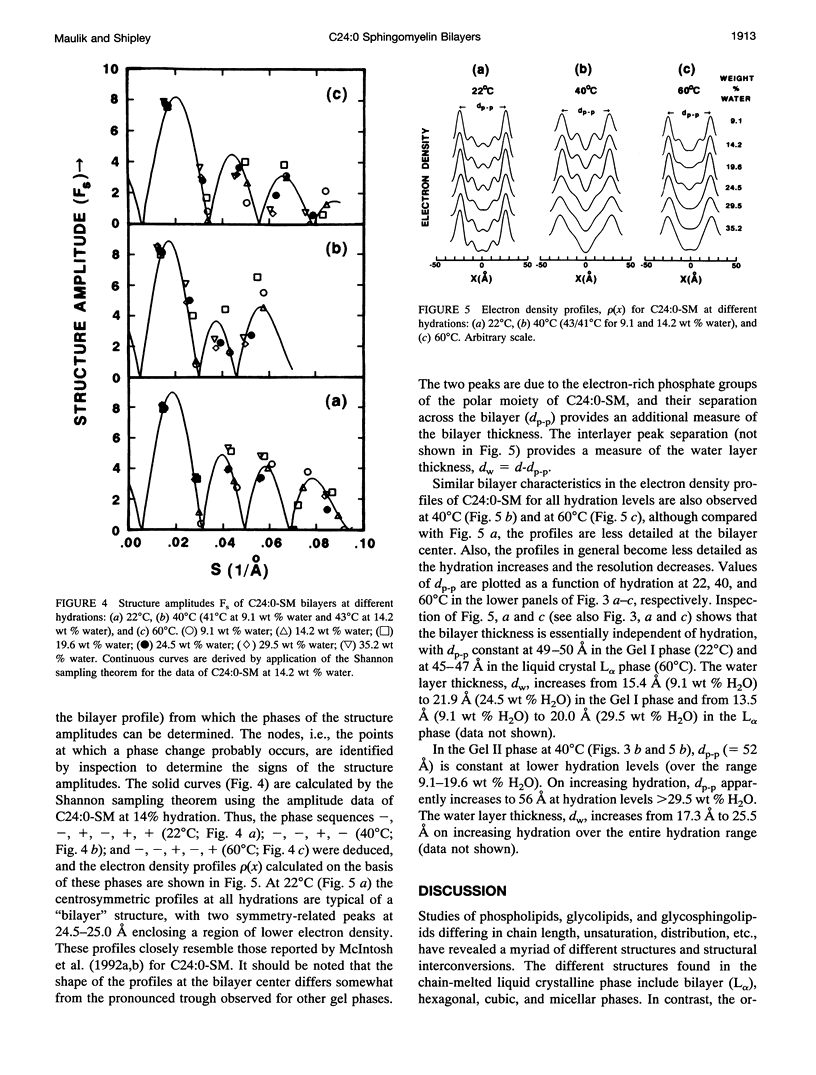
Differential scanning calorimetry and x-ray diffraction have been used to investigate hydrated multibilayers of N-lignoceryl sphingomyelin (C24:0-SM) in the hydration range 0-75 wt % H2O. Anhydrous C24:0-SM exhibits a single endothermic transition at 81.3 degrees C (delta H = 3.6 kcal/mol). At low hydration (12.1 wt % H2O), three different endothermic transitions are observed: low-temperature transition (T1) at 39.4 degrees C (transition enthalpy (delta H1) = 2.8 kcal/mol), intermediate-temperature transition (T2) at 45.5 degrees C, and high-temperature transition (T3) at 51.3 degrees C (combined transition enthalpy (delta H2 + 3) = 5.03 kcal/mol). On increasing hydration, all three transition temperatures of C24:0-SM decrease slightly to reach limiting values of 36.7 degrees C (T1), 44.4 degrees C (T2), and 48.4 degrees C (T3) at approximately 20 wt % H2O. At 22 degrees C (below T1), x-ray diffraction of C24:0-SM at different hydration levels shows two wide-angle reflections, a sharp one at 1/4.2 A-1 and a more diffuse one at 1/4.0 A-1 together with lamellar reflections corresponding to bilayer periodicities increasing from d = 65.4 A to a limiting value of 71.1 A. Electron density profiles show a constant bilayer thickness dp-p approximately 50 A. In contrast, at 40 degrees C (between T1 and T2) a single sharp wide-angle reflection at approximately 1/4.2 A-1 is observed. The lamellar reflections correspond to a larger bilayer periodicity (increasing from d = 69.3-80.2 A) and there is some increase in dp-p (52-56 A) with hydration. These structural parameters,together with calculated lipid thickness and molecular area considerations, suggest that the low temperature endotherm(T1) of hydrated C24:0-SM corresponds to a transition from a tilted, gel state (Gel I) with partially interdigitated chains to an untilted, or less tilted, gel state (Gel 11). At 600C (above T3), the usual liquid-crystalline La bilayer structure (d = 59.5-66.3A; dp p -46 A) is present at all hydrations. Comparison with the behavior of C18:0-SM indicates that the in equivalence of length of the sphingosine (C18) and lignoceryl (C24) chains results in a more complex gel phase polymorphism for the sphingosine (C18) and lignoceryl (C24) chains results in a more complex gel phase polymorphism for C24:0-SM.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad T. Y., Sparrow J. T., Morrisett J. D. Fluorine-, pyrene-, and nitroxide-labeled sphingomyelin: semi-synthesis and thermotropic properties. J Lipid Res. 1985 Sep;26(9):1160–1165. [PubMed] [Google Scholar]
- Barenholz Y., Suurkuusk J., Mountcastle D., Thompson T. E., Biltonen R. L. A calorimetric study of the thermotropic behavior of aqueous dispersions of natural and synthetic sphingomyelins. Biochemistry. 1976 Jun 1;15(11):2441–2447. doi: 10.1021/bi00656a030. [DOI] [PubMed] [Google Scholar]
- Braganza L. F., Worcester D. L. Hydrostatic pressure induces hydrocarbon chain interdigitation in single-component phospholipid bilayers. Biochemistry. 1986 May 6;25(9):2591–2596. doi: 10.1021/bi00357a047. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S., Tsai M. D. A calorimetric study of the thermotropic behavior of pure sphingomyelin diastereomers. Biochemistry. 1987 Aug 25;26(17):5364–5368. doi: 10.1021/bi00391a022. [DOI] [PubMed] [Google Scholar]
- Calhoun W. I., Shipley G. G. Fatty acid composition and thermal behavior of natural sphingomyelins. Biochim Biophys Acta. 1979 Aug 23;555(3):436–441. doi: 10.1016/0005-2736(79)90397-3. [DOI] [PubMed] [Google Scholar]
- Calhoun W. I., Shipley G. G. Sphingomyelin--lecithin bilayers and their interaction with cholesterol. Biochemistry. 1979 May 1;18(9):1717–1722. doi: 10.1021/bi00576a013. [DOI] [PubMed] [Google Scholar]
- Cohen R., Barenholz Y., Gatt S., Dagan A. Preparation and characterization of well defined D-erythro sphingomyelins. Chem Phys Lipids. 1984 Oct;35(4):371–384. doi: 10.1016/0009-3084(84)90079-3. [DOI] [PubMed] [Google Scholar]
- Dong Z., Butcher J. A., Jr An efficient route to N-palmitoyl-D-erythro-sphingomyelin and its 13C-labeled derivatives. Chem Phys Lipids. 1993 Nov;66(1-2):41–46. doi: 10.1016/0009-3084(93)90029-3. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Pacuszka T., Orlandi P. A. Gangliosides as receptors for bacterial enterotoxins. Adv Lipid Res. 1993;25:165–187. [PubMed] [Google Scholar]
- Haas N. S., Sripada P. K., Shipley G. G. Effect of chain-linkage on the structure of phosphatidyl choline bilayers. Hydration studies of 1-hexadecyl 2-palmitoyl-sn-glycero-3-phosphocholine. Biophys J. 1990 Jan;57(1):117–124. doi: 10.1016/S0006-3495(90)82512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995 Feb;20(2):73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Hui S. W., Mason J. T., Huang C. Acyl chain interdigitation in saturated mixed-chain phosphatidylcholine bilayer dispersions. Biochemistry. 1984 Nov 6;23(23):5570–5577. doi: 10.1021/bi00318a029. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Kim J. T., Mattai J., Shipley G. G. Gel phase polymorphism in ether-linked dihexadecylphosphatidylcholine bilayers. Biochemistry. 1987 Oct 20;26(21):6592–6598. doi: 10.1021/bi00395a005. [DOI] [PubMed] [Google Scholar]
- Levin I. W., Thompson T. E., Barenholz Y., Huang C. Two types of hydrocarbon chain interdigitation in sphingomyelin bilayers. Biochemistry. 1985 Oct 22;24(22):6282–6286. doi: 10.1021/bi00343a036. [DOI] [PubMed] [Google Scholar]
- Mattai J., Sripada P. K., Shipley G. G. Mixed-chain phosphatidylcholine bilayers: structure and properties. Biochemistry. 1987 Jun 16;26(12):3287–3297. doi: 10.1021/bi00386a007. [DOI] [PubMed] [Google Scholar]
- Maulik P. R., Atkinson D., Shipley G. G. X-ray scattering of vesicles of N-acyl sphingomyelins. Determination of bilayer thickness. Biophys J. 1986 Dec;50(6):1071–1077. doi: 10.1016/S0006-3495(86)83551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik P. R., Sripada P. K., Shipley G. G. Structure and thermotropic properties of hydrated N-stearoyl sphingomyelin bilayer membranes. Biochim Biophys Acta. 1991 Feb 25;1062(2):211–219. doi: 10.1016/0005-2736(91)90395-o. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Ellington J. C., Jr, Porter N. A. New structural model for mixed-chain phosphatidylcholine bilayers. Biochemistry. 1984 Aug 28;23(18):4038–4044. doi: 10.1021/bi00313a005. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Needham D., Huang C. H. Interbilayer interactions between sphingomyelin and sphingomyelin/cholesterol bilayers. Biochemistry. 1992 Feb 25;31(7):2020–2024. doi: 10.1021/bi00122a018. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Needham D., Huang C. H. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry. 1992 Feb 25;31(7):2012–2020. doi: 10.1021/bi00122a017. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck J. L., Keira T., Luzzati V. A novel packing of the hydrocarbon chains in lipids. The low temperature phases of dipalmitoyl phosphatidyl-glycerol. Biochim Biophys Acta. 1977 Sep 28;488(3):432–441. doi: 10.1016/0005-2760(77)90201-6. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Siminovitch D. J., Griffin R. G. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985 May 7;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- Serrallach E. N., Dijkman R., de Haas G. H., Shipley G. G. Structure and thermotropic properties of 1,3-dipalmitoyl-glycero-2-phosphocholine. J Mol Biol. 1983 Oct 15;170(1):155–174. doi: 10.1016/s0022-2836(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Shah J., Sripada P. K., Shipley G. G. Structure and properties of mixed-chain phosphatidylcholine bilayers. Biochemistry. 1990 May 1;29(17):4254–4262. doi: 10.1021/bi00469a030. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Avecilla L. S., Small D. M. Phase behavior and structure of aqueous dispersions of sphingomyelin. J Lipid Res. 1974 Mar;15(2):124–131. [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. Biochim Biophys Acta. 1984 Jun 13;773(1):169–172. doi: 10.1016/0005-2736(84)90562-5. [DOI] [PubMed] [Google Scholar]
- Sripada P. K., Maulik P. R., Hamilton J. A., Shipley G. G. Partial synthesis and properties of a series of N-acyl sphingomyelins. J Lipid Res. 1987 Jun;28(6):710–718. [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Torbet J., Wilkins M. H. X-ray diffraction studies of lecithin bilayers. J Theor Biol. 1976 Oct 21;62(2):447–458. doi: 10.1016/0022-5193(76)90129-6. [DOI] [PubMed] [Google Scholar]
- Untrach S. H., Shipley G. G. Molecular interactions between lecithin and sphingomyelin. Temperature- and composition-dependent phase separation. J Biol Chem. 1977 Jul 10;252(13):4449–4457. [PubMed] [Google Scholar]
- Wiener M. C., Suter R. M., Nagle J. F. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys J. 1989 Feb;55(2):315–325. doi: 10.1016/S0006-3495(89)82807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington C. R., Blaurock A. E. A structural analysis of nerve myelin. Biophys J. 1969 Jul;9(7):970–990. doi: 10.1016/S0006-3495(69)86431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


