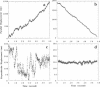
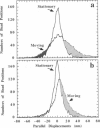
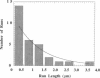
Abstract
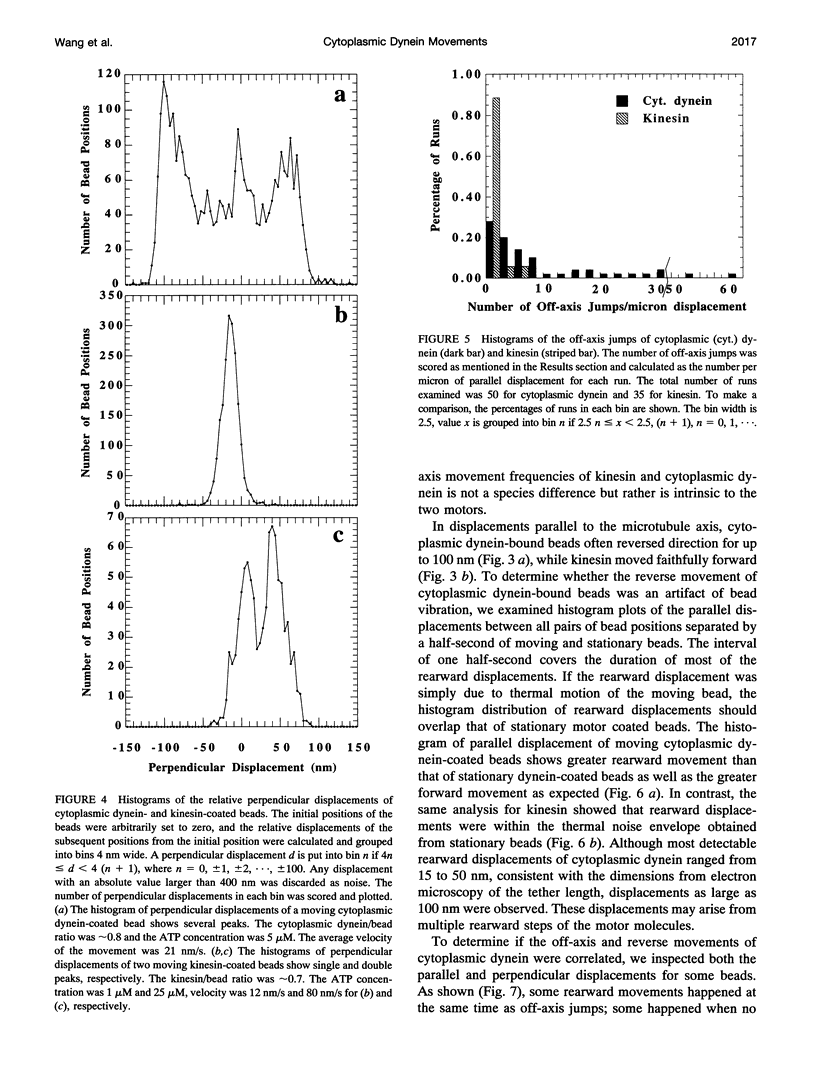
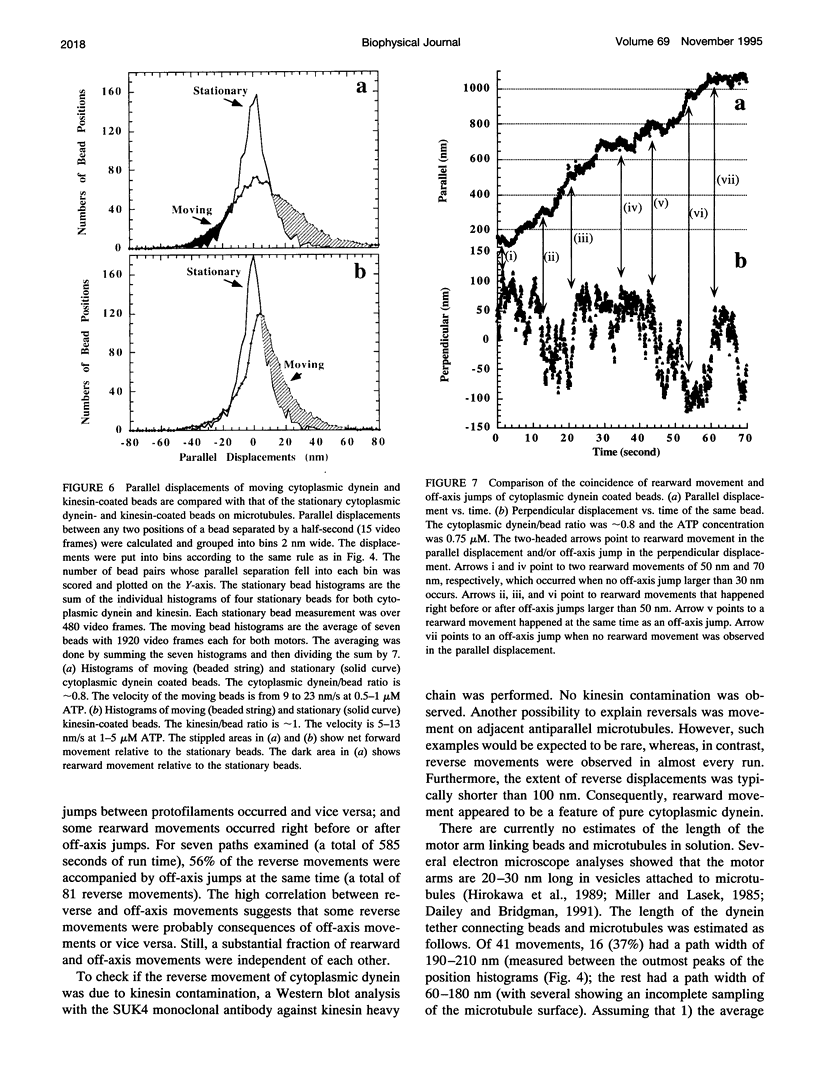
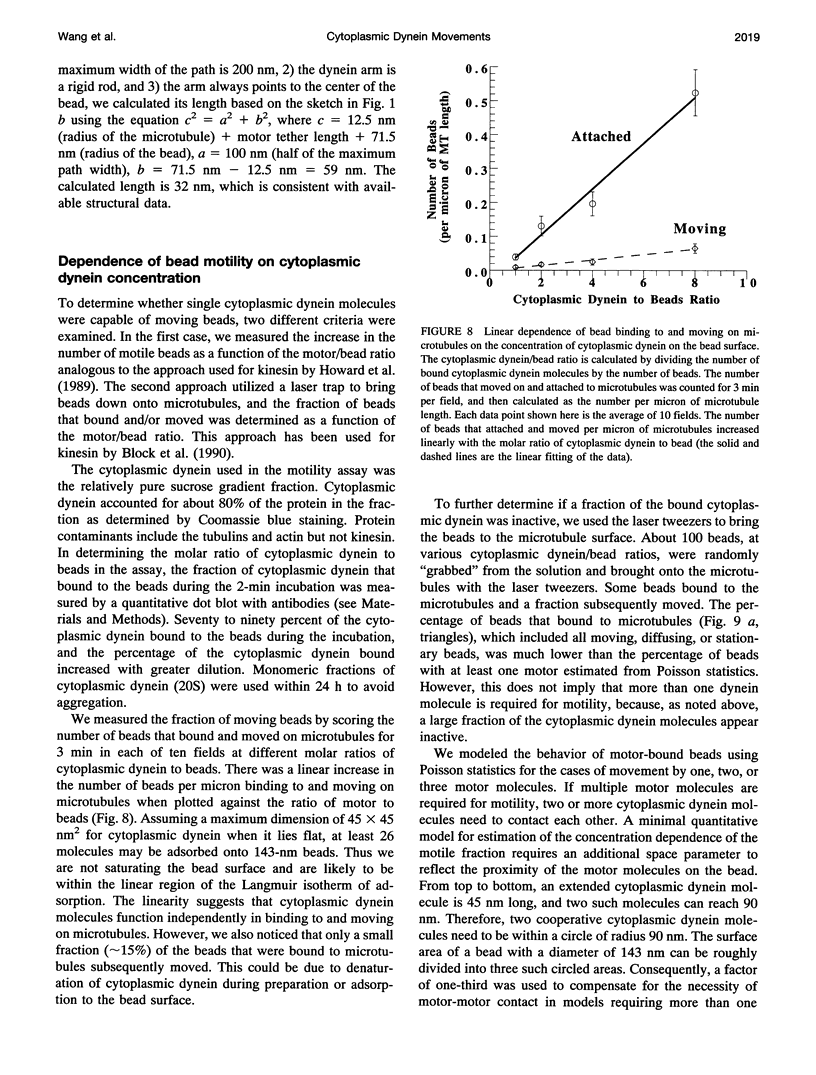
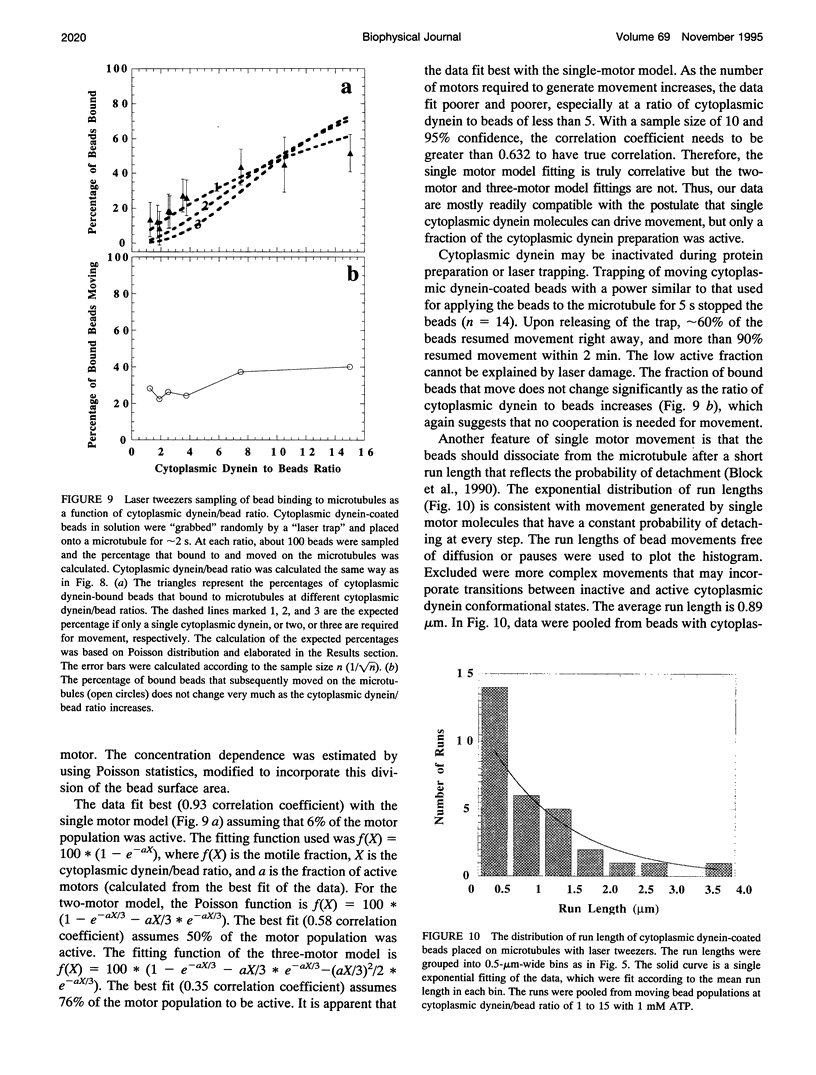
Cytoplasmic dynein is a major microtubule motor for minus-end directed movements including retrograde axonal transport. To better understand the mechanism by which cytoplasmic dynein converts ATP energy into motility, we have analyzed the nanometer-level displacements of latex beads coated with low numbers of cytoplasmic dynein molecules. Cytoplasmic dynein-coated beads exhibited greater lateral movements among microtubule protofilaments (ave. 5.1 times/microns of displacement) compared with kinesin (ave. 0.9 times/micron). In addition, dynein moved rearward up to 100 nm over several hundred milliseconds, often in correlation with off-axis movements from one protofilament to another. We suggest that single molecules of cytoplasmic dynein move the beads because 1) there is a linear dependence of bead motility on dynein/bead ratio, 2) the binding of beads to microtubules studied by laser tweezers is best fit by a first-order Poisson, and 3) the run length histogram of dynein beads follows a first-order decay. At the cellular level, the greater disorder of cytoplasmic dynein movements may facilitate transport by decreasing the duration of collisions between kinesin and cytoplasmic dynein-powered vesicles.
Full text
PDF

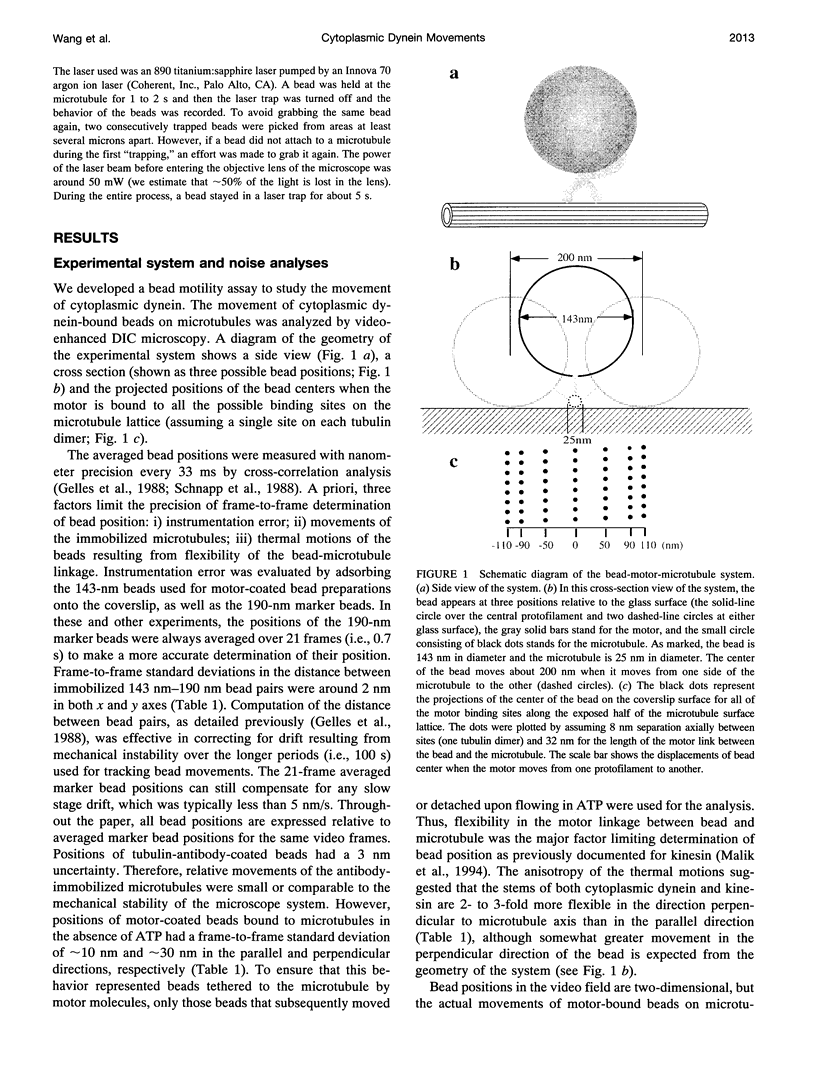
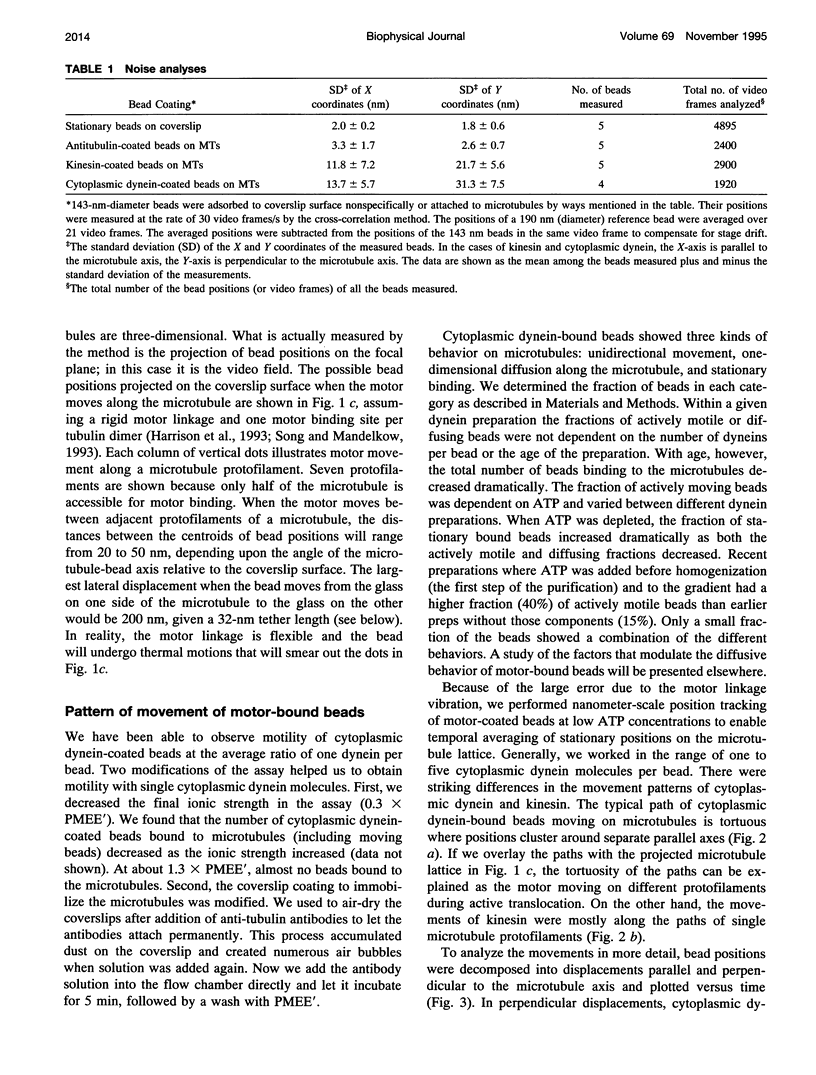
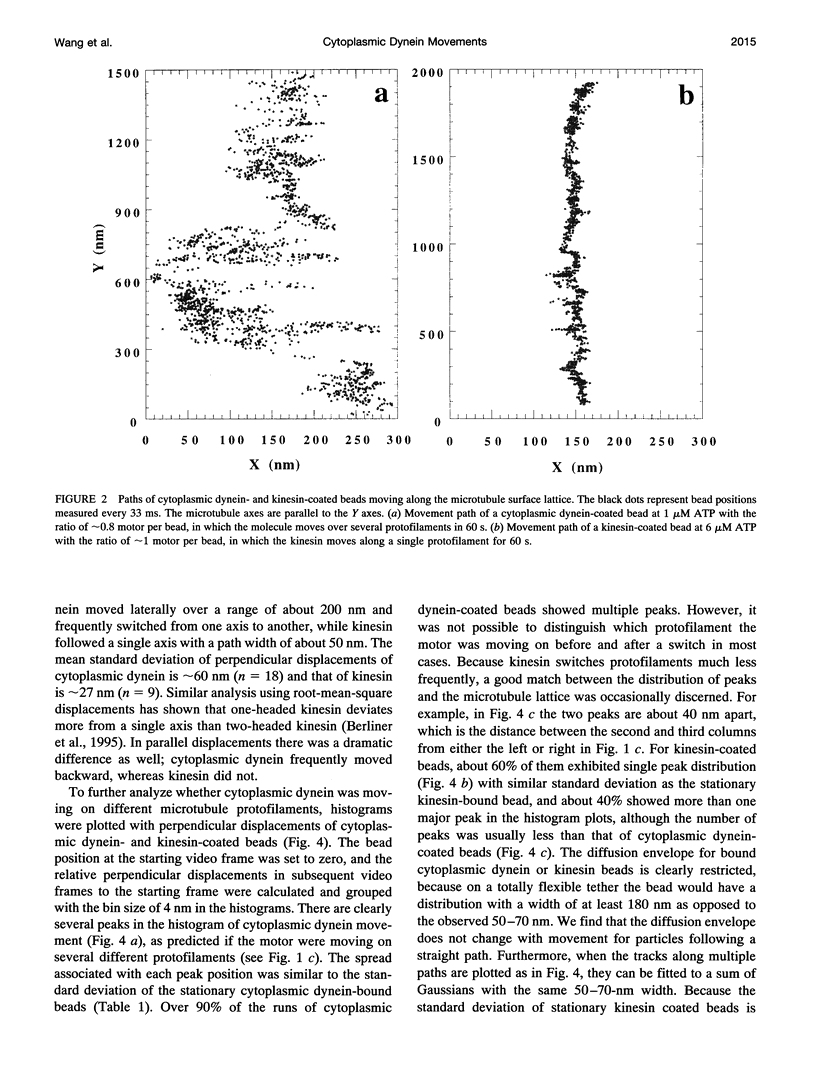
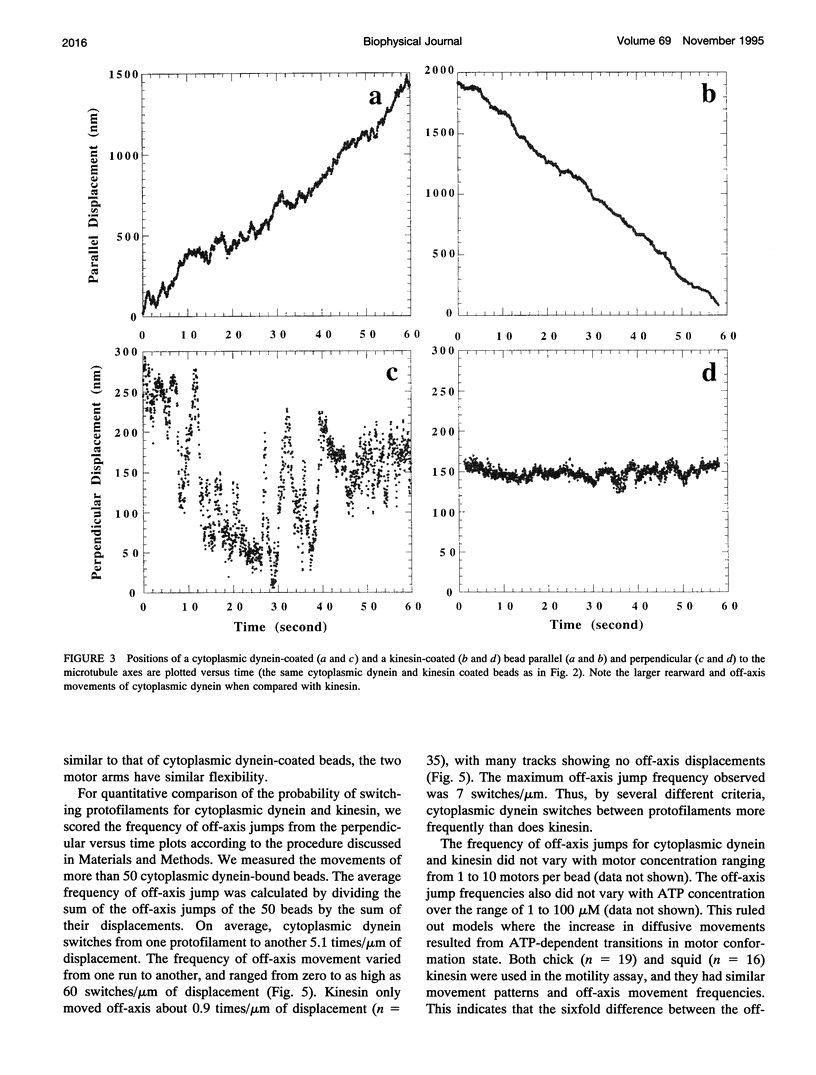



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner E., Young E. C., Anderson K., Mahtani H. K., Gelles J. Failure of a single-headed kinesin to track parallel to microtubule protofilaments. Nature. 1995 Feb 23;373(6516):718–721. doi: 10.1038/373718a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Goldstein L. S., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990 Nov 22;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- Dailey M. E., Bridgman P. C. Structure and organization of membrane organelles along distal microtubule segments in growth cones. J Neurosci Res. 1991 Sep;30(1):242–258. doi: 10.1002/jnr.490300125. [DOI] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Gelles J., Schnapp B. J., Sheetz M. P. Tracking kinesin-driven movements with nanometre-scale precision. Nature. 1988 Feb 4;331(6155):450–453. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Webb M. R., Brune M., Johnson K. A. Pathway of processive ATP hydrolysis by kinesin. Nature. 1995 Feb 23;373(6516):671–676. doi: 10.1038/373671a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B. C., Marchese-Ragona S. P., Gilbert S. P., Cheng N., Steven A. C., Johnson K. A. Decoration of the microtubule surface by one kinesin head per tubulin heterodimer. Nature. 1993 Mar 4;362(6415):73–75. doi: 10.1038/362073a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989 Mar 10;56(5):867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J., Vale R. D. Movement of microtubules by single kinesin molecules. Nature. 1989 Nov 9;342(6246):154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Ingold A. L., Cohn S. A., Scholey J. M. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988 Dec;107(6 Pt 2):2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima A., Harada Y., Kojima H., Funatsu T., Higuchi H., Yanagida T. Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem Biophys Res Commun. 1994 Mar 15;199(2):1057–1063. doi: 10.1006/bbrc.1994.1336. [DOI] [PubMed] [Google Scholar]
- Johnson K. A. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- Johnson K. A. The pathway of ATP hydrolysis by dynein. Kinetics of a presteady state phosphate burst. J Biol Chem. 1983 Nov 25;258(22):13825–13832. [PubMed] [Google Scholar]
- Kamimura S., Mandelkow E. Tubulin protofilaments and kinesin-dependent motility. J Cell Biol. 1992 Aug;118(4):865–875. doi: 10.1083/jcb.118.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. Actin-dependent organelle movement in squid axoplasm. Nature. 1992 Apr 23;356(6371):722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Malik F., Brillinger D., Vale R. D. High-resolution tracking of microtubule motility driven by a single kinesin motor. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4584–4588. doi: 10.1073/pnas.91.10.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Lasek R. J. Cross-bridges mediate anterograde and retrograde vesicle transport along microtubules in squid axoplasm. J Cell Biol. 1985 Dec;101(6):2181–2193. doi: 10.1083/jcb.101.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M. D., Erickson H. P., Boekelheide K. HMW-2, the Sertoli cell cytoplasmic dynein from rat testis, is a dimer composed of nearly identical subunits. J Biol Chem. 1990 May 25;265(15):8691–8698. [PubMed] [Google Scholar]
- Ray S., Meyhöfer E., Milligan R. A., Howard J. Kinesin follows the microtubule's protofilament axis. J Cell Biol. 1993 Jun;121(5):1083–1093. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale W. S., Satir P. The termination of the central microtubules from the cilia of Tetrahymena pyriformis. Cell Biol Int Rep. 1977 Jan;1(1):45–49. doi: 10.1016/0309-1651(77)90008-x. [DOI] [PubMed] [Google Scholar]
- Schnapp B. J., Crise B., Sheetz M. P., Reese T. S., Khan S. Delayed start-up of kinesin-driven microtubule gliding following inhibition by adenosine 5'-[beta,gamma-imido]triphosphate. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10053–10057. doi: 10.1073/pnas.87.24.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Gelles J., Sheetz M. P. Nanometer-scale measurements using video light microscopy. Cell Motil Cytoskeleton. 1988;10(1-2):47–53. doi: 10.1002/cm.970100109. [DOI] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S., Bechtold R. Kinesin is bound with high affinity to squid axon organelles that move to the plus-end of microtubules. J Cell Biol. 1992 Oct;119(2):389–399. doi: 10.1083/jcb.119.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A. Motors for fast axonal transport. Curr Opin Neurobiol. 1992 Oct;2(5):618–621. doi: 10.1016/0959-4388(92)90028-j. [DOI] [PubMed] [Google Scholar]
- Schroer T. A., Steuer E. R., Sheetz M. P. Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell. 1989 Mar 24;56(6):937–946. doi: 10.1016/0092-8674(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Song Y. H., Mandelkow E. Recombinant kinesin motor domain binds to beta-tubulin and decorates microtubules with a B surface lattice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1671–1675. doi: 10.1073/pnas.90.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E. R., Wordeman L., Schroer T. A., Sheetz M. P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990 May 17;345(6272):266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Force and velocity measured for single kinesin molecules. Cell. 1994 Jun 3;77(5):773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Organelle, bead, and microtubule translocations promoted by soluble factors from the squid giant axon. Cell. 1985 Mar;40(3):559–569. doi: 10.1016/0092-8674(85)90204-1. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Soll D. R., Gibbons I. R. One-dimensional diffusion of microtubules bound to flagellar dynein. Cell. 1989 Dec 1;59(5):915–925. doi: 10.1016/0092-8674(89)90614-4. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Toyoshima Y. Y. Rotation and translocation of microtubules in vitro induced by dyneins from Tetrahymena cilia. Cell. 1988 Feb 12;52(3):459–469. doi: 10.1016/s0092-8674(88)80038-2. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Wall J. S., Paschal B. M., Shpetner H. S. Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988 Apr 7;332(6164):561–563. doi: 10.1038/332561a0. [DOI] [PubMed] [Google Scholar]
- Walker R. A., Sheetz M. P. Cytoplasmic microtubule-associated motors. Annu Rev Biochem. 1993;62:429–451. doi: 10.1146/annurev.bi.62.070193.002241. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lee J. C. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Saxton W. M., Stewart R. J., Raff E. C., Goldstein L. S. Evidence that the head of kinesin is sufficient for force generation and motility in vitro. Science. 1990 Jul 6;249(4964):42–47. doi: 10.1126/science.2142332. [DOI] [PubMed] [Google Scholar]