Abstract
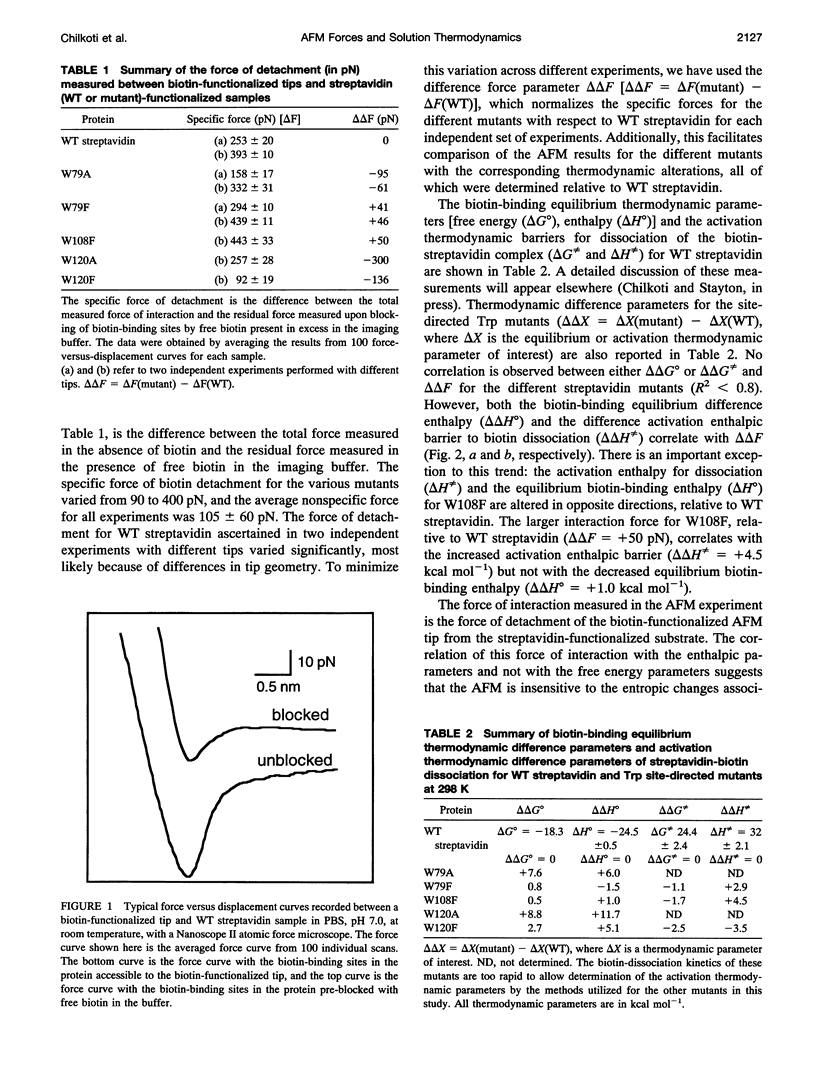
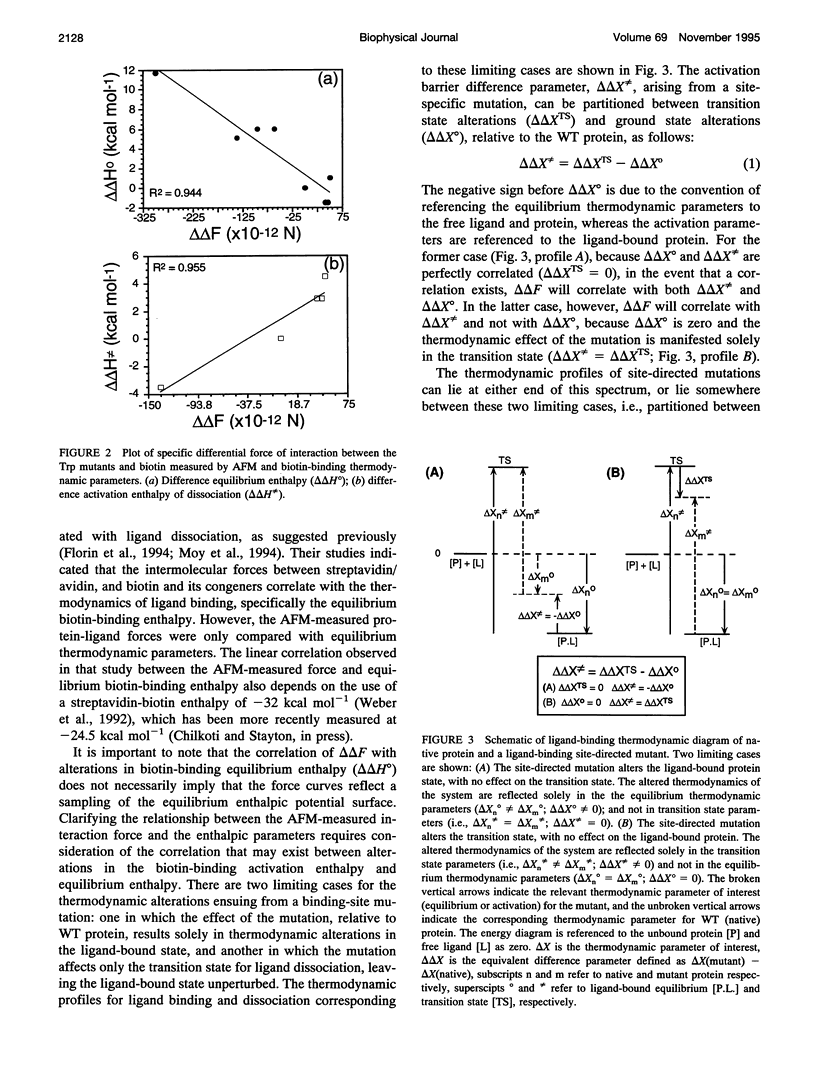
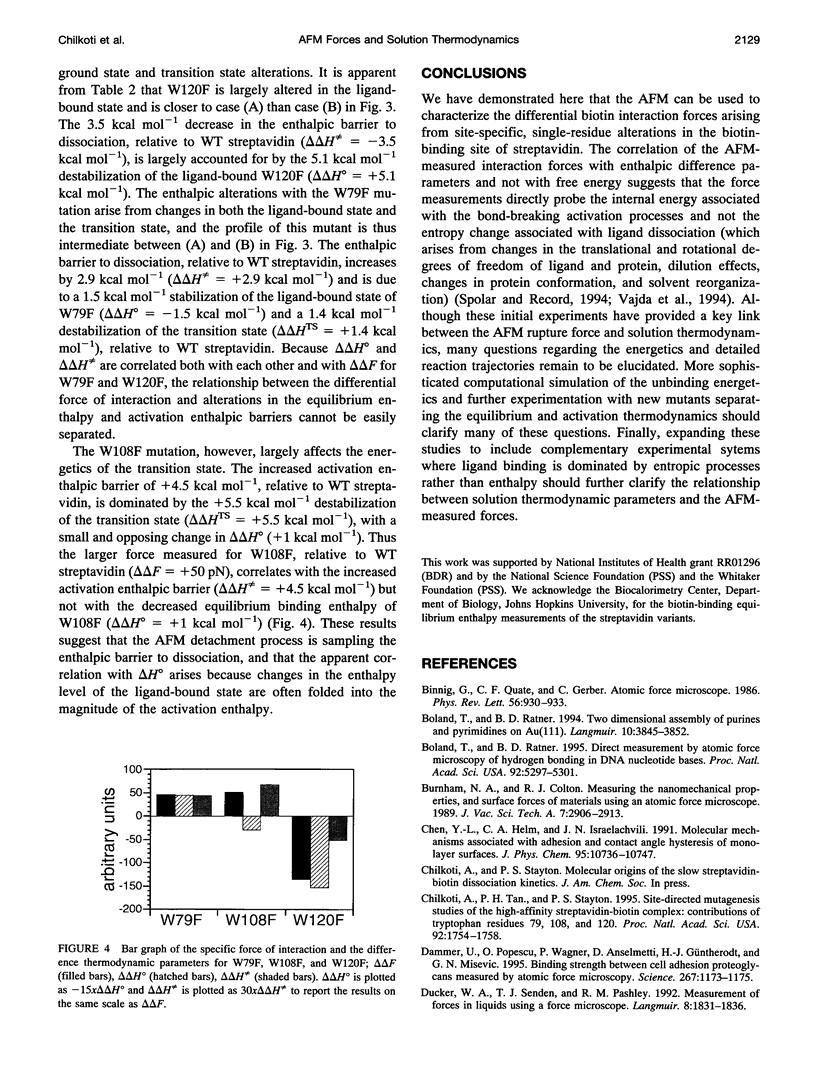
The interaction forces between biotin and a set of streptavidin site-directed mutants with altered biotin-binding equilibrium and activation thermodynamics have been measured by atomic force microscopy. The AFM technique readily discriminates differences in interaction force between the site-directed (Trp to Phe or Ala) mutants. The interaction force is poorly correlated with both the equilibrium free energy of biotin binding and the activation free energy barrier to dissociation of the biotin-streptavidin complex. The interaction force is generally well correlated with the equilibrium biotin-binding enthalpy as well as the enthalpic activation barrier, but in the one mutant where these two parameters are altered in opposite directions, the interaction force is clearly correlated with the activation enthalpy of dissociation. These results suggest that the AFM force measurements directly probe the enthalpic activation barrier to ligand dissociation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Boland T., Ratner B. D. Direct measurement of hydrogen bonding in DNA nucleotide bases by atomic force microscopy. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5297–5301. doi: 10.1073/pnas.92.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilkoti A., Tan P. H., Stayton P. S. Site-directed mutagenesis studies of the high-affinity streptavidin-biotin complex: contributions of tryptophan residues 79, 108, and 120. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1754–1758. doi: 10.1073/pnas.92.5.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammer U., Popescu O., Wagner P., Anselmetti D., Güntherodt H. J., Misevic G. N. Binding strength between cell adhesion proteoglycans measured by atomic force microscopy. Science. 1995 Feb 24;267(5201):1173–1175. doi: 10.1126/science.7855599. [DOI] [PubMed] [Google Scholar]
- Evans E., Berk D., Leung A. Detachment of agglutinin-bonded red blood cells. I. Forces to rupture molecular-point attachments. Biophys J. 1991 Apr;59(4):838–848. doi: 10.1016/S0006-3495(91)82296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin E. L., Moy V. T., Gaub H. E. Adhesion forces between individual ligand-receptor pairs. Science. 1994 Apr 15;264(5157):415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- Frisbie C. D., Rozsnyai L. F., Noy A., Wrighton M. S., Lieber C. M. Functional group imaging by chemical force microscopy. Science. 1994 Sep 30;265(5181):2071–2074. doi: 10.1126/science.265.5181.2071. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Force of single kinesin molecules measured with optical tweezers. Science. 1993 Apr 9;260(5105):232–234. doi: 10.1126/science.8469975. [DOI] [PubMed] [Google Scholar]
- Leckband D. E., Israelachvili J. N., Schmitt F. J., Knoll W. Long-range attraction and molecular rearrangements in receptor-ligand interactions. Science. 1992 Mar 13;255(5050):1419–1421. doi: 10.1126/science.1542789. [DOI] [PubMed] [Google Scholar]
- Leckband D. E., Schmitt F. J., Israelachvili J. N., Knoll W. Direct force measurements of specific and nonspecific protein interactions. Biochemistry. 1994 Apr 19;33(15):4611–4624. doi: 10.1021/bi00181a023. [DOI] [PubMed] [Google Scholar]
- Lee G. U., Chrisey L. A., Colton R. J. Direct measurement of the forces between complementary strands of DNA. Science. 1994 Nov 4;266(5186):771–773. doi: 10.1126/science.7973628. [DOI] [PubMed] [Google Scholar]
- Mate CM. Atomic-force-microscope study of polymer lubricants on silicon surfaces. Phys Rev Lett. 1992 Jun 1;68(22):3323–3326. doi: 10.1103/PhysRevLett.68.3323. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Kollman P. A. Absolute and relative binding free energy calculations of the interaction of biotin and its analogs with streptavidin using molecular dynamics/free energy perturbation approaches. Proteins. 1993 Jul;16(3):226–245. doi: 10.1002/prot.340160303. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Kollman P. A. What determines the strength of noncovalent association of ligands to proteins in aqueous solution? Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8402–8406. doi: 10.1073/pnas.90.18.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy V. T., Florin E. L., Gaub H. E. Intermolecular forces and energies between ligands and receptors. Science. 1994 Oct 14;266(5183):257–259. doi: 10.1126/science.7939660. [DOI] [PubMed] [Google Scholar]
- Spolar R. S., Record M. T., Jr Coupling of local folding to site-specific binding of proteins to DNA. Science. 1994 Feb 11;263(5148):777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Vajda S., Weng Z., Rosenfeld R., DeLisi C. Effect of conformational flexibility and solvation on receptor-ligand binding free energies. Biochemistry. 1994 Nov 29;33(47):13977–13988. doi: 10.1021/bi00251a004. [DOI] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Ohlendorf D. H., Wendoloski J. J., Salemme F. R. Structural origins of high-affinity biotin binding to streptavidin. Science. 1989 Jan 6;243(4887):85–88. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]



