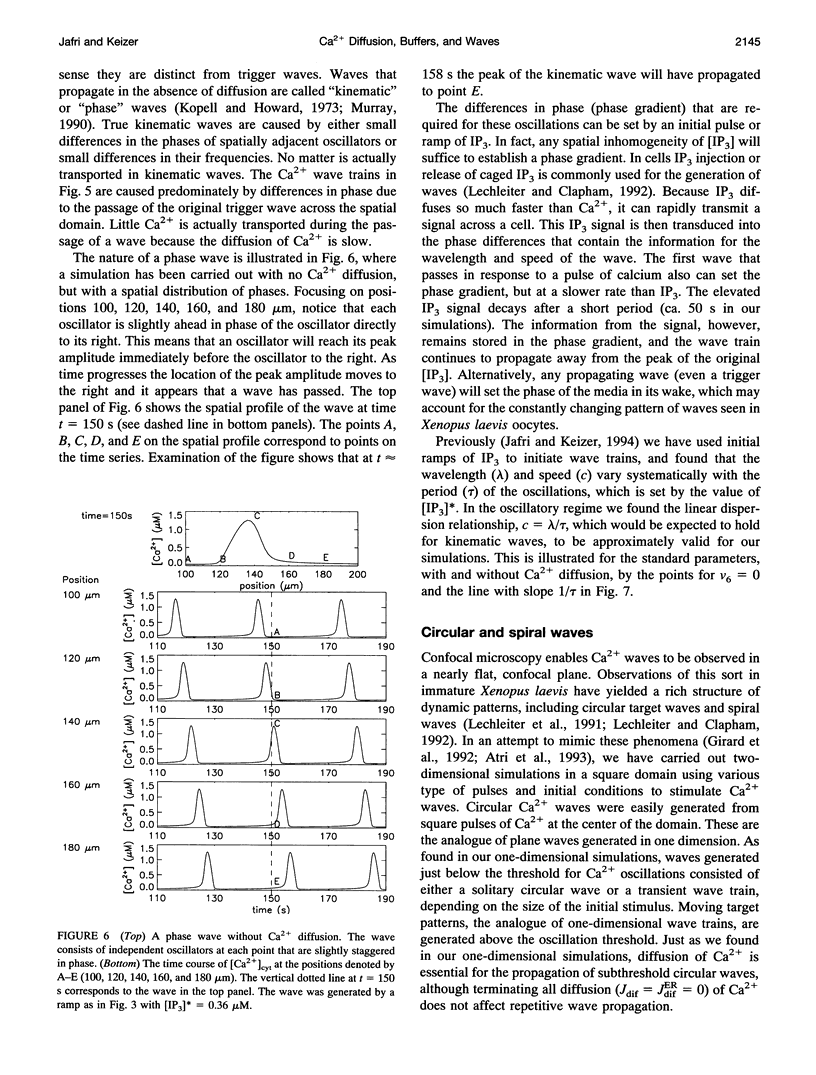
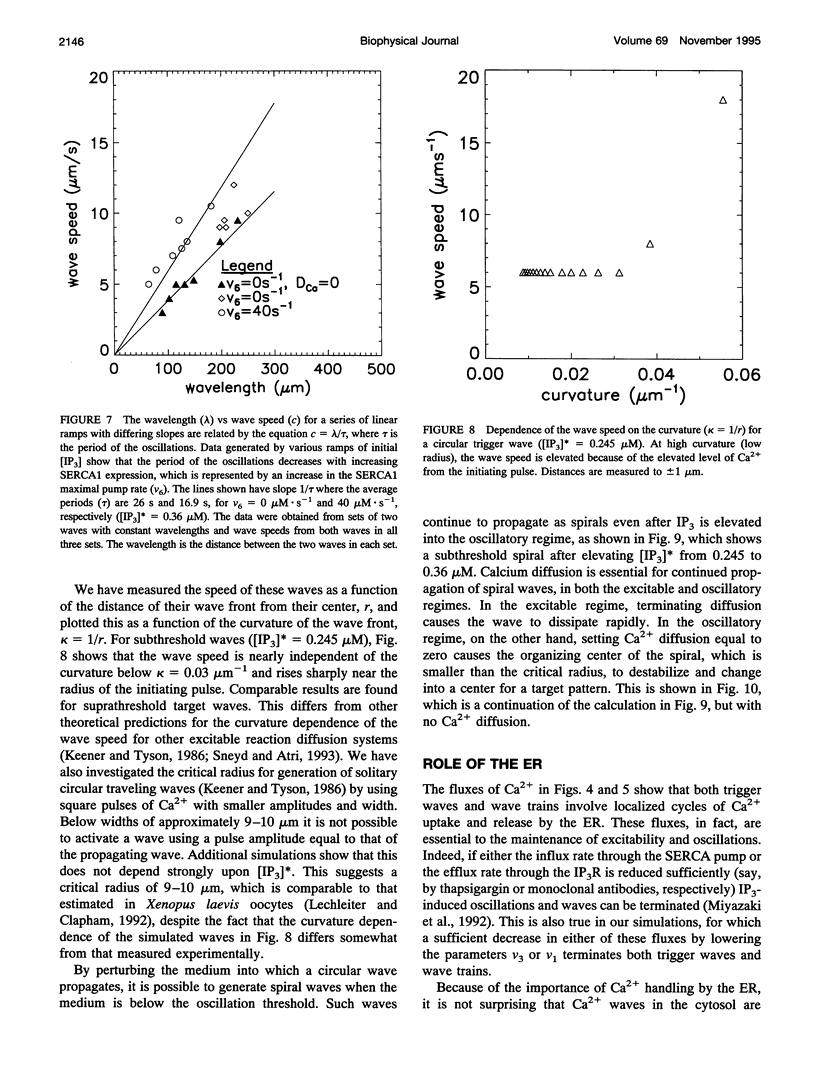
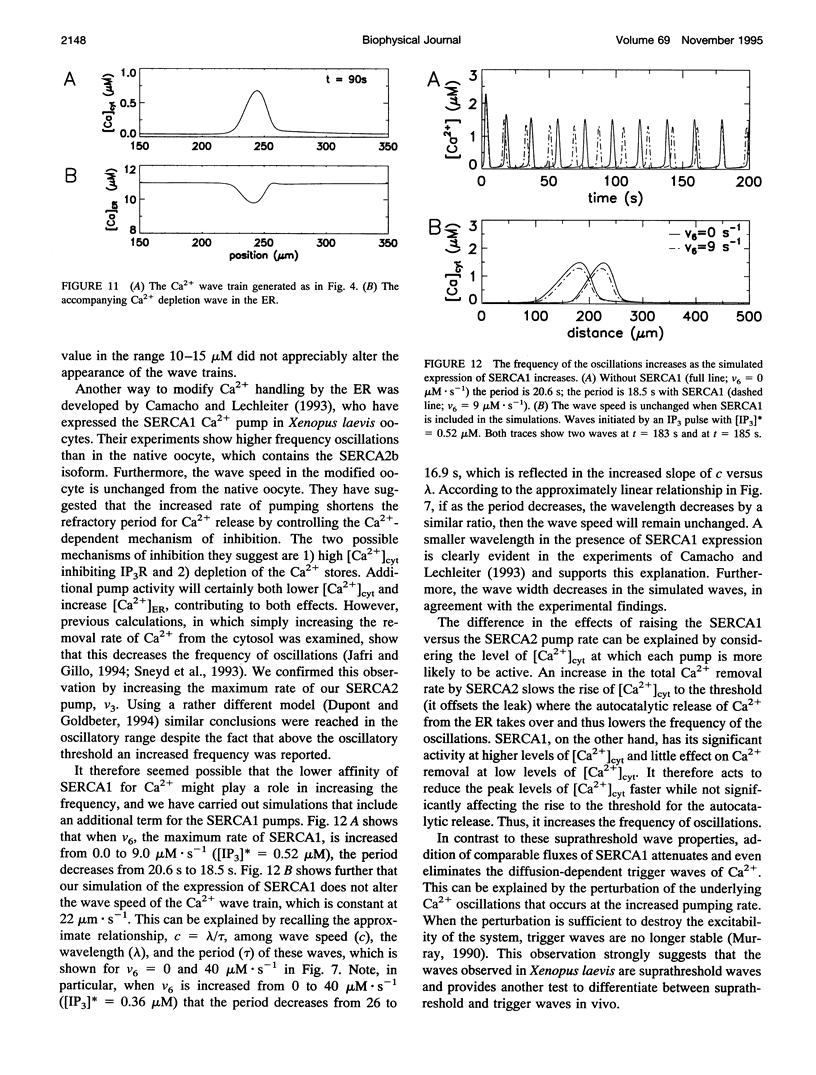
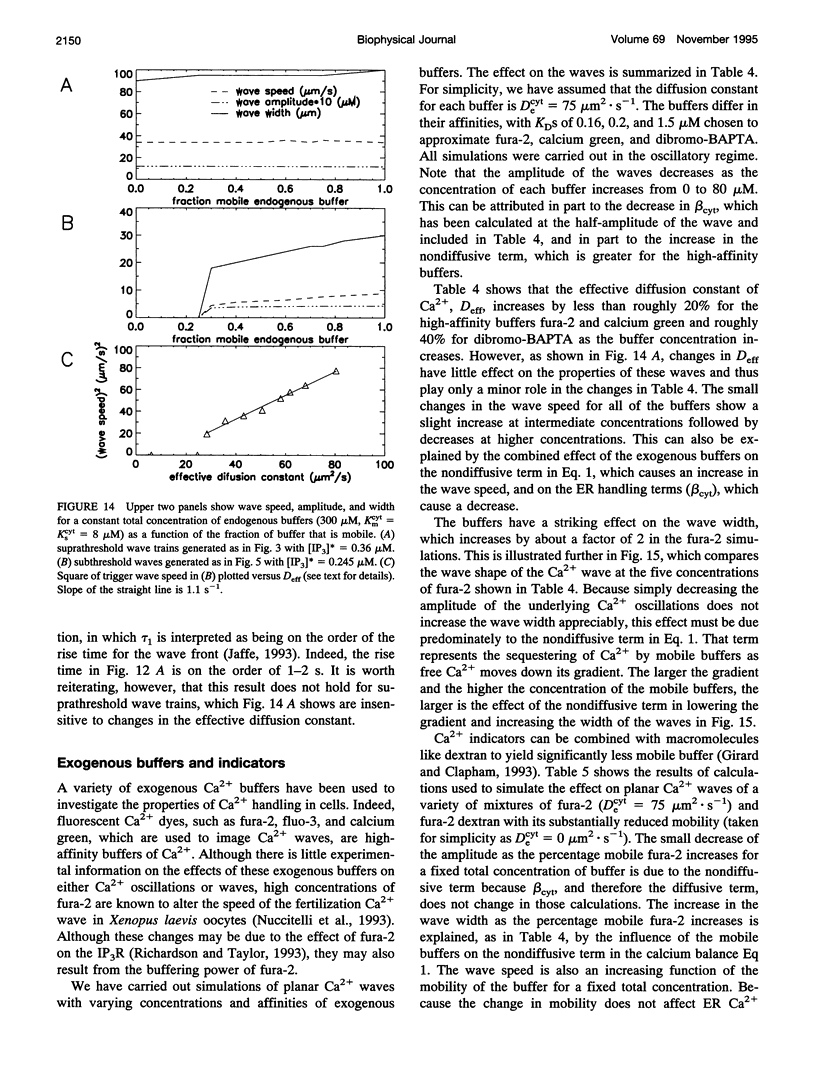
Abstract
We have investigated the effects of Ca2+ diffusion, mobile and stationary Ca2+ buffers in the cytosol, and Ca2+ handling by the endoplasmic reticulum on inositol 1,4,5-trisphosphate-induced Ca2+ wave propagation. Rapid equilibration of free and bound Ca2+ is used to describe Ca2+ sequestration by buffers in both the cytosol and endoplasmic reticulum (ER) lumen. Cytosolic Ca2+ regulation is based on a kinetic model of the inositol 1,4,5-trisphosphate (IP3) receptor of De Young and Keizer that includes activation and inhibition of the IP3 receptor Ca2+ channel in the ER membrane and SERCA Ca2+ pumps in the ER. Diffusion of Ca2+ in the cytosol and the ER and the breakdown and diffusion of IP3 are also included in our calculations. Although Ca2+ diffusion is severely limited because of buffering, when conditions are chosen just below the threshold for Ca2+ oscillations, a pulse of IP3 or Ca2+ results in a solitary trigger wave that requires diffusion of Ca2+ for its propagation. In the oscillatory regime repetitive wave trains are observed, but for this type of wave neither the wave shape nor the speed is strongly dependent on the diffusion of Ca2+. Local phase differences lead to waves that are predominately kinematic in nature, so that the wave speed (c) is related to the wavelength (lambda) and the period of the oscillations (tau) approximately by the formula c = lambda/tau. The period is determined by features that control the oscillations, including [IP3] and pump activity, which are related to recent experiments. Both solitary waves and wave trains are accompanied by a Ca2+ depletion wave in the ER lumen, similar to that observed in cortical preparations from sea urchin eggs. We explore the effect of endogenous and exogenous Ca2+ buffers on wave speed and wave shape, which can be explained in terms of three distinct effects of buffering, and show that exogenous buffers or Ca2+ dyes can have considerable influence on the amplitude and width of the waves.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993 Oct;65(4):1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Camacho P., Lechleiter J. D. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993 Apr 9;260(5105):226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. Oscillations and waves of cytosolic calcium: insights from theoretical models. Bioessays. 1992 Jul;14(7):485–493. doi: 10.1002/bies.950140711. [DOI] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. Properties of intracellular Ca2+ waves generated by a model based on Ca(2+)-induced Ca2+ release. Biophys J. 1994 Dec;67(6):2191–2204. doi: 10.1016/S0006-3495(94)80705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Girard S., Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993 Apr 9;260(5105):229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Girard S., Lückhoff A., Lechleiter J., Sneyd J., Clapham D. Two-dimensional model of calcium waves reproduces the patterns observed in Xenopus oocytes. Biophys J. 1992 Feb;61(2):509–517. doi: 10.1016/S0006-3495(92)81855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. Classes and mechanisms of calcium waves. Cell Calcium. 1993 Nov;14(10):736–745. doi: 10.1016/0143-4160(93)90099-r. [DOI] [PubMed] [Google Scholar]
- Jafri M. S. A theoretical study of cytosolic calcium waves in Xenopus oocytes. J Theor Biol. 1995 Feb 7;172(3):209–216. doi: 10.1006/jtbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- Jafri M. S., Gillo B. A membrane potential model with counterions for cytosolic calcium oscillations. Cell Calcium. 1994 Jul;16(1):9–19. doi: 10.1016/s0143-4160(05)80003-x. [DOI] [PubMed] [Google Scholar]
- Jafri M. S., Keizer J. Diffusion of inositol 1,4,5-trisphosphate but not Ca2+ is necessary for a class of inositol 1,4,5-trisphosphate-induced Ca2+ waves. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9485–9489. doi: 10.1073/pnas.91.20.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M. S., Vajda S., Pasik P., Gillo B. A membrane model for cytosolic calcium oscillations. A study using Xenopus oocytes. Biophys J. 1992 Jul;63(1):235–246. doi: 10.1016/S0006-3495(92)81583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer J., De Young G. W. Two roles of Ca2+ in agonist stimulated Ca2+ oscillations. Biophys J. 1992 Mar;61(3):649–660. doi: 10.1016/S0006-3495(92)81870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N., Howard L. N. Horizontal bands in the belousov reaction. Science. 1973 Jun 15;180(4091):1171–1173. doi: 10.1126/science.180.4091.1171. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Li Y. X., Rinzel J., Keizer J., Stojilković S. S. Calcium oscillations in pituitary gonadotrophs: comparison of experiment and theory. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):58–62. doi: 10.1073/pnas.91.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992 Jul 15;267(20):14483–14489. [PubMed] [Google Scholar]
- Milner R. E., Famulski K. S., Michalak M. Calcium binding proteins in the sarcoplasmic/endoplasmic reticulum of muscle and nonmuscle cells. Mol Cell Biochem. 1992 May 13;112(1):1–13. doi: 10.1007/BF00229637. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Luminal Ca2+ controls the activation of the inositol 1,4,5-trisphosphate receptor by cytosolic Ca2+. J Biol Chem. 1992 Nov 15;267(32):22961–22966. [PubMed] [Google Scholar]
- Miyazaki S., Yuzaki M., Nakada K., Shirakawa H., Nakanishi S., Nakade S., Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992 Jul 10;257(5067):251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R., Yim D. L., Smart T. The sperm-induced Ca2+ wave following fertilization of the Xenopus egg requires the production of Ins(1, 4, 5)P3. Dev Biol. 1993 Jul;158(1):200–212. doi: 10.1006/dbio.1993.1179. [DOI] [PubMed] [Google Scholar]
- Parys J. B., McPherson S. M., Mathews L., Campbell K. P., Longo F. J. Presence of inositol 1,4,5-trisphosphate receptor, calreticulin, and calsequestrin in eggs of sea urchins and Xenopus laevis. Dev Biol. 1994 Feb;161(2):466–476. doi: 10.1006/dbio.1994.1045. [DOI] [PubMed] [Google Scholar]
- Richardson A., Taylor C. W. Effects of Ca2+ chelators on purified inositol 1,4,5-trisphosphate (InsP3) receptors and InsP3-stimulated Ca2+ mobilization. J Biol Chem. 1993 Jun 5;268(16):11528–11533. [PubMed] [Google Scholar]
- Rooney T. A., Thomas A. P. Intracellular calcium waves generated by Ins(1,4,5)P3-dependent mechanisms. Cell Calcium. 1993 Nov;14(10):674–690. doi: 10.1016/0143-4160(93)90094-m. [DOI] [PubMed] [Google Scholar]
- Ross J., Müller S. C., Vidal C. Chemical waves. Science. 1988 Apr 22;240(4851):460–465. doi: 10.1126/science.240.4851.460. [DOI] [PubMed] [Google Scholar]
- Sneyd J., Girard S., Clapham D. Calcium wave propagation by calcium-induced calcium release: an unusual excitable system. Bull Math Biol. 1993 Mar;55(2):315–344. doi: 10.1007/BF02460886. [DOI] [PubMed] [Google Scholar]
- Sneyd J., Wetton B. T., Charles A. C., Sanderson M. J. Intercellular calcium waves mediated by diffusion of inositol trisphosphate: a two-dimensional model. Am J Physiol. 1995 Jun;268(6 Pt 1):C1537–C1545. doi: 10.1152/ajpcell.1995.268.6.C1537. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Sardet C. Demonstration of calcium uptake and release by sea urchin egg cortical endoplasmic reticulum. J Cell Biol. 1991 Nov;115(4):1031–1037. doi: 10.1083/jcb.115.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys J. 1994 Jul;67(1):447–456. doi: 10.1016/S0006-3495(94)80500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]




