Abstract
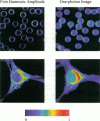
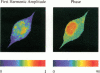
We report the development of a scanning lifetime fluorescence microscope using the asynchronous, pump-probe (stimulated emission) approach. There are two significant advantages of this technique. First, the cross-correlation signal produced by overlapping the pump and probe lasers results in i) an axial sectioning effect similar to that in confocal and two-photon excitation microscopy, and ii) improved spatial resolution compared to conventional one-photon fluorescence microscopy. Second, the low-frequency, cross-correlation signal generated allows lifetime-resolved imaging without using fast photodetectors. The data presented here include 1) determination of laser sources' threshold powers for linearity in the pump-probe signal; 2) characterization of the pump-probe intensity profile using 0.28 microns fluorescent latex spheres; 3) high frequency (up to 6.7 GHz) lifetime measurement of rhodamine B in water; and 4) lifetime-resolved images of fluorescent latex spheres, human erythrocytes and a mouse fibroblast cell stained by rhodamine DHPE, and a mouse fibroblast labeled with ethidium bromide and rhodamine DHPE.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denk W., Strickler J. H., Webb W. W. Two-photon laser scanning fluorescence microscopy. Science. 1990 Apr 6;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Dix J. A., Verkman A. S. Pyrene eximer mapping in cultured fibroblasts by ratio imaging and time-resolved microscopy. Biochemistry. 1990 Feb 20;29(7):1949–1953. doi: 10.1021/bi00459a041. [DOI] [PubMed] [Google Scholar]
- Gratton E., Limkeman M. A continuously variable frequency cross-correlation phase fluorometer with picosecond resolution. Biophys J. 1983 Dec;44(3):315–324. doi: 10.1016/S0006-3495(83)84305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H. P., Abney J. R., Verkman A. S. Determinants of the translational mobility of a small solute in cell cytoplasm. J Cell Biol. 1993 Jan;120(1):175–184. doi: 10.1083/jcb.120.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating S. M., Wensel T. G. Nanosecond fluorescence microscopy. Emission kinetics of fura-2 in single cells. Biophys J. 1991 Jan;59(1):186–202. doi: 10.1016/S0006-3495(91)82210-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśba J., Bogdanov V., Gryczynski I., Lakowicz J. R. Theory of light quenching: effects of fluorescence polarization, intensity, and anisotropy decays. Biophys J. 1994 Nov;67(5):2024–2040. doi: 10.1016/S0006-3495(94)80686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneckenburger H., Gessler P., Pavenstädt-Grupp I. Measurements of mitochondrial deficiencies in living cells by microspectrofluorometry. J Histochem Cytochem. 1992 Oct;40(10):1573–1578. doi: 10.1177/40.10.1527376. [DOI] [PubMed] [Google Scholar]