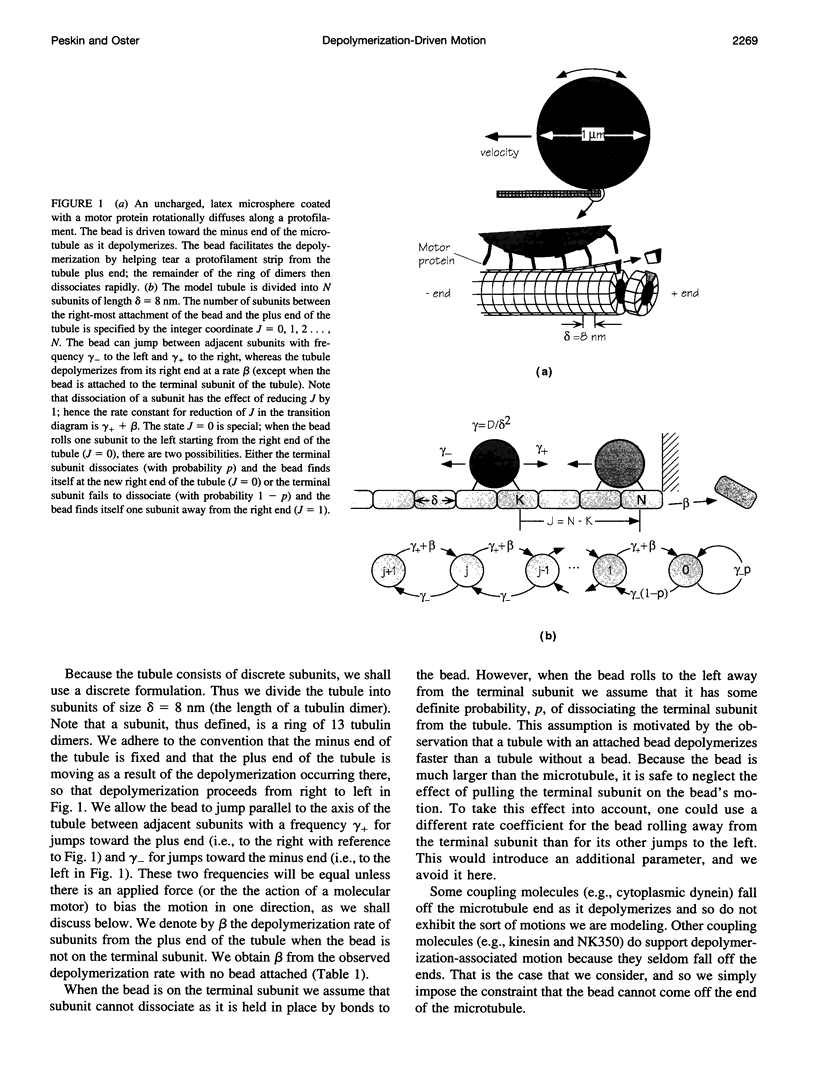
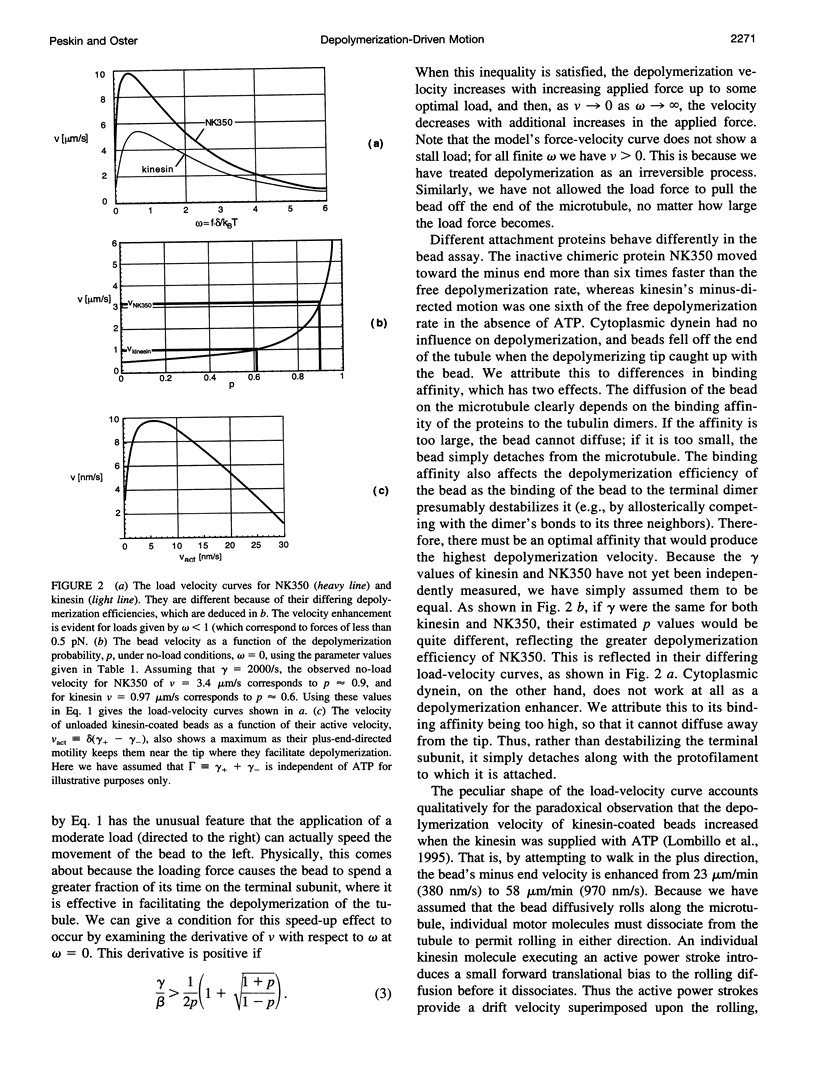
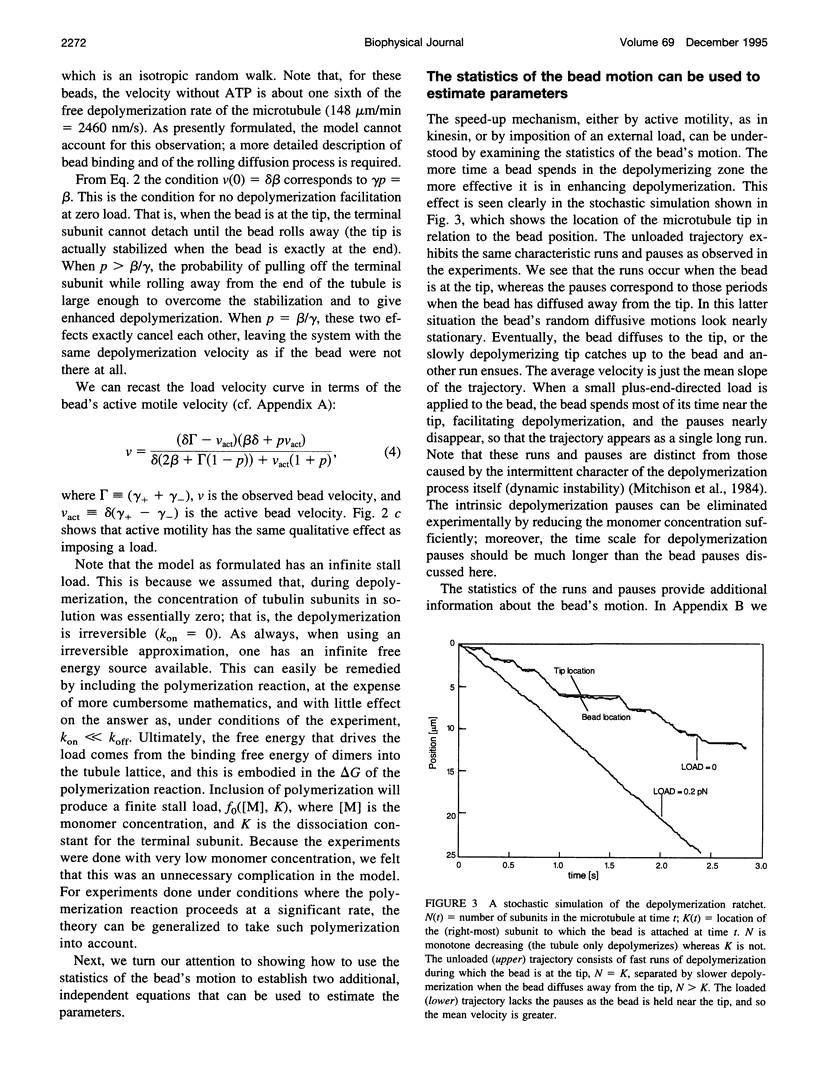
Abstract
Experiments indicate that depolymerization of microtubules generates sufficient force to produce the minus-end-directed transport of chromosomes during mitosis (Koshland et al., 1988). In vitro, analogous transport of kinesin-coated microspheres exhibits a paradoxical effect. Minus-end-directed transport of the microspheres driven by depolymerization is enhanced by the presence of ATP, which fuels the motor action of kinesin driving the microspheres in the opposite direction, toward the plus end of the microtubule. Here we present a mathematical model to explain this behavior. We postulate that a microsphere at the plus end of the microtubule facilitates depolymerization and hence enhances minus-end-directed transport. The force-velocity curve of the model is derived; it has the peculiar feature that velocity is maximal at some positive load (opposing the motion) rather than at zero load. The model is used to simulate the stochastic process of microsphere-facilitated depolymerization-driven transport. Simulated trajectories at low load show distinctive runs and pauses, the statistics of which are calculated from the model. The statistics of the process provide sufficient information to determine all of the model's parameters.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block S. M., Goldstein L. S., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990 Nov 22;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- Coue M., Lombillo V. A., McIntosh J. R. Microtubule depolymerization promotes particle and chromosome movement in vitro. J Cell Biol. 1991 Mar;112(6):1165–1175. doi: 10.1083/jcb.112.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical problems related to the attachment of microtubules to kinetochores. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Mitchison T. J., Kirschner M. W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988 Feb 11;331(6156):499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- Koshland D. Unifying forces for chromosomes in mitosis. Curr Biol. 1992 Nov;2(11):569–571. doi: 10.1016/0960-9822(92)90142-w. [DOI] [PubMed] [Google Scholar]
- Lombillo V. A., Stewart R. J., McIntosh J. R. Minus-end-directed motion of kinesin-coated microspheres driven by microtubule depolymerization. Nature. 1995 Jan 12;373(6510):161–164. doi: 10.1038/373161a0. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Unstained microtubules studied by cryo-electron microscopy. Substructure, supertwist and disassembly. J Mol Biol. 1985 Jan 5;181(1):123–135. doi: 10.1016/0022-2836(85)90330-4. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]