Abstract
Regulators of G protein signaling (RGS) accelerate intrinsic GTP hydrolysis on α subunits of trimeric G proteins and play crucial roles in the physiological regulation of G protein-mediated cell signaling. The control mechanisms of the action of RGS proteins per se are poorly clarified, however. We recently showed a physiological mode of action of a RGS protein in cardiac myocytes. The voltage-dependent formation of Ca2+/calmodulin facilitated the GTPase activity of RGS by an unidentified mechanism, which underlay the “relaxation” behavior of G protein-gated K+ (KG) channels. Here we report the mechanism which is the reversal by Ca2+/calmodulin of phosphatidylinositol-3,4,5,-trisphosphate (PIP3)-mediated inhibition of RGS. Purified RGS4 protein alone inhibited GTP-induced KG channel activity in inside-out patches from atrial myocytes. The inhibitory effect of RGS4 was reduced by PIP3 and restored by addition of Ca2+/calmodulin. The intracellular application of anti-PIP3 antibody abolished the RGS-dependent relaxation behavior of KG current in atrial myocytes. This study, therefore, reveals a general physiological control mechanism of RGS proteins by lipid–protein interaction.
Heterotrimeric G proteins mediate different intracellular signaling cascades and regulate many cellular functions (1). Although numerous kinds of G protein-coupled receptors and effectors have been identified, the existence of regulators of the G protein cycle has been neglected for a long period. Recently, a family of cytosolic proteins named “regulators of G protein signaling” (RGS proteins) has been identified (2, 3). These proteins share the conserved “RGS domain” of ≈120 aa which is responsible for accelerating GTPase activity on the G protein α subunit (4–6). RGS proteins are thought to play a central role in the physiological regulation of the G protein cycle, and their importance has been confirmed in the immune response (7) and sensory perception (8, 9). To date, more than 20 mammalian RGS proteins have been identified. These RGS proteins vary in their molecular structure, tissue distribution, and intracellular localization, and thus may play divergent functional roles in different tissues.
We recently found that RGS proteins were responsible not only for the acceleration of the deactivation time course upon agonist washout (10), but also for the characteristic gating behavior of G protein-activated inward-rectifier K+ channels (KG) in cardiac atrial myocytes (11–13). KG channels are directly activated by the βγ subunits released from pertussis toxin-sensitive G proteins upon receptor stimulation, and contribute to acetylcholine (ACh)-induced deceleration of heart beat and neurotransmitter-evoked slow inhibitory postsynaptic potentials in neurons (14). When membrane potential is suddenly hyperpolarized from positive potentials, the KG current in myocytes first instantaneously and then slowly increases. The latter time-dependent current change is termed “relaxation” and is characteristic for native KG current (15). We recently showed that coexpression of RGS protein was mandatory for reconstituting the relaxation behavior of KG current in Xenopus laevis oocytes (11, 12), and we further revealed that this characteristic could be imputable to the facilitation of the action of RGS by depolarization-induced Ca2+ entry and resultant formation of Ca2+/calmodulin (CaM) in native atrial myocytes (13). The molecular mechanism of how Ca2+/CaM facilitates the action of a RGS protein, however, remains unresolved.
A recent report showed that phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) can bind to the RGS domain of the RGS4 protein. This binding inhibits the GTPase-accelerating activity of the protein, and Ca2+/CaM reverses the inhibitory effect of PIP3 in vitro (16). The physiological role of this regulatory mechanism, however, was not clarified. In the present study, we found that PIP3- and Ca2+/CaM-dependent modulation of RGS4 accounted for the Ca2+/CaM-induced facilitation of the action of RGS4 and thus the voltage-dependent behavior of native KG current in cardiac atrial myocytes. This study, therefore, identifies a probably general physiological control mechanism of RGS proteins by lipid–protein interaction.
Materials and Methods
Expression and Purification of Glutathione S-Transferase (GST)-Fusioned RGS4 Proteins.
GST fusion constructs of RGS4 were prepared by PCR tagging of RGS4 cDNA (kindly provided by C. Doupnik, University of South Florida, Tampa, FL) with BamHI and EcoRI sites at the 5′ and 3′ end, respectively, and were subcloned into pGEX-2T vector (Amersham Pharmacia). GST-RGS4 and GST were expressed in Escherichia coli and purified from the cell lysates through a glutathione-Sepharose column (Amersham Pharmacia). The point mutation N128H in RGS4 cDNA was introduced by mutagenic oligonucleotide primers by using the QuickChange Site-Directed Mutagenesis Kit (Stratagene). The sequences were verified by ABI Dye terminator cycle sequencing with the ABI PRISM 310 GENETIC ANALYZER (Applied Biosystems).
Electrophysiological Measurements.
Experiments were performed in accordance with the guidelines for the use of laboratory animals of Osaka University. Single atrial myocytes were enzymatically isolated from hearts removed from adult male Wister–Kyoto rats as described (13, 17). G protein-gated K+ channel currents were recorded both in the inside-out configuration and in the whole-cell configuration of the patch-clamp technique (18). The tips of patch electrodes were coated with Sylgard and fire-polished. The tip resistance was 5–8 MΩ (single-channel recording) or 2–3 MΩ (whole-cell recording) when filled with the pipette solution. All recordings from inside-out patches were made at a holding potential of −70 mV. All experiments were performed at room temperature (≈25°C). The channel current was recorded with a patch-clamp amplifier (Axon 200A, Axon Instruments, Foster City, CA) and stored on a videotape through a PCM converter system (VR-10B; Instrutech, Great Neck, NY). For analysis, data were reproduced, low-pass-filtered at 1 kHz (−3 dB) by an 8-pole Bessel filter, digitized by an AD converter (ITC-16; Instrutech), continuously acquired on a computer (Macintosh Quadra; Apple Computer, Inc., Cupertino, CA) with PULSE program (HEKA Electronics, Lambrecht, Germany) and analyzed with a commercially available software (PATCH ANALYST PRO; MT Corporation, Hyogo, Japan). The ACh-induced muscarinic K+ (KG) currents in whole-cell recordings were obtained by digitally subtracting currents recorded under control conditions from those recorded in the presence of ACh. Results are shown as mean values obtained from n myocytes and error bars represent SEM. Statistical differences were evaluated by Student's unpaired t test. Statistical probability of P < 0.05 was taken as significant.
Solutions and Chemical Materials.
For single-channel recordings, the pipette (external) solution contained 150 mM KCl/1 mM CaCl2/1 mM MgCl2/5 mM Hepes-KOH (pH 7.4) and 0.3 μM acetylcholine chloride (Sigma-Aldrich). During inside-out patch-clamp recordings, the bath was perfused with the “internal” solution composed of 140 mM KCl/2 mM MgCl2/5 mM EGTA/5 mM Hepes-KOH (pH 7.3). For whole-cell recordings, the pipette (internal) solution contained 150 mM KCl/5 mM EGTA/2 mM MgCl2/3 mM K2ATP/0.1 mM Na2GTP/5 mM Hepes-KOH (pH 7.3). The bathing solution contained 115 mM NaCl/20 mM KCl/1.8 mM CaCl2/0.53 mM MgCl2/5.5 mM glucose/5.5 mM Hepes-NaOH (pH 7.4). Various concentrations of guanosine 5′-triphosphate (GTP, Na salt, Sigma-Aldrich) and its nonhydrolyzable analogue, guanosine 5′-O-(3-thiophosphate) (GTPγS, Li salt, Boehringer Mannheim) were added to the internal solution. When AlF4− was applied, a mixture of 100 μM AlCl3 (Sigma-Aldrich) and 10 mM NaF (Sigma-Aldrich) was added to the solution. GST-RGS4, CaM (Sigma-Aldrich), and purified Gβγ subunit from bovine brain (19) were diluted into experimental solutions just before use. Phosphatidylinositol-3,4,5-trisphosphate (PIP3), phosphatidylinositol-4,5-bisphosphate (PIP2), phosphatidylinositol-4-phosphate (PIP) were purchased from Biomol Research Laboratories (Plymouth Meeting, PA), and phosphatidylinositol (PI) was purchased from Sigma-Aldrich. Phospholipids were sonicated in icy-cold water for more than 30 min before application. Monoclonal anti-PIP3 antibody and control antibody (Molecular Probes) were diluted 100-fold into experimental solutions.
Protein–Lipid Overlay Assay.
Protein–lipid overlay assays were performed as described (20). In brief, membrane arrays (PIP-Strips) were purchased from Echelon Research Laboratories (Salt Lake City, UT). Membranes were blocked with 3% (wt/vol) fatty acid-free BSA (Sigma-Aldrich; A-7030) in TBST [150 mM NaCl/10 mM Tris⋅HCl (pH 8.0), and 0.1% (vol/vol) Tween-20] for 1 h at 4°C. Blocked membranes were incubated for 2 h at 4°C with 100 ng/ml GST-RGS4 in the presence or absence of 100 μM Ca2+/1 μM CaM. The membranes were then washed five times for 5 min each with TBST. After washing, membranes were incubated with anti-GST goat antibody (Amersham Pharmacia) for 1 h at 1:2000 dilution, followed by additional washing and incubation with horseradish peroxidase-conjugated anti-goat IgG rabbit antibody (Wako Pure Chemical, Osaka). After final washing, enhanced chemiluminescence was then used to detect binding of GST fusions to phospholipids.
Results
The Effects of GST-RGS4, PIP3, and Ca2+/CaM on KG Channel Activity.
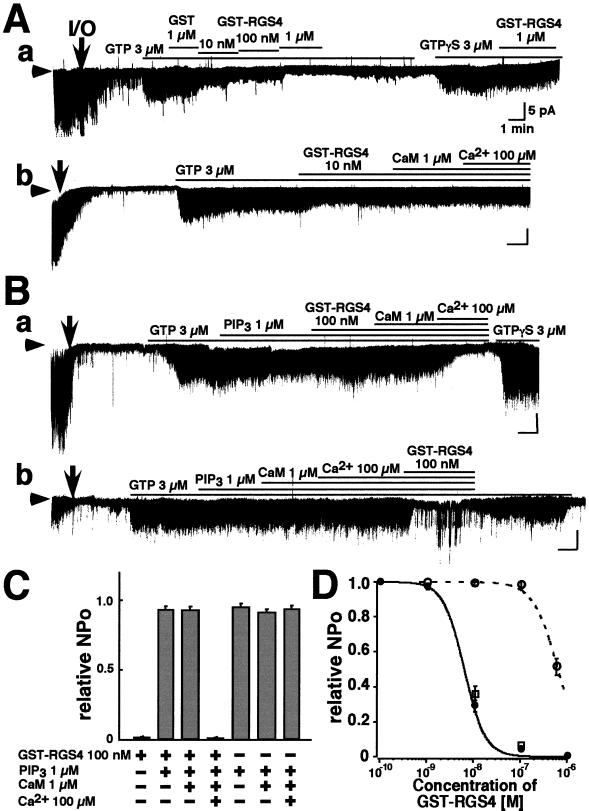
To examine the direct effect of RGS4 protein on KG channel activity, purified GST-RGS4 protein was applied to inside-out membrane patches excised from atrial myocytes. KG channel activity was stimulated by including ACh (0.3 μM) in the external pipette solution and superfusing GTP across the exposed internal surface of the patch (Fig. 1). The fusion protein GST-RGS4 was applied to the internal surface of patches and inhibited KG channel activity in a dose-dependent manner (Fig. 1 Aa and D). The GST construct alone had no effect (Fig. 1Aa); neither did boiled GST-RGS4 (n = 4, not shown) nor did the mutant GST-RGS4 (N128H) (n = 4, not shown), which lacks GTPase-activating protein activity (13, 21). Inhibition of KG channel activity by 1 μM GST-RGS4 was essentially irreversible upon washout of the protein (Fig. 1Aa), although channel activity could be recovered by the application of GTPγS (Fig. 1Aa), after which GST-RGS4 was without effect. GST-RGS4 was also without effect when KG channels were activated by the application of either Gβγ subunit or AlF4− (n = 3, not shown). These results indicate that GTP hydrolysis is required for GST-RGS4 to reduce KG channel activity and that GST-RGS4 may therefore accelerate the intrinsic GTPase activity of Gα.
Figure 1.
The effects of RGS4, PIP3, and Ca2+/CaM upon single-channel KG currents in patches from atrial myocytes. Experiments were performed on excised inside-out membrane patches in symmetrical 150 mM K+ solutions with 0.3 μM ACh in the pipette. Arrowheads indicate the zero current level. I/O indicates the excision of the patch from the myocyte. Compounds were applied in bath solution to the internal surface of patches for the periods indicated by the bars above the current records. (A) The effects of RGS4 and Ca2+/CaM on channel activity. (a) Concentration-dependent inhibitory effect of GST-RGS4 on KG channel currents. (b) The addition of 1 μM CaM and 100 μM Ca2+ had no effect on the inhibition of KG channels by 10 nM GST-RGS4. (B) The effect of PIP3. (a) PIP3 (1 μM) severely reduced the inhibitory effect of GST-RGS4, an effect that is reversed by Ca2+/CaM. (b) In PIP3 and Ca2+/CaM the effect of GST-RGS4 was not inhibited. (C) The NPo of KG channel currents recorded under different conditions (indicated below) relative to that seen in 3 μM GTP. N represents the number of KG channels in a patch, and Po represents the open probability of each channel. Symbols and bars indicate the mean ± SEM; n = 10 for each. (D) Dose-dependent inhibition by GST-RGS4 of KG channel currents in the absence (closed circles) or presence (open circles) of 1 μM PIP3, and in the presence of 1 μM PIP3 and Ca2+/CaM (open squares). Symbols and bars indicate the mean ± SEM, n = 6 for each. Vertical bars represent 5 pA, and horizontal bars represent 1 min.
The effect of RGS4 on KG channels in intact atrial myocytes is facilitated by Ca2+/CaM (13) by an unknown mechanism. We therefore applied Ca2+/CaM to excised membrane patches (Fig. 1Ab). This application was without effect on the activity of KG channels in the presence of 10 nM (n = 3, Fig. 1Ab) or 100 nM GST-RGS4. In excised membrane patches, therefore, the effect of RGS4 was not facilitated by Ca2+/CaM.
Popov et al. (16) reported that the phospholipid PIP3 inhibits the GTPase-accelerating activity of RGS proteins. The effect is reversed by Ca2+/CaM in vitro. PIP3 (1 μM) was itself without effect on KG channel activity (Fig. 1B), but PIP3 reduced the effect of 100 nM GST-RGS4 (Fig. 1Ba) in an apparently competitive manner (Fig. 1D). It required the addition of CaM and Ca2+ to reestablish the inhibition of KG channels by GST-RGS4 (Fig. 1 Ba, C, and D). CaM and Ca2+ themselves had no effect on channel activity in the presence of PIP3 (Fig. 1 Bb and C), and it required the addition of GST-RGS4 to inhibit channel opening (Fig. 1Bb).
These data can be summarized as follows (Fig. 1D). Purified GST-RGS4 protein inhibited KG channel activity (IC50 ≈80 nM; Hill coefficient ≈1.4). Ca2+/CaM had no effect on this action in excised membrane patches. On the other hand, the phospholipid PIP3 blocked the effect of GST-RGS4. The effect of the phospholipid was reversed by Ca2+/CaM. These data suggest that modulation of the effect of RGS4 on KG channel activity by Ca2+/CaM (13) occurs not at the level of RGS4 but by its interaction with PIP3.
Functional Specificity of PIP3.
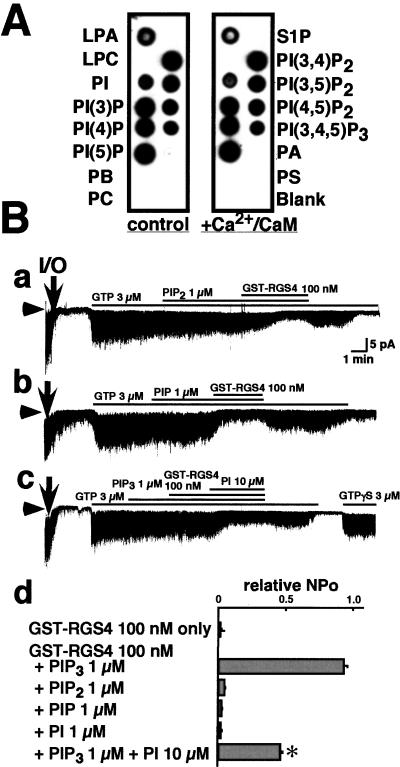
To evaluate the specificity of the interaction between PIP3 and RGS4, we first examined the binding of GST-RGS4 to various phospholipids with a protein–lipid overlay assay (Fig. 2A). GST-RGS4 could bind to all phosphatidylinositols (PI, PIP, PIP2, and PIP3) and lysophosphatidic acid. GST did not bind to any of these phospholipids (not shown). Binding was not significantly altered in the presence of Ca2+/CaM.
Figure 2.
Interactions between different phospholipids and RGS4. (A) Protein–lipid overlay assay of GST-RGS4. GST-RGS4 binds specifically to phosphatidylinositols and LPA (control: Left), and this binding is not affected by the addition of Ca2+/CaM (Right). The spotted phospholipids are identified on either side of the assays. LPA, lysophosphatidic acid; LPC, lysophosphocholine; S1P, sphingosine-1-phosphate; PA, phosphatidic acid; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PS, phosphatidylserine. (B) Functional specificity of the interaction between PIP3 and RGS4. Neither 1 μM PI(4,5)P2 (PIP2) (a) nor 1 μM PI(4)P (PIP) (b) block the inhibitory effect of GST-RGS4 on GTP-induced KG channel activity. PI (10 μM) competitively antagonizes the effect of 1 μM PIP3 on GST-RGS4 inhibition of KG channels (c). (d) The NPo of KG channels recorded under different conditions (indicated on the left) relative to that seen in the presence of 3 μM GTP alone. Bars indicate the mean ± SEM, n = 5 for each. ✻, P < 0.05.
We next examined the functional specificity of these phospholipids with KG channel activity. PIP2, PIP, and PI alone could not block the inhibitory effect of RGS4 on KG channel activity (Fig. 2B). However, PI (10 μM) could compete with PIP3 (1 μM) to prevent RGS-mediated inhibition of KG channel activity (Fig. 2 Bc and Bd). Therefore, although all of the tested phosphatidylinositols could bind to RGS4, only PIP3 inhibited the effect of RGS4.
Depletion of Endogenous PIP3 Abolished the Relaxation of Native KG Currents.
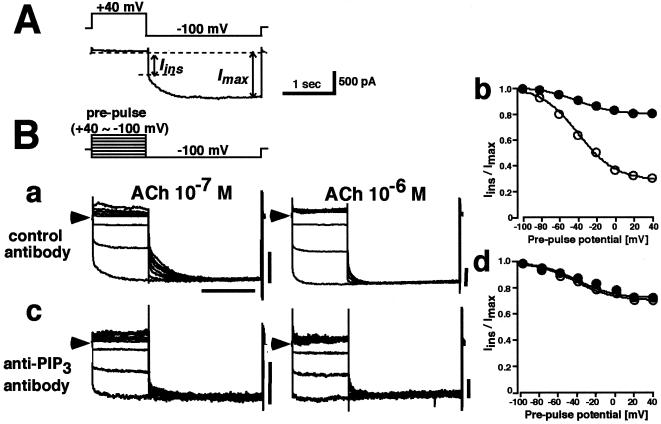
In native atrial myocytes, the whole-cell KG current is known to exhibit characteristic behavior, named “relaxation” (Fig. 3A). When the membrane potential is clamped to +40 mV for 1 s, little outward current flows through KG channels. Upon hyperpolarization to −100 mV, the inward KG current immediately jumps to one level (Iins) and then slowly increases to a steady level (Imax). The immediate increase in current reflects the rapid relief from the blockade of outward KG current by intracellular Mg2+ and/or polyamines, which confers the inwardly rectifying property to all inward rectifier K+ channels. The subsequent slow increase, named “relaxation”, is a unique characteristic of the KG current and reflects a time-dependent recovery from the reduction in available KG channel numbers associated with depolarization. Fig. 3Ba exhibits a typical agonist-concentration dependence of relaxation behavior in the control condition. At 10−7 M ACh (Left), when the prepulse was applied to more depolarization potentials, the amplitude of Iins became smaller without affecting Imax. This effect essentially disappeared at 10−6 M ACh (Right). The ratio Iins/Imax shows the amount of KG current available during each prepulse relative to that at −100 mV. Thus, at 10−7 M ACh, KG channel availability was reduced with depolarization such that at +40 mV the availability was ≈30% of that at −100 mV (Fig. 3Bb, open circles), whereas at 10−6 M ACh, KG channel availability at +40 mV had been increased to ≈80% (Fig. 3Bb, closed circles). In summary, at low concentrations of ACh, KG channels are inhibited upon depolarization, and the slow increase in current (relaxation) upon hyperpolarization reflects relief from this inhibition. Increasing KG channel activity with 10−6 M ACh overcomes this inhibition and channel activity then becomes practically voltage-independent.
Figure 3.
Functional role of endogenous PIP3 in the voltage-dependent relaxation of KG currents in atrial myocytes. (A) Voltage-dependent relaxation of ACh-induced KG current in atrial myocytes. Voltage-clamp protocol (Upper) and a typical ACh-induced KG current (Lower) recorded during a prepulse to +40 mV followed by stepping to −100 mV. Inward current upon stepping to −100 mV changes first instantaneously (Iins) and then slowly increases to a steady-state (Imax); the time-dependent decay is called “relaxation”. (B) KG currents were evoked by 10−7 M (Left) or 10−6 M (Right) ACh when control antibody (a) or anti-PIP3 antibody (c) were contained in the pipette. Currents at −100 mV were recorded after prepulses to between −100 and +40 mV in steps of 20 mV (Inset, voltage protocol ). (b and d) Relationship between the prepulse voltage and the Iins/Imax ratio for currents elicited by either 10−7 M (open circles) or 10−6 M (closed circles) ACh, with either control antibody (b) or anti-PIP3 antibody (d) in the pipette. n = 5 for each. In each family of currents arrowheads indicate the zero current level, and horizontal scale bars represent 1 s, and vertical scale bars represent 500 pA.
The experiments shown in Fig. 3B were designed to investigate the influence of PIP3 on this process by infusing an anti-PIP3 antibody into the cells. In the presence of the control antibody (Fig. 3Ba), typical voltage- and agonist concentration-dependent relaxation of the current was recorded (14, 15). Fig. 3Bb shows that depolarization decreased KG channel availability at 10−7 M ACh, an effect which was largely absent at 10−6 M ACh. In the presence of the anti-PIP3 antibody (Fig. 3Bc), on the other hand, Iins at 10−7 M ACh had been increased and the currents showed little voltage-dependent relaxation. Fig. 3Bd shows that, in the presence of the anti-PIP3 antibody, the voltage-dependent decrease of KG channel availability seen at 10−7 M ACh had been essentially abolished. Boiled anti-PIP3 antibody had no effect (n = 3, not shown). Therefore, it is concluded that, in intact atrial myocytes, endogenous PIP3 is required to allow the voltage-dependent reduction in KG channel availability with relatively low agonist stimulation.
Discussion
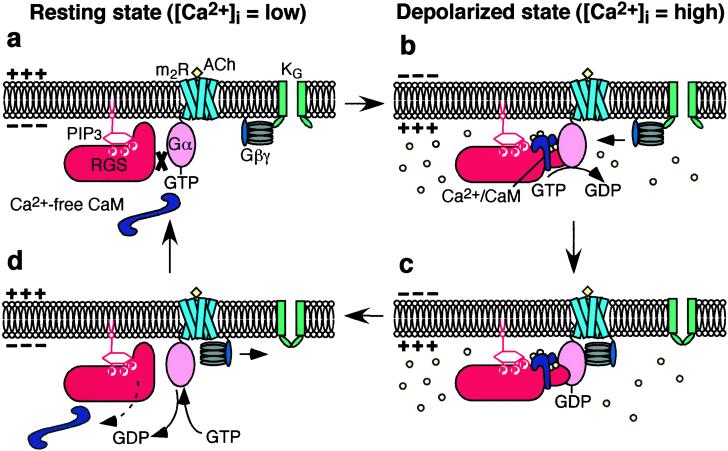
In cardiac myocytes the physiological mode of action of RGS4 involves the voltage-dependent formation of Ca2+/CaM which facilitates GTPase activity of RGS4 by an unidentified mechanism (13). The present study strongly suggests that this mechanism is the reversal by Ca2+/CaM of the inhibition of RGS4 by PIP3, which is similar to the effect of Ca2+/CaM on endothelial nitric oxide synthase where Ca2+/CaM removes the inhibitory molecule caveolin-1 from the enzyme (22). We propose the following reaction for the mode of action of the RGS4 protein (Fig. 4). In resting conditions PIP3 in the plasma membrane binds to RGS4 and inhibits its action (Fig. 4a). When the concentration of Ca2+ beneath the membrane is raised by depolarization or possibly by other signaling pathways, the formation of Ca2+/CaM would reverse the effect of PIP3 and the GTPase-accelerating activity of RGS4 would resume (Fig. 4b). The hydrolysis of GTP on Gα to form GDP-bound Gα would be accelerated. This acceleration would result in the reduction of Gβγ and thus a decrease in active KG channels (Fig. 4b). Upon hyperpolarization the reverse reactions would occur and the number of active KG channels would increase, which would account for the time-dependent relaxation of the KG current (Fig. 4 c and d).
Figure 4.
Schematic representation of Ca2+/CaM-dependent facilitation of the action of RGS proteins. (a) In a resting (low Ca2+) state, the action of RGS is inhibited by PIP3. (b) Once intracellular Ca2+ concentration is elevated, e.g., upon depolarization, Ca2+/CaM binds to RGS proteins and reverses the inhibitory effect of PIP3. (c) Consequently, the G protein cycle is negatively regulated. (d) When the Ca2+ concentration decreases to the steady level, CaM dissociates from RGS proteins and their action is once again inhibited by PIP3.
Because Ca2+/CaM did not change the binding of RGS4 and PIP3 in vitro (Fig. 2A), the effects of PIP3 and CaM on RGS may be allosteric. Although the binding sites for Ca2+/CaM and PIP3 on RGS have not been fully identified, clusters of positively charged residues in the α4≈α5 helix of the RGS domain have been suggested to be responsible for the binding of PIP3 (16). According to the crystal structure of the RGS domain of RGS4 (23), this area is far from the RGS–Gα contact interface. To further elucidate the molecular mechanism of PIP3- and Ca2+/CaM-dependent modulation of RGS4, an approach with use of structural-biology techniques may be required.
Classically PIP2 is considered to be the substance from which inositol-(1,4,5)-triphosphate and diacylglycerol are produced by phospholipase C. Recently evidence has appeared that PIP2 itself regulates several proteins. Ion channels and exchangers, such as the Na+/Ca2+ exchanger, the ATP-sensitive K+ channel, and also the KG channel, have been shown to be regulated by PIP2 (24, 25). PIP2 activates reconstituted KG channel in X. laevis oocytes (26, 27). Recent studies also revealed that depletion of PIP2 by activation of phospholipase C-Gq-coupled receptor causes slow inhibition of KG channel currents in native myocytes (28, 29). On the other hand, in inside-out patches PIP2 rather inhibited native KG channel activity by uncoupling the channel and G protein (30). The present study shows that PIP3, another phosphatidylinositol, regulates RGS action, and thus KG channel activity. Therefore, the physiological roles of phosphatidylinositols in the control of KG current in native cells may be more complex than proposed so far and needs to be reevaluated.
PIP3 is usually generated from PIP2 by phosphatidylinositol 3-kinase. The activity of phosphatidylinositol 3-kinase is regulated by receptor-dependent tyrosine phosphorylation (31, 32). Therefore, receptors that influence phosphatidylinositol 3-kinase activity, such as insulin-like growth factor (33) and tumor necrosis factor (34), may also modulate RGS proteins. Furthermore, considering the fact that Ca2+/CaM also regulates RGS proteins, RGS may be at the center of cross-talk between Ca2+, phospholipids, tyrosine kinase, and G protein-signaling systems. Further studies are needed to evaluate this concept of cell signaling.
Acknowledgments
We thank Dr. Ian Findlay (Université de Tours, Tours, France) for critically reading this manuscript. This work was supported by Grant-in-Aid 12144207 for Specific Research on Priority Area (B) (to Y.K.), by Grant-in-Aid 13770044 for Encouragement of Young Scientists (to M.I.) from the Ministry of Education, Science, Sports and Culture of Japan, by Grant-in-Aid 96L00302 from the Research for the Future Program of the Japan Society for the Promotion of Science (to Y.K.), and by the Japan Heart Foundation Dr. Hiroshi Irisawa Commemorative Research Grant (to M.I.).
Abbreviations
- RGS
regulators of G protein-signaling
- KG
G protein gated K+ channel
- CaM
calmodulin
- PI
phosphatidylinositol
- PIP
phosphatidylinositol-4-phosphate
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- ACh
acetylcholine
- GST
glutathione S-transferase
References
- 1.Gilman A G. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.De Vries L, Mousli M, Wurmser A, Farquhar M G. Proc Natl Acad Sci USA. 1995;92:11916–11920. doi: 10.1073/pnas.92.25.11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman D M, Wilkie T M, Gilman A G. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 4.De Vries L, Farquhar M G. Trends Cell Biol. 1999;9:138–144. doi: 10.1016/s0962-8924(99)01515-9. [DOI] [PubMed] [Google Scholar]
- 5.Hepler J R. Trends Pharmacol Sci. 1999;20:376–382. doi: 10.1016/s0165-6147(99)01369-3. [DOI] [PubMed] [Google Scholar]
- 6.Ross E M, Wilkie T M. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 7.Kehrl J H. Immunity. 1998;8:1–10. doi: 10.1016/s1074-7613(00)80453-7. [DOI] [PubMed] [Google Scholar]
- 8.He W, Cowan C W, Wensel T G. Neuron. 1998;20:95–102. doi: 10.1016/s0896-6273(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 9.Sinnarajah S, Dessauer C W, Srikumar D, Chen J, Yuen J, Yilma S, Dennis J C, Morrison E E, Vodyanoy V, Kehrl J H. Nature (London) 2001;409:1051–1055. doi: 10.1038/35059104. [DOI] [PubMed] [Google Scholar]
- 10.Doupnik C A, Davidson N, Lester H A, Kofuji P. Proc Natl Acad Sci USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita S, Inanobe A, Chachin M, Aizawa Y, Kurachi Y. J Physiol (London) 2000;526:341–347. doi: 10.1111/j.1469-7793.2000.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inanobe A, Fujita S, Makino Y, Matsushita K, Ishii M, Chachin M, Kurachi Y. J Physiol (London) 2001;535:133–143. doi: 10.1111/j.1469-7793.2001.t01-1-00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii M, Inanobe A, Fujita S, Makino Y, Hosoya M, Kurachi Y. Circ Res. 2001;89:1045–1050. doi: 10.1161/hh2301.100815. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Inanobe A, Kurachi Y. Pharmacol Rev. 1998;50:723–760. [PubMed] [Google Scholar]
- 15.Noma A, Trautwein W. Pflügers Arch. 1978;377:193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- 16.Popov S G, Krishna U M, Falck J R, Wilkie T M. J Biol Chem. 2000;275:18962–18968. doi: 10.1074/jbc.M001128200. [DOI] [PubMed] [Google Scholar]
- 17.Bünemann M, Pott L. J Physiol (London) 1995;482:81–92. doi: 10.1113/jphysiol.1995.sp020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi I, Shibasaki H, Takahashi K, Tohyama K, Kurachi Y, Ito H, Ui M, Katada T. Eur J Biochem. 1990;191:499–506. doi: 10.1111/j.1432-1033.1990.tb19149.x. [DOI] [PubMed] [Google Scholar]
- 20.Cheever M L, Sato T K, de Beer T, Kutateladze T G, Emr S D, Overdulin M. Nat Cell Biol. 2001;3:613–618. doi: 10.1038/35083000. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasa S P, Watson N, Overton M C, Blumer K J. J Biol Chem. 1998;273:1529–1533. doi: 10.1074/jbc.273.3.1529. [DOI] [PubMed] [Google Scholar]
- 22.Michel T, Feron O. J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesmer J J G, Berman D M, Gilman A G, Sprang S R. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 24.Hilgemann D W, Ball R. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 25.Huang C-L, Feng S, Hilgemann D W. Nature (London) 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 26.Sui J L, Petit-Jacques J, Logothetis D E. Proc Natl Acad Sci USA. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho I H M, Murrell-Lagnado R D. J Biol Chem. 1999;274:8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- 28.Meyer T, Wellner-Kienitz M-C, Biewald A, Bender K, Eickel A, Pott L. J Biol Chem. 2001;276:5650–5658. doi: 10.1074/jbc.M009179200. [DOI] [PubMed] [Google Scholar]
- 29.Lei Q, Talley E M, Bayliss D A. J Biol Chem. 2001;276:16720–16730. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Bang H. J Physiol (London) 1999;517:59–74. doi: 10.1111/j.1469-7793.1999.0059z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leevers S J, Vanhaesebroeck B, Waterfield M D. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 32.Cantrell D A. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 33.Valentinis B, Baserga R. Mol Pathol. 2001;54:133–137. doi: 10.1136/mp.54.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiraoka E, Kawashima S, Takahashi T, Rikitake Y, Kitamura T, Ogawa W, Yokoyama M. Am J Physiol. 2001;280:H1861–H1868. doi: 10.1152/ajpheart.2001.280.4.H1861. [DOI] [PubMed] [Google Scholar]