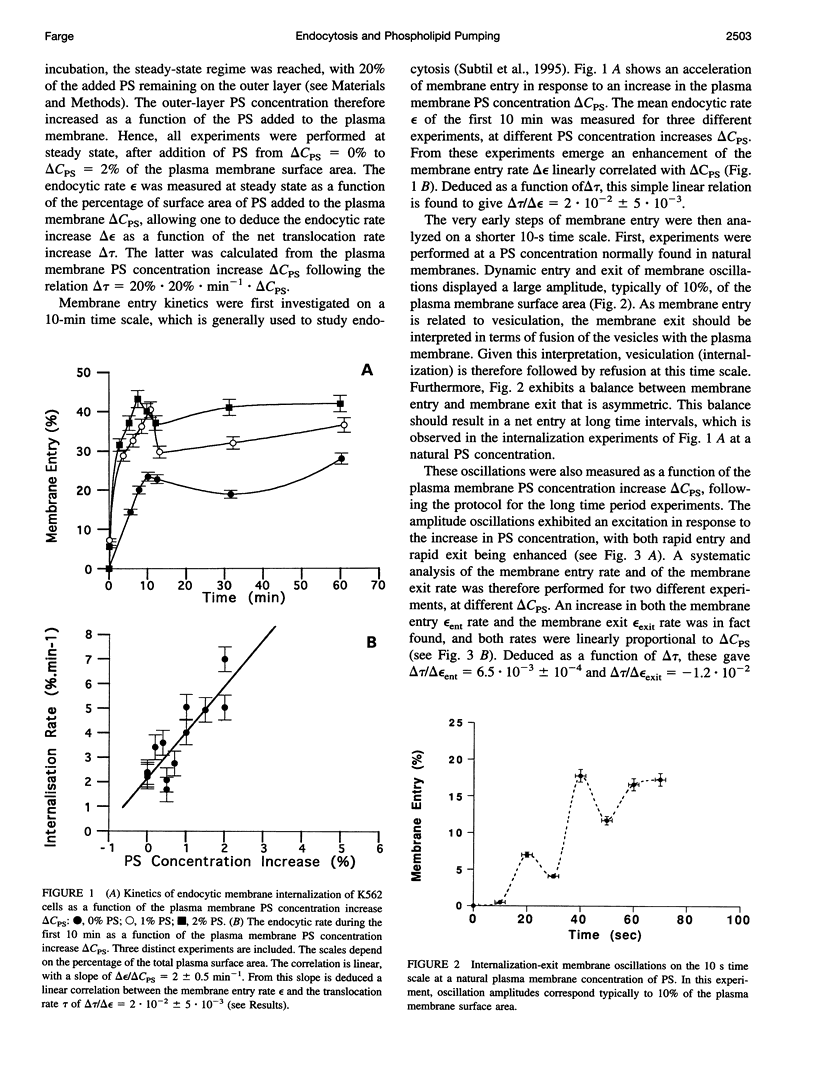
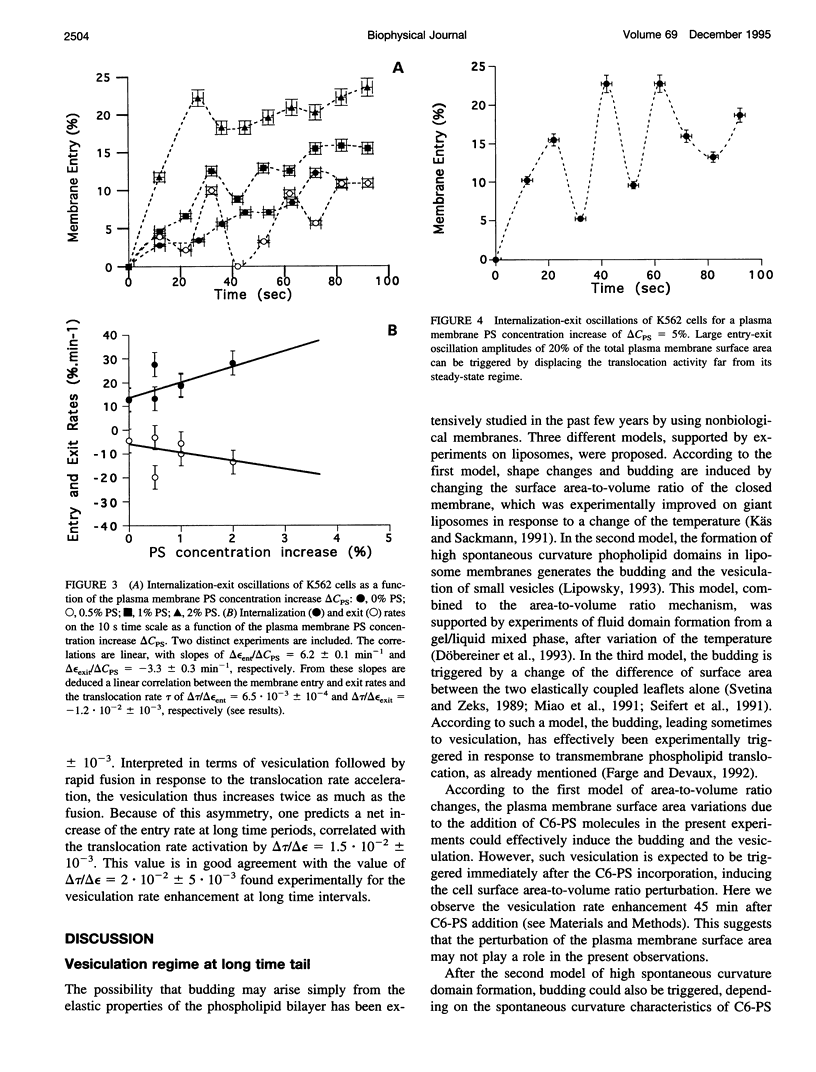
Abstract
Endocytosis vesiculation consists of local membrane invaginations, continuously generated on the plasma membrane surface of living cells. This vesiculation process was found to be activated in vivo by the generation of a transmembrane surface area asymmetry in the plasma membrane bilayer, after enhancement of transbilayer phospholipid translocation. The observed enhancement was shown to be in good quantitative agreement with a theoretical model of elastic equilibrium describing stabilization of 100-nm vesicles in response to phospholipid redistribution. Very rapid dynamic vesiculation and direct re-fusion of the vesicles, both dependent on the phospholipid translocation activity, were found on a time scale of seconds. Both vesiculation and re-fusion were shown to result in a steady-state population of internal vesicles at long time points. The plasma membrane appears to be a dynamic structure, oscillating between two distinct curvature states, the 10 microns-1 "vesicle" and the 0.1 micron-1 "plasma membrane" curvature states. This dynamic behavior is discussed in terms of an elastic control of the membranes curvature state by the phospholipid translocation activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auland M. E., Roufogalis B. D., Devaux P. F., Zachowski A. Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10938–10942. doi: 10.1073/pnas.91.23.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribier S., Sainte-Marie J., Devaux P. F. Quantitative comparison between aminophospholipid translocase activity in human erythrocytes and in K562 cells. Biochim Biophys Acta. 1993 May 14;1148(1):85–90. doi: 10.1016/0005-2736(93)90163-t. [DOI] [PubMed] [Google Scholar]
- Cupers P., Veithen A., Kiss A., Baudhuin P., Courtoy P. J. Clathrin polymerization is not required for bulk-phase endocytosis in rat fetal fibroblasts. J Cell Biol. 1994 Nov;127(3):725–735. doi: 10.1083/jcb.127.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P. F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991 Feb 5;30(5):1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Döbereiner H. G., Käs J., Noppl D., Sprenger I., Sackmann E. Budding and fission of vesicles. Biophys J. 1993 Oct;65(4):1396–1403. doi: 10.1016/S0006-3495(93)81203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E., Devaux P. F. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys J. 1992 Feb;61(2):347–357. doi: 10.1016/S0006-3495(92)81841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellmann P., Zachowski A., Devaux P. F. Synthesis and use of spin-labeled lipids for studies of the transmembrane movement of phospholipids. Methods Mol Biol. 1994;27:161–175. doi: 10.1385/0-89603-250-7:161. [DOI] [PubMed] [Google Scholar]
- Jin A. J., Nossal R. Topological mechanisms involved in the formation of clathrin-coated vesicles. Biophys J. 1993 Oct;65(4):1523–1537. doi: 10.1016/S0006-3495(93)81189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käs J., Sackmann E. Shape transitions and shape stability of giant phospholipid vesicles in pure water induced by area-to-volume changes. Biophys J. 1991 Oct;60(4):825–844. doi: 10.1016/S0006-3495(91)82117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky R. Domain-induced budding of fluid membranes. Biophys J. 1993 Apr;64(4):1133–1138. doi: 10.1016/S0006-3495(93)81479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky R, Leibler S. Unbinding transitions of interacting membranes. Phys Rev Lett. 1986 Jun 9;56(23):2541–2544. doi: 10.1103/PhysRevLett.56.2541. [DOI] [PubMed] [Google Scholar]
- Marsh D., Smith I. C. An interacting spin label study of the fluidizing and condensing effects of cholesterol on lecithin bilayers. Biochim Biophys Acta. 1973 Mar 16;298(2):133–144. doi: 10.1016/0005-2736(73)90345-3. [DOI] [PubMed] [Google Scholar]
- Mayor S., Presley J. F., Maxfield F. R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993 Jun;121(6):1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L, Fourcade B, Rao M, Wortis M, Zia RK. Equilibrium budding and vesiculation in the curvature model of fluid lipid vesicles. Phys Rev A. 1991 Jun 15;43(12):6843–6856. doi: 10.1103/physreva.43.6843. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Alvarez de Toledo G., Fernandez J. M. Tension in secretory granule membranes causes extensive membrane transfer through the exocytotic fusion pore. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7804–7808. doi: 10.1073/pnas.87.20.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Cassagne C. Phospholipid trafficking and membrane biogenesis. Biochim Biophys Acta. 1994 Dec 9;1197(3):257–290. doi: 10.1016/0304-4157(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Mutz M, Helfrich W. Unbinding transition of a biological model membrane. Phys Rev Lett. 1989 Jun 12;62(24):2881–2884. doi: 10.1103/PhysRevLett.62.2881. [DOI] [PubMed] [Google Scholar]
- Nezil F. A., Bayerl S., Bloom M. Temperature-reversible eruptions of vesicles in model membranes studied by NMR. Biophys J. 1992 May;61(5):1413–1426. doi: 10.1016/S0006-3495(92)81947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Seifert U, Berndl K, Lipowsky R. Shape transformations of vesicles: Phase diagram for spontaneous- curvature and bilayer-coupling models. Phys Rev A. 1991 Jul 15;44(2):1182–1202. doi: 10.1103/physreva.44.1182. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A., Hémar A., Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci. 1994 Dec;107(Pt 12):3461–3468. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- Svetina S., Zeks B. Membrane bending energy and shape determination of phospholipid vesicles and red blood cells. Eur Biophys J. 1989;17(2):101–111. doi: 10.1007/BF00257107. [DOI] [PubMed] [Google Scholar]
- Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J. 1993 Aug 15;294(Pt 1):1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]


