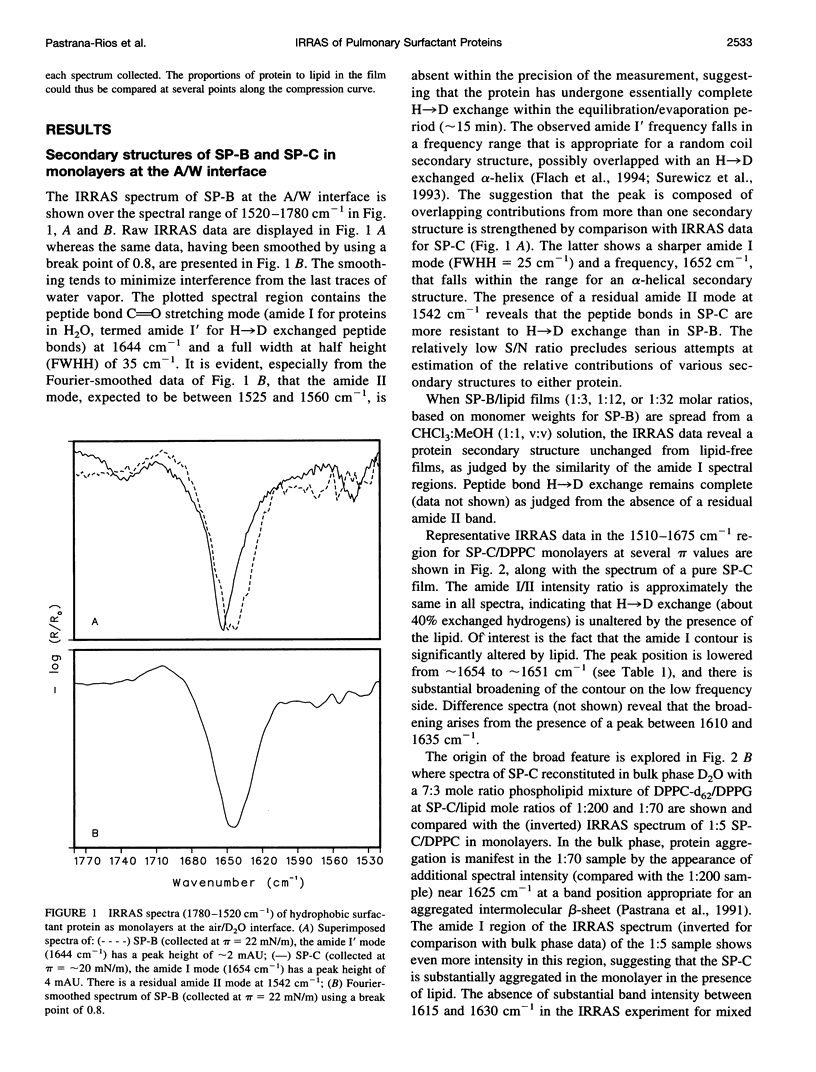
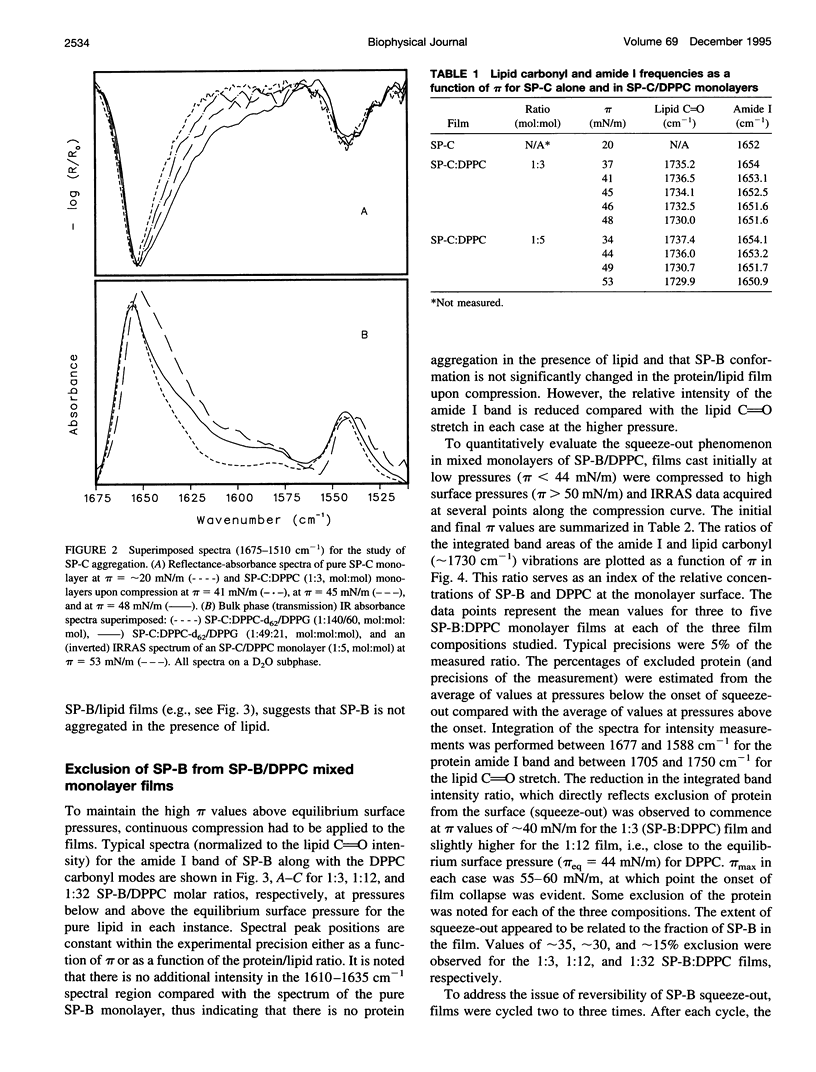
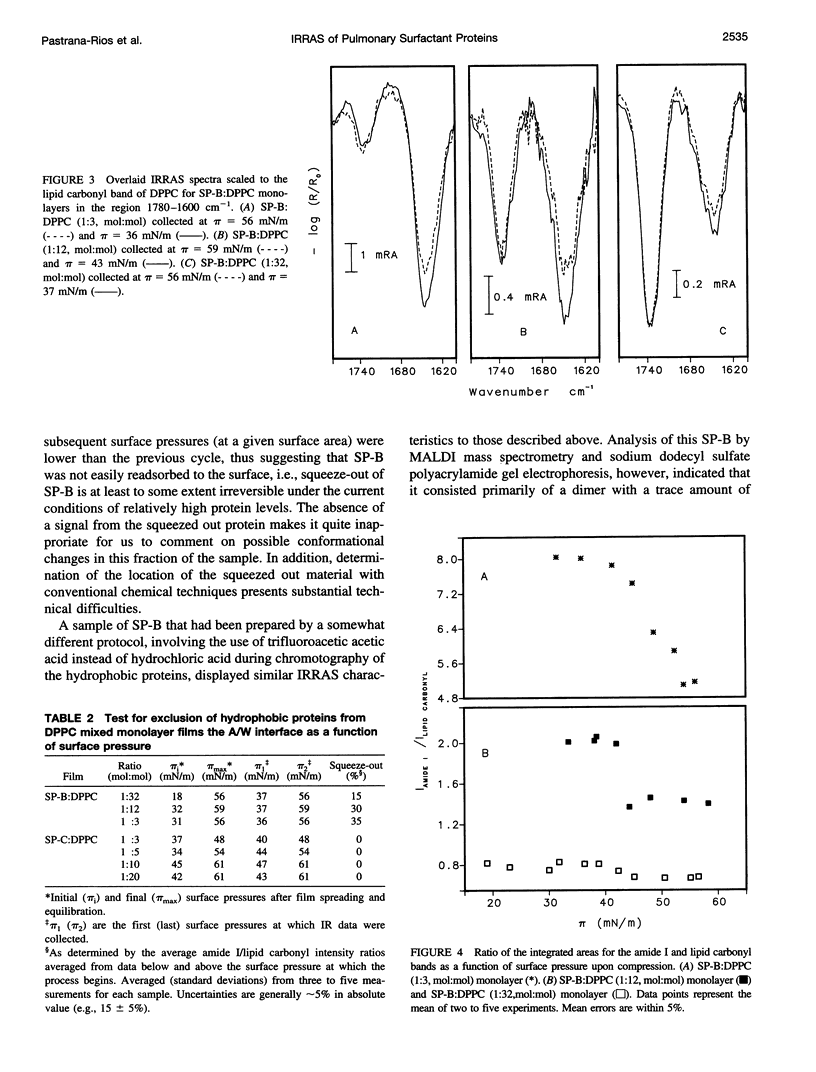
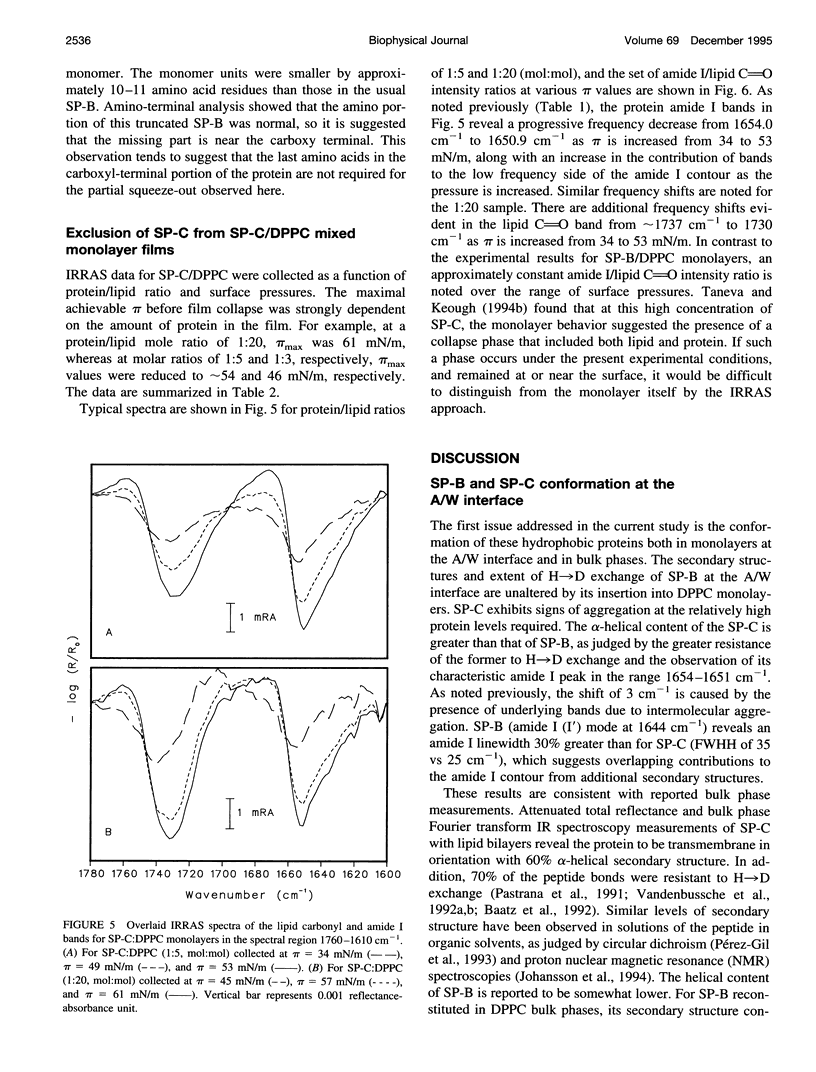
Abstract
The interactions of the hydrophobic pulmonary surfactant proteins SP-B and SP-C with 1,2-dipalmitoylphosphatidylcholine in mixed, spread monolayer films have been studied in situ at the air/water interface with the technique of external reflection absorption infrared spectroscopy (IRRAS). SP-C has a mostly alpha-helical secondary structure both in the pure state and in the presence of lipids, whereas SP-B secondary structure is a mixture of alpha-helical and disordered forms. When films of SP-B/1,2-dipalmitoylphosphatidylcholine are compressed to surface pressures (pi) greater than approximately 40-43 mN/m, the protein is partially (15-35%) excluded from the surface, as measured by intensity ratios of the peptide bond amide l/lipid C==O stretching vibrations. The extent of exclusion increases as the protein/lipid ratio in the film increases. In contrast, SP-C either remains at the surface at high pressures or leaves accompanied by lipids. The amide l peak of SP-C becomes asymmetric as a result of the formation of intermolecular sheet structures (1615-1630 cm-1) suggestive of peptide aggregation. The power of the IRRAS experiment for determination of film composition and molecular structure, i.e., as a direct test of the squeeze-out hypothesis of pulmonary surfactant function, is evident from this work.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baatz J. E., Elledge B., Whitsett J. A. Surfactant protein SP-B induces ordering at the surface of model membrane bilayers. Biochemistry. 1990 Jul 17;29(28):6714–6720. doi: 10.1021/bi00480a022. [DOI] [PubMed] [Google Scholar]
- Baatz J. E., Smyth K. L., Whitsett J. A., Baxter C., Absolom D. R. Structure and functions of a dimeric form of surfactant protein SP-C: a Fourier transform infrared and surfactometry study. Chem Phys Lipids. 1992 Nov;63(1-2):91–104. doi: 10.1016/0009-3084(92)90026-l. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clements J. A. Functions of the alveolar lining. Am Rev Respir Dis. 1977 Jun;115(6 Pt 2):67–71. doi: 10.1164/arrd.1977.115.S.67. [DOI] [PubMed] [Google Scholar]
- Egberts J., Sloot H., Mazure A. Minimal surface tension, squeeze-out and transition temperatures of binary mixtures of dipalmitoylphosphatidylcholine and unsaturated phospholipids. Biochim Biophys Acta. 1989 Mar 14;1002(1):109–113. doi: 10.1016/0005-2760(89)90072-6. [DOI] [PubMed] [Google Scholar]
- Flach C. R., Brauner J. W., Taylor J. W., Baldwin R. C., Mendelsohn R. External reflection FTIR of peptide monolayer films in situ at the air/water interface: experimental design, spectra-structure correlations, and effects of hydrogen-deuterium exchange. Biophys J. 1994 Jul;67(1):402–410. doi: 10.1016/S0006-3495(94)80495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawco M. W., Coolbear K. P., Davis P. J., Keough K. M. Exclusion of fluid lipid during compression of monolayers of mixtures of dipalmitoylphosphatidylcholine with some other phosphatidylcholines. Biochim Biophys Acta. 1981 Aug 6;646(1):185–187. doi: 10.1016/0005-2736(81)90286-8. [DOI] [PubMed] [Google Scholar]
- Hawco M. W., Davis P. J., Keough K. M. Lipid fluidity in lung surfactant: monolayers of saturated and unsaturated lecithins. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):509–515. doi: 10.1152/jappl.1981.51.2.509. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Hamilton R. L., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry. 1985 Jan 1;24(1):184–190. doi: 10.1021/bi00322a026. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebran J. N., Goerke J., Clements J. A. Pulmonary surface film stability and composition. J Appl Physiol Respir Environ Exerc Physiol. 1979 Sep;47(3):604–611. doi: 10.1152/jappl.1979.47.3.604. [DOI] [PubMed] [Google Scholar]
- Hillenkamp F., Karas M., Beavis R. C., Chait B. T. Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal Chem. 1991 Dec 15;63(24):1193A–1203A. doi: 10.1021/ac00024a002. [DOI] [PubMed] [Google Scholar]
- Johansson J., Szyperski T., Curstedt T., Wüthrich K. The NMR structure of the pulmonary surfactant-associated polypeptide SP-C in an apolar solvent contains a valyl-rich alpha-helix. Biochemistry. 1994 May 17;33(19):6015–6023. doi: 10.1021/bi00185a042. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Pérez-Gil J., Simatos G., Boland C., Stewart J., Absolom D., Sarin V., Keough K. M. Pulmonary surfactant-associated protein SP-B has little effect on acyl chains in dipalmitoylphosphatidylcholine dispersions. Biochemistry. 1993 Apr 27;32(16):4397–4402. doi: 10.1021/bi00067a032. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Taneva S., Simatos G. A., Allwood L. A., Keough K. M. 2H NMR studies of the effect of pulmonary surfactant SP-C on the 1,2-dipalmitoyl-sn-glycero-3-phosphocholine headgroup: a model for transbilayer peptides in surfactant and biological membranes. Biochemistry. 1993 Oct 26;32(42):11338–11344. doi: 10.1021/bi00093a010. [DOI] [PubMed] [Google Scholar]
- Nag K., Keough K. M. Epifluorescence microscopic studies of monolayers containing mixtures of dioleoyl- and dipalmitoylphosphatidylcholines. Biophys J. 1993 Sep;65(3):1019–1026. doi: 10.1016/S0006-3495(93)81155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana-Rios B., Flach C. R., Brauner J. W., Mautone A. J., Mendelsohn R. A direct test of the "squeeze-out" hypothesis of lung surfactant function. External reflection FT-IR at the air/water interface. Biochemistry. 1994 May 3;33(17):5121–5127. doi: 10.1021/bi00183a016. [DOI] [PubMed] [Google Scholar]
- Pastrana B., Mautone A. J., Mendelsohn R. Fourier transform infrared studies of secondary structure and orientation of pulmonary surfactant SP-C and its effect on the dynamic surface properties of phospholipids. Biochemistry. 1991 Oct 15;30(41):10058–10064. doi: 10.1021/bi00105a033. [DOI] [PubMed] [Google Scholar]
- Pérez-Gil J., Cruz A., Casals C. Solubility of hydrophobic surfactant proteins in organic solvent/water mixtures. Structural studies on SP-B and SP-C in aqueous organic solvents and lipids. Biochim Biophys Acta. 1993 Jul 1;1168(3):261–270. doi: 10.1016/0005-2760(93)90181-8. [DOI] [PubMed] [Google Scholar]
- Pérez-Gil J., Nag K., Taneva S., Keough K. M. Pulmonary surfactant protein SP-C causes packing rearrangements of dipalmitoylphosphatidylcholine in spread monolayers. Biophys J. 1992 Jul;63(1):197–204. doi: 10.1016/S0006-3495(92)81582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Gil J., Tucker J., Simatos G., Keough K. M. Interfacial adsorption of simple lipid mixtures combined with hydrophobic surfactant protein from pig lung. Biochem Cell Biol. 1992 May;70(5):332–338. doi: 10.1139/o92-051. [DOI] [PubMed] [Google Scholar]
- Schürch S., Goerke J., Clements J. A. Direct determination of surface tension in the lung. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4698–4702. doi: 10.1073/pnas.73.12.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Curstedt T., Grossmann G., Kobayashi T., Nilsson R., Nohara K., Robertson B. The role of the low-molecular weight (less than or equal to 15,000 daltons) apoproteins of pulmonary surfactant. Eur J Respir Dis. 1986 Nov;69(5):336–345. [PubMed] [Google Scholar]
- Takahashi A., Waring A. J., Amirkhanian J., Fan B., Taeusch H. W. Structure-function relationships of bovine pulmonary surfactant proteins: SP-B and SP-C. Biochim Biophys Acta. 1990 May 1;1044(1):43–49. doi: 10.1016/0005-2760(90)90216-k. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., Tatulian S. A. Orientation of functional and nonfunctional PTS permease signal sequences in lipid bilayers. A polarized attenuated total reflection infrared study. Biochemistry. 1993 Aug 3;32(30):7720–7726. doi: 10.1021/bi00081a017. [DOI] [PubMed] [Google Scholar]
- Taneva S., Keough K. M. Pulmonary surfactant proteins SP-B and SP-C in spread monolayers at the air-water interface: I. Monolayers of pulmonary surfactant protein SP-B and phospholipids. Biophys J. 1994 Apr;66(4):1137–1148. doi: 10.1016/S0006-3495(94)80895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneva S., Keough K. M. Pulmonary surfactant proteins SP-B and SP-C in spread monolayers at the air-water interface: II. Monolayers of pulmonary surfactant protein SP-C and phospholipids. Biophys J. 1994 Apr;66(4):1149–1157. doi: 10.1016/S0006-3495(94)80896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneva S., Keough K. M. Pulmonary surfactant proteins SP-B and SP-C in spread monolayers at the air-water interface: III. Proteins SP-B plus SP-C with phospholipids in spread monolayers. Biophys J. 1994 Apr;66(4):1158–1166. doi: 10.1016/S0006-3495(94)80897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche G., Clercx A., Clercx M., Curstedt T., Johansson J., Jörnvall H., Ruysschaert J. M. Secondary structure and orientation of the surfactant protein SP-B in a lipid environment. A Fourier transform infrared spectroscopy study. Biochemistry. 1992 Sep 29;31(38):9169–9176. doi: 10.1021/bi00153a008. [DOI] [PubMed] [Google Scholar]
- Vandenbussche G., Clercx A., Curstedt T., Johansson J., Jörnvall H., Ruysschaert J. M. Structure and orientation of the surfactant-associated protein C in a lipid bilayer. Eur J Biochem. 1992 Jan 15;203(1-2):201–209. doi: 10.1111/j.1432-1033.1992.tb19848.x. [DOI] [PubMed] [Google Scholar]
- Watkins J. C. The surface properties of pure phospholipids in relation to those of lung extracts. Biochim Biophys Acta. 1968 Mar 4;152(2):293–306. doi: 10.1016/0005-2760(68)90037-4. [DOI] [PubMed] [Google Scholar]
- Yu S. H., Possmayer F. Role of bovine pulmonary surfactant-associated proteins in the surface-active property of phospholipid mixtures. Biochim Biophys Acta. 1990 Oct 1;1046(3):233–241. doi: 10.1016/0005-2760(90)90236-q. [DOI] [PubMed] [Google Scholar]
- Zhang Y. P., Lewis R. N., Hodges R. S., McElhaney R. N. FTIR spectroscopic studies of the conformation and amide hydrogen exchange of a peptide model of the hydrophobic transmembrane alpha-helices of membrane proteins. Biochemistry. 1992 Nov 24;31(46):11572–11578. doi: 10.1021/bi00161a041. [DOI] [PubMed] [Google Scholar]
- van Liempd J. P., Boonman A. A., Demel R. A., Gieles P. M., Gorree T. C. Nonselective squeeze-out of dioleoylphosphatidylcholine and dioleoylphosphatidylglycerol from binary mixed monolayers with dipalmitoylphosphatidylcholine. Biochim Biophys Acta. 1987 Mar 12;897(3):495–501. doi: 10.1016/0005-2736(87)90447-0. [DOI] [PubMed] [Google Scholar]


