Abstract
Chemotherapy of human sleeping sickness, a fatal disease caused by the protozoan parasite Trypanosoma brucei, is in a dismal state, and the identification and characterization of new drug targets is an urgent prerequisite for an improvement of the dramatic situation in the field. Over the last several years, inhibitors of cyclic nucleotide-specific phosphodiesterases have proven to be highly successful drug candidates for an assortment of clinical conditions. Their potential as antiparasitic drugs has not been explored so far. This study reports the characterization of a cAMP-specific phosphodiesterase from T. brucei, TbPDE2C. This enzyme is a class I phosphodiesterase, and it is a member of a small enzyme family in T. brucei, TbPDE2. Inhibitors of this enzyme block the proliferation of bloodstream form trypanosomes in culture. RNA interference experiments demonstrated that the TbPDE2 family, and in particular TbPDE2C, are essential for maintaining intracellular cAMP concentrations within a physiological range. Bloodstream form trypanosomes are exquisitely sensitive to elevated concentrations of intracellular cAMP, and a disruption of TbPDE2C function quickly leads to the disruption of nuclear and cellular cell division, and to cell death. TbPDE2C might represent a novel drug target for the development of new and effective trypanocidal drugs.
Cyclic nucleotide-specific phosphodiesterases (PDEs) are key regulators of cAMP signaling pathways by controlling the spatial and temporal shapes of cyclic nucleotide signals as well as the steady-state levels of intracellular cAMP (1–3). PDEs are cogently involved in a large variety of physiological processes, such as the control of metabolic activities, cell aggregation or quorum sensing in unicellular organisms, or the modulation of the immune response, insulin secretion, aldosterone production, vascular resistance, inflammation, reproduction, olfaction, and vision in metazoa. Genetic defects in some of the PDE genes lead to hereditary disease in humans and other mammals (4–6).
In eukaryotes, the PDEs represent a large and diverse group of enzymes. Two classes of eukaryotic PDEs have been identified (7, 8): Class I includes all currently known families of mammalian PDEs (see below), as well as a number of PDEs from lower eukaryotes, such as PDE2 from the yeast Saccharomyces cerevisiae or the product of the regA gene of Dictyostelium discoideum. Class II PDEs show little sequence similarity to class I enzymes, usually have a much higher Km, and are represented by genes identified in S. cerevisiae, Candida albicans, or D. discoideum.
In mammals, 11 distinct class I PDE families have been identified so far based on DNA sequence analysis and on their biochemical and pharmacological characteristics (9, 10). At the amino acid level, family members exhibit >50% sequence identity within a conserved catalytic domain of about 250 aa length. Between families, the sequence identity drops to 30–40% in the same region (11). Family members also share extensive similarity in regions outside the catalytic domain, whereas no significant similarity can be detected between these regions of members of different families.
In clinical pharmacology, the PDEs have become highly attractive targets for drug development over the last few years. A growing number of highly family-specific inhibitors were developed, despite considerable sequence homology between the catalytic domains of the different PDE families. PDE inhibitors are under exploration, or already in clinical use, as therapeutic agents for diseases ranging from autoimmune disease (12), arthritis (13), asthma (14), and inflammatory diseases (15) to impotency (16) and cancer (17). The ongoing development of new and ever more subtype-specific inhibitors holds great promise for achieving more potent and specific drug action with fewer side effects, as well as for discovering new areas of application for PDE inhibitors.
Although PDE inhibitors have met with considerable success in many clinical applications, their potential as anti-parasitic drugs remains to be established. In view of the current deplorable state of human sleeping sickness chemotherapy (18, 19), in which the protozoan parasite Trypanosoma brucei can cause a fatal infection of the central nervous system, the identification of new potential drug targets in trypanosomes is necessary and urgent. Drug targets that are already being exploited successfully in other organisms, such as the PDEs in humans, are of particular interest in this context.
The role of PDEs in the cell biology of Trypanosoma brucei still remains largely unexplored (20). A first PDE from T. brucei was recently identified by complementation screening in yeast and was shown to represent a class I PDE with an unusually high Km for its specific substrate, cAMP (TbPDE1; (S. Kunz, T. Kloeckner, B. Bieger, L. O. Essen, T.S., and M. Boshart, unpublished results). This enzyme is not essential for the proliferation of T. brucei in culture, nor for the infection of its natural vector, the tsetse fly (21). Two members of a different class I family of T. brucei, TbPDE2, have been recently identified and characterized [TbPDE2A (22); TbPDE2B (A. Rascón, S. H. Soderling, and I. A. Beovo, personal communication].
The current study describes the identification of a previously undocumented member of the TbPDE2 family, TbPDE2C, and demonstrates that this family, and in particular its member TbPDE2C, are essential for bloodstream trypanosome proliferation. RNA interference experiments demonstrated that the inactivation of TbPDE2 reduces total PDE activity, increases intracellular cAMP and is lethal to bloodstream trypanosomes. The data presented validate the TbPDE2 family, and in particular TbPDE2C, as potential drug targets and indicate that PDE inhibitors might be useful for anti-protozoal chemotherapy.
Materials and Methods
Plasmid Construction.
DNA fragments representing the N-terminal regions of the ORFs of TbPDE1 (S. Kunz, T. Kloeckner, B. Bieger, L. O. Essen, T.S., and M. Boshart, unpublished results; AF253418), TbPDE2A (ref. 22; AF263280), TbPDE2B (A. Rascón, S. H. Soderling, and I. A. Beovo, personal communication; AF192755), and TbPDE2C (this study), as well as a region that is fully conserved between the catalytic regions of all TbPDE2 family members, were amplified by PCR from T. brucei 427 genomic DNA, using different sets of primers containing XhoI and HindIII linkers. The PCR fragments were cloned into pGEM-T-Easy (Promega), verified by sequencing, and ligated into the XhoI and HindIII sites of the RNA interference (RNAi) vector pZJM (23).
Trypanosome Growth and Transformation.
The procyclic RNAi host strain 29-13 (24) was grown in SDM-79 (25) supplemented with 15% FCS in the presence of G418 (15 μg/ml) and hygromycin (50 μg/ml) to maintain the integrated genes for T7 RNA polymerase and tetracycline repressor, respectively. Monomorphic bloodstream forms of strain 221 (MITat 1.2) and the corresponding RNAi host strain 13-90 (23) were cultivated as described by Hesse et al. (26). Strain 13-90 was cultured in the presence of G418 (1.0 μg/ml) and hygromycin (1.0 μg/ml) to maintain the integrated genes for T7 RNA polymerase and tetracycline repressor, respectively.
For stable transfection of the procyclic host strain 29-13 and the bloodstream host strain 13-90 (23) via integration into an rDNA spacer region, the RNAi constructs were linearized by NotI digestion. Transfectants were selected by using 2.5 μg/ml (for procyclics) or 1 μg/ml (for bloodstream forms) of phleomycin. RNAi was induced by adding 1 μg/ml tetracycline to the medium.
cAMP Determination.
Intracellular cAMP concentrations were determined by using an enzyme-linked immunoassay kit (Biomol, Plymouth Meeting, PA) with 5 × 106 procyclic trypanosomes before and after the induction of RNAi as described earlier (22). Cultures were always harvested at similar cell densities (around 3.5 × 106/ml), all assays were done in triplicate, and at least two independent experiments using different batches of cells were performed.
Cytotoxicity Determination.
Cytotoxicity of 8-bromo-cAMP and N6,O2′-dioctanoyl-cAMP was determined for bloodstream forms in culture by using alamarBlue (BioSource International, Camarillo, CA) as a metabolic indicator (27). Exponentially growing bloodstream forms 221 (MiTat2.1) were seeded into microtiter wells (99 μl/well) containing 1.0 μl of inhibitor or solvent control. Plates were incubated for 40 and 70 h at 37°C in a humidified atmosphere containing 5% CO2. At the end of the growth period, 10 μl of alamarBlue was added per well, and the incubation was continued for another 3 h at 37°C. The plates were read on a SPECTRAmax Gemini fluorescence plate reader (Molecular Devices) using an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Data analysis was performed by using the prism software package of GraphPad (San Diego). Assays were done in triplicate, and two independent experiments using different batches of cells were performed.
Phosphodiesterase Assay.
Expression of TbPDE2C in S. cerevisiae and analysis of the activity of the recombinant enzyme were done exactly as described previously (22). Total PDE activity in trypanosome lysates was determined as follows: 2 × 107 parasites from exponentially growing cultures of similar cell densities were harvested and washed once in cold HHB buffer (Hanks' balanced salts, containing 50 mM Hepes, pH 7.5). The washed cell pellets were suspended in 500 μl of cold HHB containing a protease inhibitor mixture (Complete, Roche Diagnostics) and were sonicated three times for 15 s in an ice bath (Soniprep 150, amplitude 6 μm). After centrifugation in an Eppendorf 5415C centrifuge at 12,000 rpm for 15 min, glycerol was added to the resulting supernatants to a final concentration of 25% (vol/vol), and lysates were stored at −70°C. PDE assays were done by using the ZnSO4/Ba(OH)2 precipitation method of Schilling et al. (28) as described earlier (22). All assays were carried out in triplicate and with three independent enzyme preparations.
RNA Extraction and Reverse Transcription (RT–PCR).
Total RNA was purified from 3 × 107 parasites from the cultures with similar densities (StrataPrep Total RNA microprep kit; Stratagene). RNA was tested for the absence of genomic DNA by PCR analysis. Randomly primed cDNA synthesis was carried out with 5 μg of total RNA in a final volume of 50 μl (ProSTAR First-Strand RT-PCR Kit; Stratagene). One microliter of cDNA product was then used as a template in PCRs (1 time at 94°C for 1 min; 25 times at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min; and 1 time at 72°C for 5 min) to amplify the specific regions of different RNAs. The number of amplification cycles was adjusted so that the reactions were in the linear range. All reactions also contained primers for the simultaneous amplification of a 500 bp calmodulin gene fragment as an internal control and standard for quantification. The primers should not amplify the double-stranded RNAs produced by the various RNAi vectors. Quantification of the PCR products was performed by using metamorph software (Universal Imaging, Media, PA) using data from three independent RT-PCR experiments. The following primers were used: for TbPDE2A, 2A-FX: 5′-TACTCGAGATGTATGTGCACGACGTACGCATGTTC-3′ and Pde2.gen.rev: 5′-TTCAACCCCATATGATCAAGATCATGCACCAG-3′; for TbPDE2B, 2B-FRN: 5′-GAGCAACATGAAGCTCGACGAAG-3′ and 2B-RRN: 5′-TGGTGATGATTCATCGAGTGCAG-3′; for TbPDE2C, 2C-FRN: 5′-CGTTTGCCATCACTGAAGCAATC-3′ and 2C-RRN: 5′-ATCAATCAATGCTGCCGTGTCGT-3′; for TbPDE1, RNA1-f-a: 5′-GAGAGAGGATCCACCATGTTTGCCGTTACATC-3′ and RNA1-r: 5′-GAGAGAGAATTCCTCTTCTCGCTACAGTCCTC-3′; for the common catalytic domain of all TbPDE2 enzymes, PDE2-FRN: 5′-TTGTACAGGGGAAATGTGTATGAGAAG-3′ and PDE2ir-XhoI: 5′-GATACTCGAGTTCCAGAACTCGCTCCCAC-3′; and for calmodulin, CMD5′: 5′-GAGAGTCGACCCATATGGCCGATCAACTCTCC-3′ and CMD5′-r: 5′-TCTCAAGCTTGGATCCTATTTGCTCATCATCA-3′.
Microscopy.
Cells were collected by low speed centrifugation, washed twice with PBS, and allowed to settle onto poly(l-lysine)-coated slides. They were fixed for 15 min with 4% paraformaldehyde-PBS-20% FCS. After fixation, the cells were washed three times in PBS containing 20% FCS and were permeabilized by incubation in the same solution containing 0.025% saponin for 10 min. After washing, the cells were embedded in mounting medium containing 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Vectashield; Vector Laboratories) and were visualized in a Leitz Laborlux fluorescence microscope equipped with a charge-coupled device (CCD) camera.
Results
Sequence Organization of TbPDE2C.
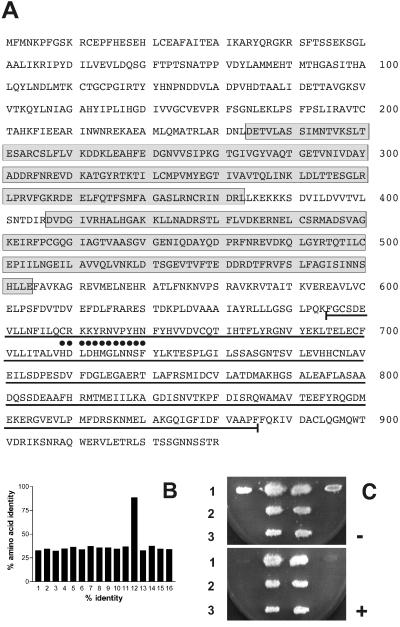
The identification and characterization of two members of the TbPDE2 family have already been reported [TbPDE2A (22); TbPDE2B (A. Rascón, S. H. Soderling, and I. A. Beovo, personal communication]. Hybridization screening of genomic and cDNA libraries with a DNA probe representing the catalytic region of TbPDE2A revealed the presence of additional family members in the genome of T. brucei. One of these, TbPDE2C, was cloned and sequenced. The ORF of TbPDE2C is preceded by a 5′-untranslated region of 77 nucleotides length, as determined by the presence of a miniexon (29) at nucleotide −77. The ORF is predicted to code for a 930-aa protein with a calculated molecular weight of 103,694 (Fig. 1A). Amino acids 234–379 and 407–552 represent GAF domains, ubiquitous conserved structures that are involved in binding low-molecular-weight ligands (30). The catalytic domain is predicted to start around amino acid 580, with the highly conserved catalytic core region (31, 32) extending from Phe-645 to Phe-877. The amino acid sequence identity of this core of TbPDE2C with the corresponding regions of TbPDE2A and TbPDE2B is 91.8% and 99.6%, respectively, whereas sequence conservation with the corresponding regions of mammalian and other eukaryotic PDEs ranges from 30 to 40% (Fig. 1B). The two GAF domains of TbPDE2C share 47.7% sequence identity. The N-terminal GAF-A is 94.4% identical with GAF-A of TbPDE2B (A. Rascón, S. H. Soderling, and I. A. Beovo, personal communication) and 53.4% identical with the single GAF domain of TbPDE2A (21). GAF-B shares 97.3% sequence identity with GAF-B of TbPDE2B and 97.3% identity with the single GAF domain of TbPDE2A (22).
Figure 1.
(A) Predicted amino acid sequence of TbPDE2C. Boxed sequences represent GAF domains A and B. The central core of the catalytic domain is underlined, and the PDE signature sequence is marked by black dots. (B) Amino acid sequence identity between the catalytic cores of class I PDE families. 1, human PDE 1C3A (accession no. U40372); 2, human PDE2A (NP002590); 3, human PDE 3A (M91667); 4, human PDE4A4B (AAC35012); 5, human PDE5A (NM_001083); 6, human PDE6B (NM_000283); 7, mouse PDE7A2 (U68171); 8, mouse PDE8 (NM_008803); 9, mouse PDE 9A (AF031147); 10, human PDE 10A (AF127479); 11, human PDE 11A1 (AJ251509); 12, T. brucei TbPDE2A (AF263280); 13, T. brucei TbPDE1 (AF253418); 14, D. discoideum regA (U60170); 15, D. melanogaster dunce (P12252); 16, S. cerevisiae pde2 (M14563). (C) Complementation of the heat-shock phenotype of S. cerevisiae PP5-12 by TbPDE2C. Row 1, First and last patches: PP5-12 containing the empty vector pLT1 (negative control); second and third patches: PP5-12 expressing TbPDE1 (positive control). Row 2, Two patches of PP5-12 expressing untagged TbPDE2C. Row 3, Two patches of PP5-12 expressing TbPDE2C with a C-terminal hemagglutinin tag. −, No heat shock; +, with heat shock.
Catalytic Properties of TbPDE2C.
The biological activity of TbPDE2C was explored by functional complementation of a PDE-deficient S. cerevisiae strain (PP5-12; refs. 22 and 33). Full-length TbPDE2C and a full-length version of TbPDE2C containing a hemagglutinin-tag at its C terminus (-TyrProTyrAspTyrProAspTyrAlaGlyIleProMet-stop) were expressed from plasmid pLT1 (S. Kunz, T. Kloeckner, B. Bieger, L. O. Essen, T.S., and M. Boshart, unpublished results) under the control of a strong TEF2-promotor (34). Restoration of the heat shock resistance phenotype was assessed (Fig. 1C). Both untagged and C-terminally tagged TbPDE2C rendered the PP5-12 host cells heat-shock resistant. In addition, the flocculate growth of the PP5-12 mutant strain (S. Kunz, T. Kloeckner, B. Bieger, L. O. Essen, T.S., and M. Boshart, unpublished results) was restored to smooth growth in suspension culture by both constructs. These results established that TbPDE2C is enzymatically active in yeast, and that it represents a PDE that can use cAMP as its substrate.
Hemagglutinin-tagged TbPDE2C is completely stable under the conditions of yeast cell breakage and enzyme assay. Lysates from the yeast strain PP5-12 expressing untagged TbPDE2C were then used to characterize the catalytic activity and inhibitor profile of TbPDE2C. The results (Table 1) demonstrated that TbPDE2C exhibits characteristics that are similar to those described earlier for TbPDE2A (22). This result was not unexpected, given the almost perfect sequence identity between the catalytic domains of TbPDE2A and TbPDE2C. TbPDE2C is a cAMP-specific PDE with a Km for cAMP of around 8 μM. cGMP neither competes for cAMP as a substrate, nor does it modulate the activity of TbPDE2C. From a panel of inhibitors tested, trequinsin, dipyridamol, ethaverine, etazolate, and sildenafil were the most potent, with IC50s in the low micromolar range, whereas rolipram or IBMX exhibited IC50 values of >500 μM.
Table 1.
Potency of selected PDE inhibitors against TbPDE2C
| TbPDE2A | TbPDE2C | |
|---|---|---|
| Km, μM | 2.28 | 7.97 ± 2.32 (n = 4) |
| IC50, μM | ||
| IBMX | 545 | 1704 ± 721 (n = 3) |
| Sildenafil | 9.4 | 42.2 ± 5.7 (n = 3) |
| Dipyridamole | 5.9 | 14.6 ± 2.8 (n = 3) |
| Etazolate | 30.3 | 30.6 ± 4.3 (n = 3) |
| Ethaverine | 14.2 | 26.8 ± 3.0 (n = 3) |
| Trequinsin | 5.4 | 13.3 ± 2.9 (n = 3) |
IC50 values were determined at a cAMP concentration of 1 μM. Values for TbPDE2A are taken from ref. 22.
RNA Interference.
Earlier experiments had demonstrated that inhibitors of the TbPDE2 family raised the intracellular cAMP concentration and inhibited proliferation of bloodstream form trypanosomes in culture (22). To genetically validate these results, attempts were made to construct genetic TbPDE2A knock-outs in bloodstream trypanosomes. Heterozygous knock-out clones were readily recovered. These cells looked completely normal and proliferated well as long as they were kept in continuous culture. However, none of the clones could be kept alive for more than a week after freezing and rethawing. Numerous attempts to delete the second allele of TbPDE2A always led to cells that survived the antibiotic selection, but invariably died after maximally 3 weeks. Microscopic inspection of DAPI-stained cells revealed that they were odd shaped and contained multiple nuclei. These observations suggested that TbPDE2A is an essential enzyme, and that it exhibits marked haploid insufficiency.
In view of the failure to produce TbPDE2A knock-outs, the biological significance of the TbPDE2 family was then investigated by using RNAi as an alternative strategy. A bloodstream and a procyclic cell line of T. brucei that express the tetracycline repressor and the bacterial T7 RNA polymerase (24) were generously provided by G. A. M. Cross (The Rockefeller University). The inactivating fragments for RNAi were inserted between the two opposing T7 promoters of the pZJM vector that are both regulated by tetracycline repressors (23). The fragments were designed to specifically inactivate the individual mRNAs for either TbPDE2A, -B, or -C, or to simultaneously inactivate all TbPDE2 mRNAs. The family-member-specific fragments were derived from the 5′-ends of the ORFs where the three genes differ considerably in sequence. The RNAi probe for TbPDE2A corresponded to nucleotides 1–177 of its coding region (accession no. AF263280); the TbPDE2B-specific probe represented nucleotides 75–453 of its coding region (accession no. AF192755); and the TbPDE2C-specific probe corresponded to nucleotides 3–340 of its ORF. The pan-PDE2 RNAi probe designed to simultaneously inactivate all TbPDE2 mRNAs was directed against a highly conserved region of the catalytic domain of all three family members, represented by nucleotides 774-1432 of the ORF of TbPDE2A. A control construct was directed against a different PDE family, TbPDE1 (S. Kunz, T. Kloeckner, B. Bieger, L. O. Essen, T.S., and M. Boshart, unpublished results), by using nucleotides 165–673 of its ORF (accession no. AF253418) as an RNAi probe.
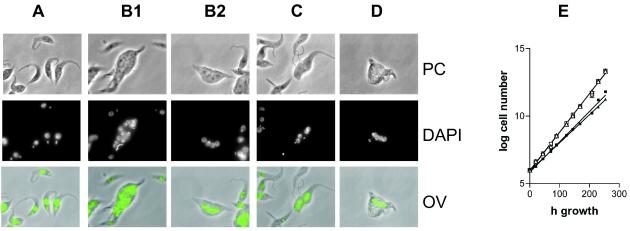
Transfection of the bloodstream form cell line 90-13 (24, 35) with RNAi constructs directed against either TbPDE2A or TbPDE2B resulted in cells that survived phleomycin selection and that grew for about 21–24 days after transfection, after which the invariably died. Microscopic inspection of the cells that survived the antibiotic selection showed that they all were odd-shaped and multinucleate, similarly to what had been observed with the homozygous TbPDE2A knock-outs. Transfection with constructs directed against TbPDE2C or against the common catalytic region of all three family members produced the same phenotype (Fig. 2D), but the cells died even earlier, between days 10–13 after transfection. Again, all cells were odd shaped and multinucleated. This effect was manifest even without induction of RNAi by tetracycline, confirming earlier observations that the pZJM vector is somewhat leaky (35). In the case of the TbPDE2 family, this low level of leakiness appears to be sufficient to produce a significant phenotype. When cells were transfected with control constructs, they always survived selection, proliferated indefinitely, and exhibited normal shapes and growth rates.
Figure 2.
Effect of RNAi on cell shape and proliferation. (A) Procyclic strain 29-13 (control). (B1 and B2) RNAi against TbPDE2C. (C) RNAi against the entire TbPDE2 family. (D) Temporarily surviving bloodstream form trypanosomes expressing RNAi against TbPDE2C. PC, Phase contrast; DAPI, nuclear staining; OV, overlay. (E) Growth curve of procyclic RNAi constructs. Open squares, Control strain, no tetracycline; open triangles, control strain, 1 μg/ml tetracycline; filled squares, RNAi against TbPDE2C, 1 μg/ml tetracycline; filled triangles, RNAi against entire TbPDE2 family, 1 μg/ml tetracycline. The graph represents one of three similar experiments.
In contrast to the bloodstream forms, procyclic trypanosomes survived transformation with all RNAi constructs. In procyclic transformants, only a proportion of the cells (10–20%) were multinucleate, oversized, and/or odd-shaped (Fig. 2 B1, B2, and C). When production of double-stranded RNA was induced by tetracycline in such cultures, the proportion of aberrant cells doubled, although normally shaped cells always constituted the majority of the culture. Under both conditions, the cultures exhibited stable exponential growth, although their population doubling time was significantly increased (e.g., 10.4 ± 0.06 h for the control 29-13 strain vs. 13.13 ± 0.21 h without and 14.43 ± 0.18 h with tetracycline induction of RNAi against TbPDE2C, respectively; Fig. 2E).
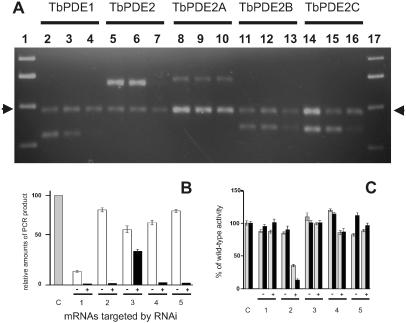
The effectiveness of RNAi on mRNA levels in procyclic cells was analyzed by RT-PCR (Fig. 3 A and B). In the absence of the inducer tetracycline, the target mRNAs were readily detectable by RT-PCR. After induction, the concentrations of the specific mRNAs were strongly diminished or dropped below detectability, except for TbPDE2A, where the mRNA level was reduced by only 40%. In cells expressing the pan-PDE2 construct, RT-PCR with a primer pair designed to amplify the mRNA fragments representing the catalytic domain of all three family members demonstrated their complete disappearance after induction (Fig. 3A, lanes 5–7). Additional RT-PCR with primer pairs specific for the mRNAs of each family member further confirmed that the levels of all of them were similarly reduced. The disappearance of the target mRNAs after induction of RNAi was also reflected when whole procyclic cell lysates were assayed for total PDE activity (Fig. 3C). Induction of RNAi against TbPDE1 did not cause a significant reduction of total PDE activity. This finding is in agreement with earlier experiments where the homozygous deletion of the TbPDE1 gene led to only a very slight reduction of total cellular PDE activity (21). RNAi targeted individually against TbPDE2B or TbPDE2C each reduced the total PDE activity, whereas a construct directed against the entire TbPDE2 family led to a cumulative reduction of overall PDE activity. The RNAi construct directed against TbPDE2A produced no significant effect on overall PDE activity. This finding is in agreement with the observation that this construct only slightly reduces the corresponding mRNA level (Fig. 3A). No significant differences were seen between the activities determined at 48 h or 120 h of induction, indicating that the turnover of PDE mRNAs and protein is essentially complete after 48 h and, secondly, that the effect of RNAi is stable for at least 120 h. This conclusion is supported by the RT-PCR analyses where very similar results were obtained at either 48 h or 120 h after induction. The residual PDE activity observed even after 120 h of RNAi against the entire TbPDE2 family is most likely due to TbPDE1 (about 15% of total PDE activity; ref. 21) and possibly to other PDEs that have recently been identified in T. brucei (S. Kunz and S. Templer, personal communication).
Figure 3.
Effect of RNAi on mRNA concentrations and PDE activity in procyclic trypanosomes. (A) Analysis of PCR products 120 h after induction. Lanes 1 and 17, Molecular weight markers; lanes 2–4, RNAi against TbPDE1, source of RNA; lane 2, wild-type cells; lane 3, cells carrying the RNAi construct, but not induced; lane 4, cells carrying the RNAi construct, induced for 120 h with 1 μg/ml tetracycline; lanes 5–7, pan-PDE2 RNAi directed against the entire TbPDE2 family, same arrangement; lanes 8–10, RNAi against TbPDE2A; lanes 11–13, RNAi against TbPDE2B; lanes 14–16, RNAi against TbPDE2C. Arrows indicate the calmodulin-specific PCR product used as an internal control. The primer pairs used for each set are detailed in Materials and Methods and are specific for the mRNA targeted by the respective RNAi constructs. (B) Quantification of RT-PCR products. Means and SEMs from three data sets similar to those shown in A. Sources of RNA: gray bar, control cells; white bars, cells carrying the respective RNAi constructs, no induction; black bars, cells carrying the respective RNAi constructs and induced for 48 h with 1 μg/ml tetracycline. C, Control cells. RNAi constructs directed against: 1, TbPDE1; 2, TbPDE2 family; 3, TbPDE2A; 4, TbPDE2B; and 5, TbPDE2C. Amounts of PCR product in each reaction were normalized with respect to the calmodulin PCR fragment in the same reaction. (C) Reduction of total PDE activity after RNAi. Total PDE activity was determined in whole cell lysates by using 1 μM cAMP as a substrate. Minus and plus signs designate uninduced and induced cultures, respectively. Gray bars, 48 h induction; black bars, 120 h induction. C, control cells. RNAi constructs directed against: 1, TbPDE1; 2, TbPDE2 family; 3, TbPDE2A; 4, TbPDE2B; 5, TbPDE2C.
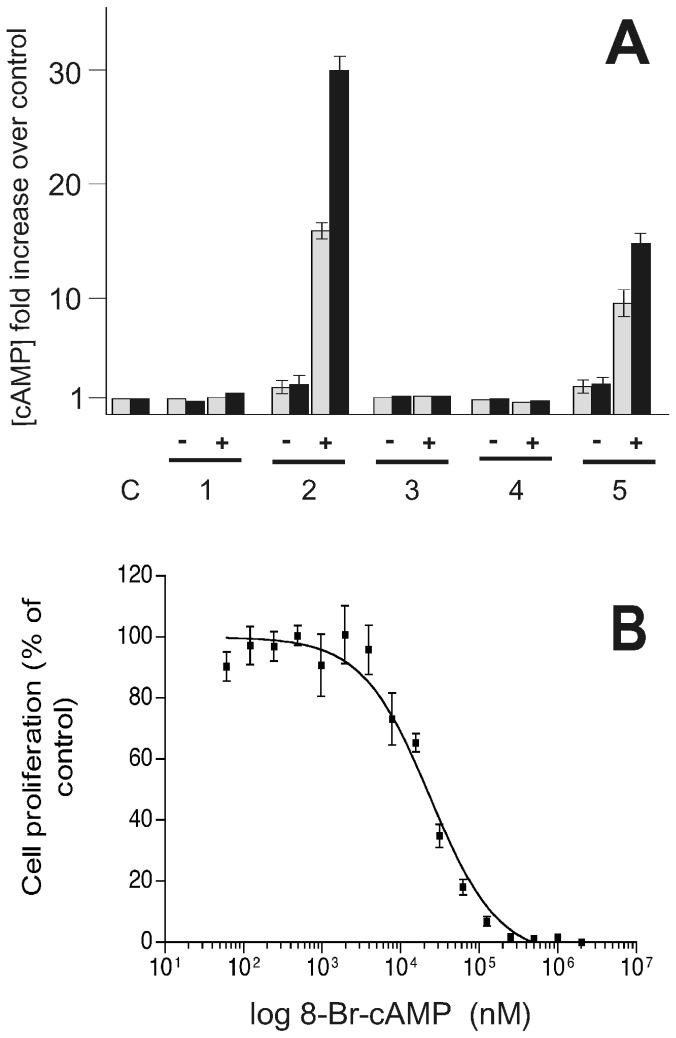
The effect of RNAi on intracellular cAMP levels after 48 h and 120 h of induction was determined in procyclic cells (Fig. 4A). The intracellular cAMP level in control cells was 64 ± 11 pmol/109 trypanosomes (intracellular concentration ≈1 μM), in agreement with the range of concentrations determined earlier in this and other laboratories (22, 36). On induction of RNAi, the pattern of cAMP levels closely correlated to what was observed for total PDE activities. RNAi against either TbPDE1 or against TbPDE2A and -B did not significantly alter the level of intracellular cAMP, either with or without induction by tetracycline. In contrast, an increase in cAMP was detectable with RNAi against TbPDE2C even in the absence of induction. After induction by tetracyline, a strong additional increase in cAMP (about 15-fold over the uninduced level) was observed. Targeting the entire TbPDE2 family led to a further increase in cAMP to more than 30-fold the steady-state level of wild-type cells. Whereas the overall PDE activity showed no further decrease after 48 h (see Fig. 3C), longer inductions led to a further increase in intracellular cAMP concentrations, consistent with a continuous accumulation of cAMP because of the activity of adenylyl cyclases in the absence of PDEs. These findings agree with the earlier observations that TbPDE2 inhibitors led to an increase in intracellular cAMP (22), and they support the view that the TbPDE2 family, and particularly TbPDE2C, are essential for maintaining the intracellular cAMP levels in trypanosomes.
Figure 4.
(A) Increase in intracellular cAMP in procyclic trypanosomes after RNAi-mediated inactivation of the TbPDE2 family. C, procyclic strain 29-13 (control). RNAi directed against: 1, TbPDE1; 2, the entire TbPDE2 family; 3, TbPDE2A; 4, TbPDE2B; 5, TbPDE2C. −, No induction of RNAi; +, RNAi induced by 1 μg/ml tetracycline. Gray bars, 48 h after induction; black bars, 120 h after induction. Intracellular cAMP level in control cells was 64 ± 11 pmol/109 trypanosomes. (B) Susceptibility of the proliferation of bloodstream form trypanosomes to 8-bromo-cAMP.
Increased cAMP Concentrations Are Toxic for Bloodstream Trypanosomes.
The proliferation of procyclic trypanosomes is only slightly affected by the high cAMP levels induced by RNAi against TbPDE2. This finding is in agreement with earlier observations that procyclic trypanosome proliferation appeared unchanged by even high concentrations of membrane-permeable cAMP-analogs (R. Schaub, personal communication). In contrast, the observations that bloodstream form trypanosomes are very sensitive to even a partial inactivation of TbPDE2 indicated that they might be much more susceptible to increased intracellular cAMP. This hypothesis was experimentally confirmed when bloodstream form trypanosomes were cultivated in the presence of different concentrations of membrane-permeable cAMP analogs. A representative dose-response curve for 8-bromo-cAMP is given in Fig. 4B. Very similar results were obtained with the chemically different cAMP analog N6,O2′-dioctanoyl-cAMP. The respective IC50 values of both compounds decreased with increasing time of exposure to the cells, from 76.2 ± 1.8 (after 40 h) to 25.1 ± 2.4 μM (after 70 h) for 8-bromo-cAMP and from 11.7 ± 0.9 (after 40 h) to 8.1 ± 0.7 μM (after 70 h) for N6,O2′-dioctanoyl-cAMP, respectively. When analyzed microscopically, the cells displayed the same odd shapes and multinuclear appearance as was seen with RNAi (see Fig. 2D). The data demonstrate that bloodstream form trypanosomes are indeed exquisitely sensitive to the elevation of intracellular cAMP. This result is in agreement with earlier findings that inhibitors of the TbPDE2 family elevate intracellular cAMP and concomitantly inhibit the proliferation of bloodstream form trypanosomes (22). The TbPDE2 family, and particularly TbPDE2C, apparently play a pivotal role in maintaining the steady-state level of intracellular cAMP in procyclic trypanosomes, and inactivation of these enzymes can be lethal for the parasite.
Discussion
T. brucei contains several families of class I PDEs, TbPDE1 (S. Kunz, T. Kloeckner, B. Bieger, L. O. Essen, T.S., and M. Boshart, unpublished results), TbPDE2 (ref. 22; A. Rascón, S. H. Soderling, and I. A. Beovo, personal communication; this paper), and presumably the additional families TbPDE3 and TbPDE4 (S. Templer and S. Kunz, personal communication). TbPDE1 was recently characterized (S. Kunz, T. Kloeckner, B. Bieger, L. O. Essen, T.S., and M. Boshart, unpublished results), but its biological function still remains unclear (21). The current study demonstrates that the TbPDE2 family, and particularly TbPDE2C, are essential for the proliferation of bloodstream form trypanosomes.
Eliminating the mRNAs of either individual family members or of the entire TbPDE2 family in procyclic trypanosomes generated cells that were able to proliferate, but where a sizeable proportion of the cells exhibited severely distorted shapes and were multinucleated. This phenotype was already seen in the absence of RNAi induction, confirming the reported low level of leakiness of the pZJM vector (35). Induction of RNAi in these cells by tetracycline greatly increased the proportion of odd-shaped cells, although the majority of cells in these clonal populations still retained their normal shape. When the intracellular cAMP concentrations were determined, inactivation of TbPDE2C alone strongly increased the steady-state level of intracellular cAMP. This increase was even more marked when the entire TbPDE2 family was inactivated. The relative significance of the three family members analyzed could not be assessed unambiguously because their respective mRNAs were eliminated by RNAi with very different efficiencies. This marked difference was consistently observed and suggests a degree of sequence selectivity by the RNAi mechanism of T. brucei. The finding that elimination of the TbPDE2C mRNA alone produced a phenotype that was almost as strong as that produced by the elimination of the entire family indicated that TbPDE2C is probably the most important family member in procyclics, at least as far as the total PDE activity and the control of intracellular cAMP levels is concerned. The long-term increase in cAMP that results from the inactivation of the TbPDE2 family leads to a disruption of cytokinesis and cell division in many but not all of the cells of a given population. Such populations still exhibited exponential growth, although with increased population doubling times.
In contrast to the situation in procyclic forms, the members of the TbPDE2 family were found to be essential for bloodstream form trypanosome proliferation. Heterozygous knock-outs of TbPDE2A of bloodstream forms exhibit marked haploid insufficiency, and the failure to obtain homozygous knockouts that grew for more than 2 or 3 weeks demonstrated the importance of this enzyme for bloodstream form proliferation. Similarly, bloodstream form trypanosomes were extremely sensitive to the effect of RNAi, even without induction of double-strand RNA synthesis. This finding indicated that even the low levels of double-strand RNA that are produced in uninduced cells because of the slight leakiness of the repressor (35) are sufficient to generate a lethal phenotype. The absence of TbPDE2 activity could also be mimicked by membrane-permeable cAMP analogs. The IC50s of these compounds corresponded to about a 10-fold level of physiological cAMP concentrations in trypanosomes. These results demonstrated that bloodstream form trypanosomes are exquisitely sensitive to an elevation of intracellular cAMP levels beyond a narrowly defined physiological level, and they indicate that the phenotype produced by inactivation of the TbPDE2 family is due to the elevation of intracellular cAMP. This sensitivity to increased cAMP levels is reminiscent of what has been reported in mammalian cells where excessive levels of cAMP can induce apoptosis and where cAMP is involved in the control of tumor cell proliferation (37).
This study demonstrates that the TbPDE2 family, and particularly TbPDE2C, are essential for the proliferation of bloodstream form T. brucei. This finding demonstrates an essential role of cAMP signaling for the proliferation in a protozoal pathogen. The TbPDE2 family, and particularly TbPDE2C, may represent a promising target for the development of PDE inhibitors as a new generation of trypanocidal drugs.
Acknowledgments
We thank G. A. M. Cross (The Rockefeller University) for generously providing his trypanosome strains 29-13 and 13-90, P. Englund (Johns Hopkins University) for the RNAi vector pZJM, Ana Rascón for communicating unpublished data, Min Ku for her careful editing of the text, and Isabel Roditi for many helpful discussions. This work was supported by Grant 31-058927.99 of the Swiss National Science Foundation, Grant C98.0060 of Cooperation in Science and Technology program B9 of the European Union, and by the United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases. R.Z. was the recipient of a fellowship of the Ministry of Culture and Higher Education of the Islamic Republic of Iran.
Abbreviations
- PDE
cyclic nucleotide specific phosphodiesterase
- RNAi
RNA interference
- RT-PCR
reverse transcription–PCR
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY028446).
References
- 1.Boekhoff I, Michel W C, Breer H, Ache B W. J Neurosci. 1994;14:3304–3309. doi: 10.1523/JNEUROSCI.14-05-03304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hempel C M, Vincent P, Adams S R, Tsien R Y, Selverston A I. Nature (London) 1996;384:166–169. doi: 10.1038/384166a0. [DOI] [PubMed] [Google Scholar]
- 3.Ma P, Wera S, VanDijk P, Thevelein J M. Mol Biol Cell. 1999;10:91–104. doi: 10.1091/mbc.10.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Secchiero P, Zella D, Curreli S, Mirandola P, Capitani S, Gallo R C, Zauli G. Proc Natl Acad Sci USA. 2000;97:14620–14625. doi: 10.1073/pnas.011512398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson-Jones S M, Entz D D, Sargan D R. Invest Ophthalmol Vis Sci. 1999;40:1637–1644. [PubMed] [Google Scholar]
- 6.Strettoi E, Pignatelli V. Proc Natl Acad Sci USA. 2000;97:11020–11025. doi: 10.1073/pnas.190291097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikawa J, Sass P, Wigler M. Mol Cell Biol. 1987;7:3629–3636. doi: 10.1128/mcb.7.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sass P, Field P, Nikawa J, Toda T, Wigler M. Proc Natl Acad Sci USA. 1986;83:9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis S H, Turko I V, Corbin J D. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 10.Soderling S H, Beavo J A. Curr Opin Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 11.Beavo J A. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 12.Bielekova B, Lincoln A, McFarland H, Martin R. J Immunol. 2000;164:1117–1124. doi: 10.4049/jimmunol.164.2.1117. [DOI] [PubMed] [Google Scholar]
- 13.Kiely P D, Gillespie K M, Oliveira D B. Eur J Immunol. 1995;25:2899–2906. doi: 10.1002/eji.1830251029. [DOI] [PubMed] [Google Scholar]
- 14.Barnette M S. Prog Drug Res. 1999;53:193–229. doi: 10.1007/978-3-0348-8735-9_5. [DOI] [PubMed] [Google Scholar]
- 15.Barnes P J. Novartis Found Symp. 2001;234:255–267. [PubMed] [Google Scholar]
- 16.Langtry H D, Markham A. Drugs. 1999;57:967–989. doi: 10.2165/00003495-199957060-00015. [DOI] [PubMed] [Google Scholar]
- 17.Marko D, Pahlke G, Merz K H, Eisenbrand G. Chem Res Toxicol. 2000;10:944–948. doi: 10.1021/tx000090l. [DOI] [PubMed] [Google Scholar]
- 18.Seebeck T, Naula C, Shalaby T, Gong K W. Nova Acta Leopold. 1999;307:227–241. [Google Scholar]
- 19.Keiser J, Stich A, Burri C. Trends Parasitol. 2001;17:42–49. doi: 10.1016/s1471-4922(00)01829-8. [DOI] [PubMed] [Google Scholar]
- 20.Naula C, Seebeck T. Parasitol Today. 2000;16:39–42. doi: 10.1016/s0169-4758(99)01582-3. [DOI] [PubMed] [Google Scholar]
- 21.Gong K W, Kunz S, Zoraghi R, Kunz Renggli C, Brun R, Seebeck T. Mol Biochem Parasitol. 2001;116:229–232. doi: 10.1016/s0166-6851(01)00315-2. [DOI] [PubMed] [Google Scholar]
- 22.Zoraghi R, Kunz S, Gong K, Seebeck T. J Biol Chem. 2001;276:11559–11566. doi: 10.1074/jbc.M005419200. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Morris J C, Drew M E, Englund P T. J Biol Chem. 2000;275:40174–40179. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- 24.Wirtz E, Leal S, Ochatt C, Cross G A M. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 25.Brun R, Schönenberger M. Acta Trop. 1979;36:289–292. [PubMed] [Google Scholar]
- 26.Hesse F, Selzer P M, Mühlstadt K, Duszenko M. Mol Biochem Parasitol. 1995;70:157–166. doi: 10.1016/0166-6851(95)00027-x. [DOI] [PubMed] [Google Scholar]
- 27.Räz B, Iten M, Grether-Bühler Y, Kaminsky R, Brun R. Acta Trop. 1997;68:139–147. doi: 10.1016/s0001-706x(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 28.Schilling R J, Morgan D R, Kilpatrick B F. Anal Biochem. 1994;216:154–158. doi: 10.1006/abio.1994.1019. [DOI] [PubMed] [Google Scholar]
- 29.Sutton R E, Boothroyd J C. Mol Cell Biol. 1988;8:494–496. doi: 10.1128/mcb.8.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho Y S, Burden L M, Hurley J H. EMBO J. 2000;19:5288–5299. doi: 10.1093/emboj/19.20.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAllister-Lucas L M, Sonnenburg W K, Kadlecek A, Seger D, Trong H L, Colbran J L, Thomas M K, Walsh K A, Francis S H, Corbin J D, et al. J Biol Chem. 1993;268:22863–22873. [PubMed] [Google Scholar]
- 32.McAllister-Lucas L M, Haik T L, Colbran J L, Sonneburg W K, Seger D, Turko I V, Beavo J A, Francis S H, Corbin J D. J Biol Chem. 1995;270:30671–30679. doi: 10.1074/jbc.270.51.30671. [DOI] [PubMed] [Google Scholar]
- 33.Pillai R, Kytle K, Reyes A, Colicelli J. Proc Natl Acad Sci USA. 1993;90:11970–11974. doi: 10.1073/pnas.90.24.11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumberg D, Muller R, Funk M. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 35.Morris J C, Wang Z, Drew M E, Paul K S, Englund P T. Mol Biochem Parasitol. 2001;117:111–113. doi: 10.1016/s0166-6851(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 36.Vassella E, Reuner B, Yuzi B, Boshart M. J Cell Sci. 1997;110:2661–2671. doi: 10.1242/jcs.110.21.2661. [DOI] [PubMed] [Google Scholar]
- 37.Lerner A, Kim D H, Lee R. Leuk Lymphoma. 2000;37:39–51. doi: 10.3109/10428190009057627. [DOI] [PubMed] [Google Scholar]