Abstract
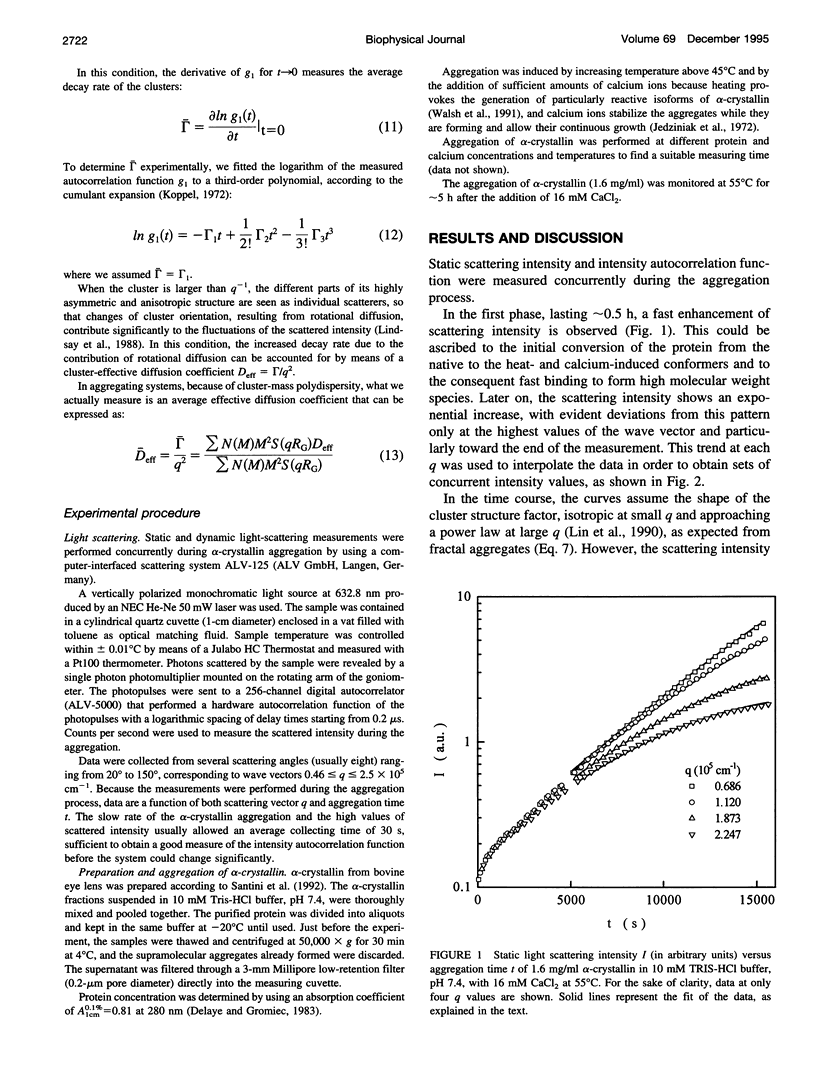
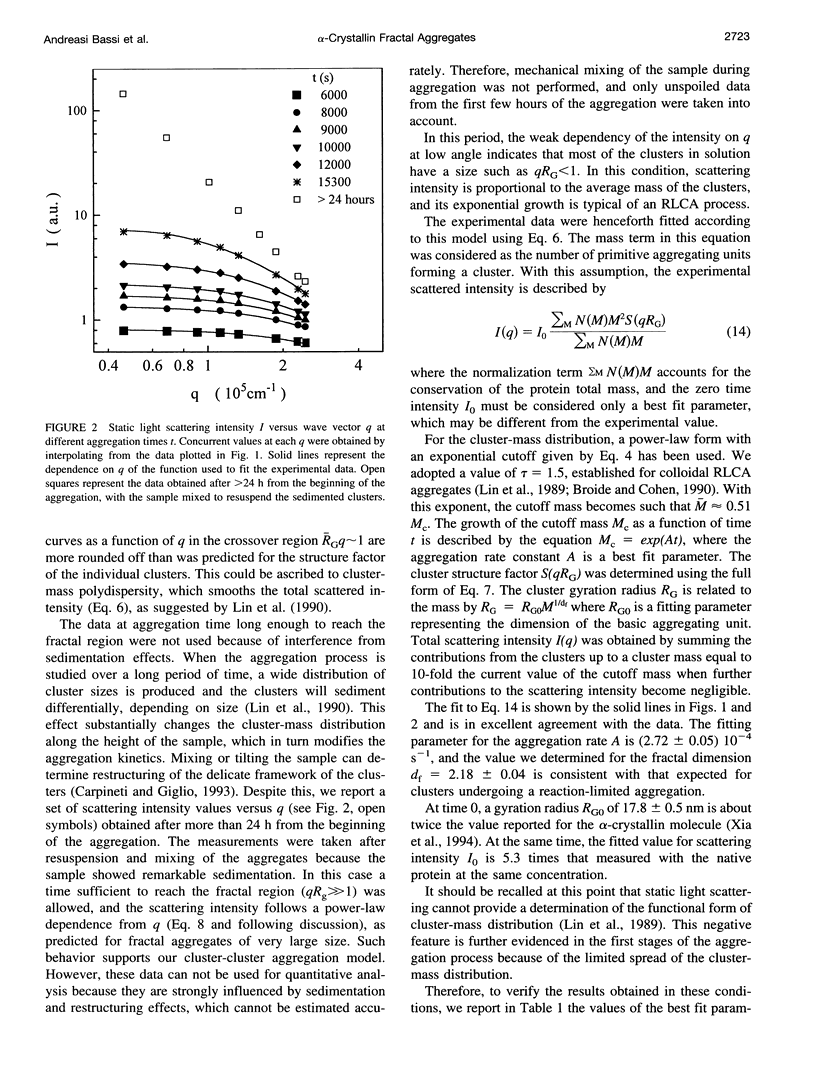
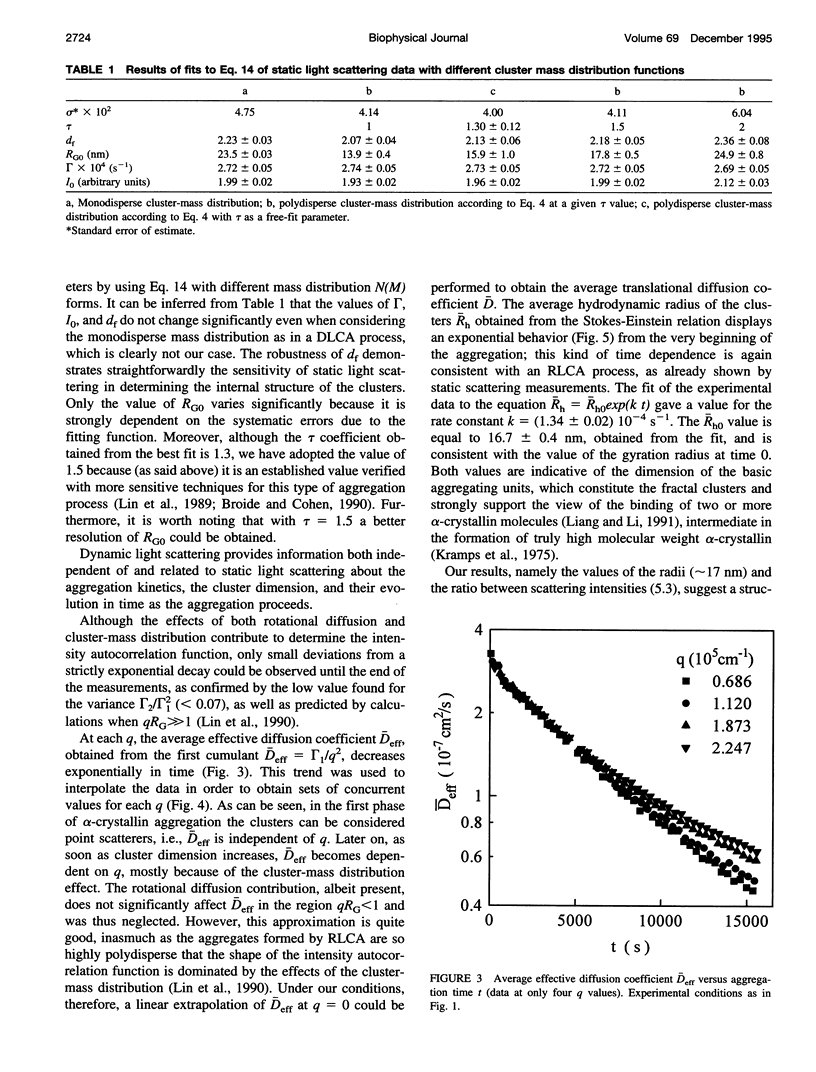
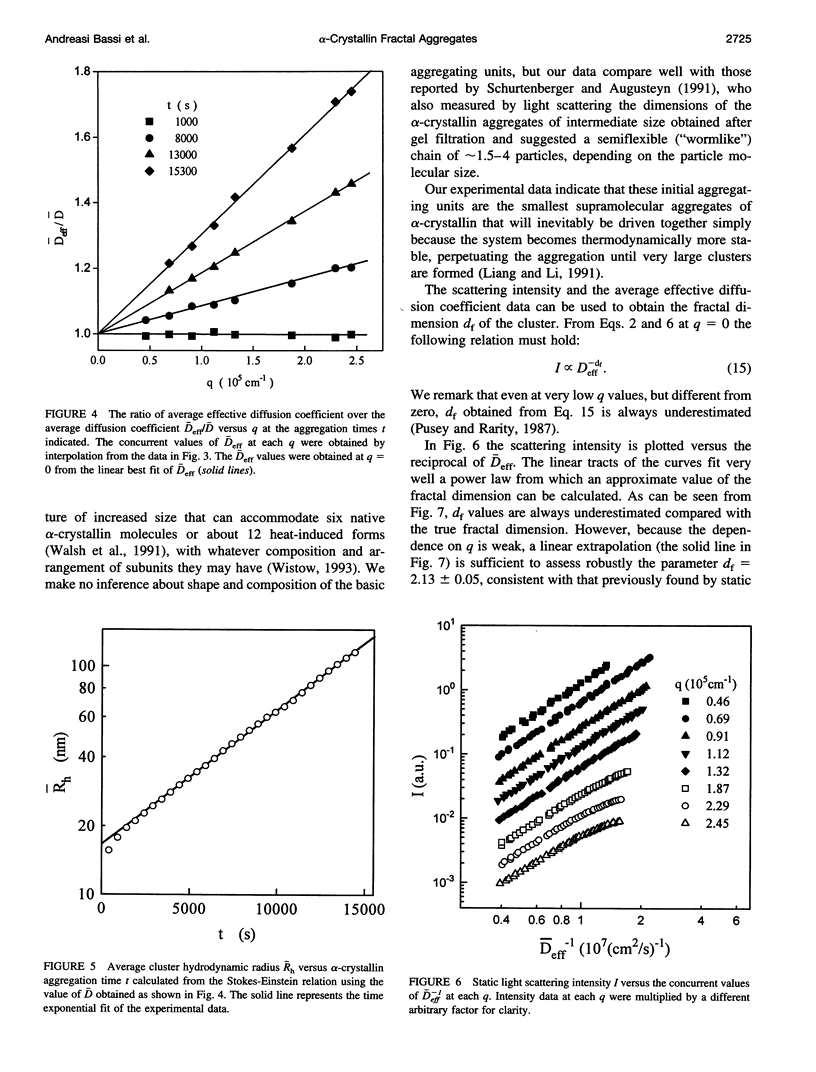
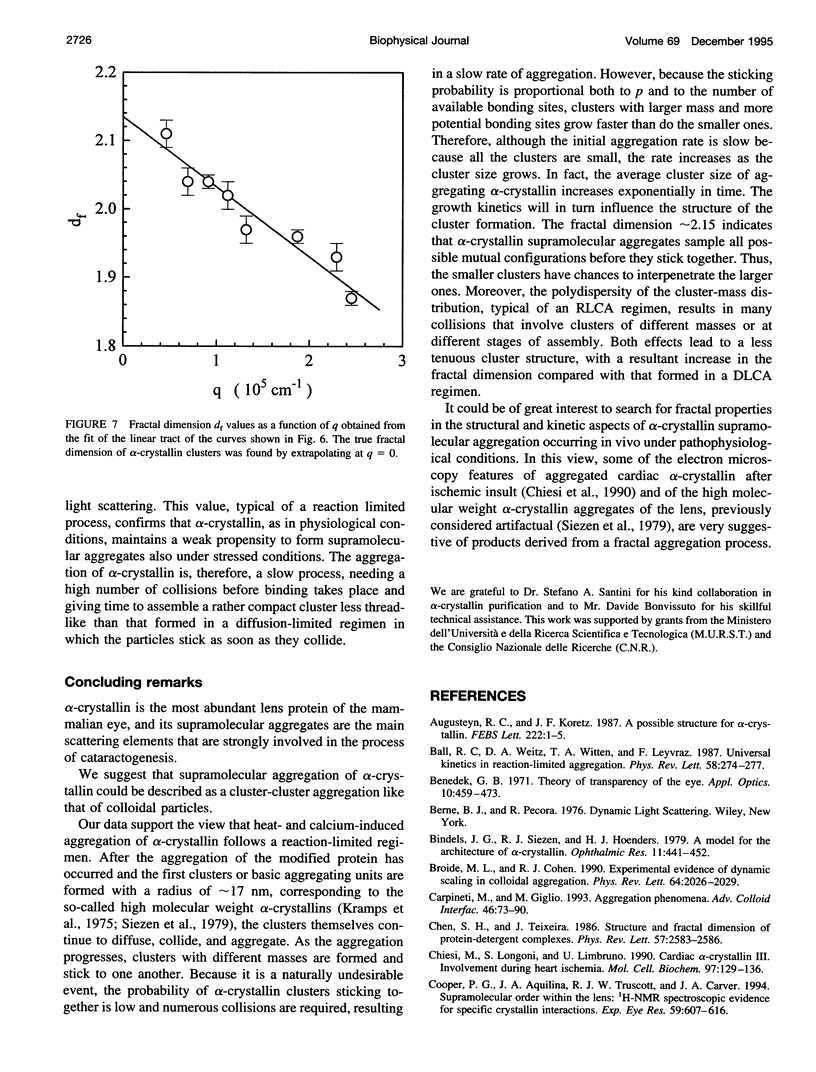
The supramolecular aggregation of alpha-crystallin, the major protein of the eye lens, was investigated by means of static and dynamic light scattering. The aggregation was induced by generating heat-modified alpha-crystallin forms and by stabilizing the clusters with calcium ions. The kinetic pattern of the aggregation and the structural features of the clusters can be described according to the reaction limited cluster-cluster aggregation theory previously adopted for the study of colloidal particles aggregation systems. Accordingly, the average mass and the hydrodynamic radius of alpha-crystallin supramolecular aggregates grow exponentially in time. The structure factor of the clusters is typical of fractal aggregates. A fractal dimension df approximately 2.15 was determined, indicating a low probability of sticking together of the primitive aggregating particles. As a consequence, the slow-forming clusters assemble a rather compact structure. The basic units forming the fractal aggregates were found to have a radius about twice (approximately 17 nm) that of the native protein and 5.3 times its size, which is consistent with an intermediate molecular assembly corresponding to the already known high molecular weight forms of alpha-crystallin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusteyn R. C., Koretz J. F. A possible structure for alpha-crystallin. FEBS Lett. 1987 Sep 28;222(1):1–5. doi: 10.1016/0014-5793(87)80180-1. [DOI] [PubMed] [Google Scholar]
- Ball RC, Weitz DA, Witten TA, Leyvraz F. Universal kinetics in reaction-limited aggregation. Phys Rev Lett. 1987 Jan 19;58(3):274–277. doi: 10.1103/PhysRevLett.58.274. [DOI] [PubMed] [Google Scholar]
- Broide ML, Cohen RJ. Experimental evidence of dynamic scaling in colloidal aggregation. Phys Rev Lett. 1990 Apr 23;64(17):2026–2029. doi: 10.1103/PhysRevLett.64.2026. [DOI] [PubMed] [Google Scholar]
- Chen SH, Teixeira J. Structure and fractal dimension of protein-detergent complexes. Phys Rev Lett. 1986 Nov 17;57(20):2583–2586. doi: 10.1103/PhysRevLett.57.2583. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Longoni S., Limbruno U. Cardiac alpha-crystallin. III. Involvement during heart ischemia. Mol Cell Biochem. 1990 Sep 21;97(2):129–136. doi: 10.1007/BF00221054. [DOI] [PubMed] [Google Scholar]
- Cooper P. G., Aquilina J. A., Truscott R. J., Carver J. A. Supramolecular order within the lens: 1H NMR spectroscopic evidence for specific crystallin-crystallin interactions. Exp Eye Res. 1994 Nov;59(5):607–616. doi: 10.1006/exer.1994.1146. [DOI] [PubMed] [Google Scholar]
- Delaye M., Clark J. I., Benedek G. B. Identification of the scattering elements responsible for lens opacification in cold cataracts. Biophys J. 1982 Mar;37(3):647–656. [PMC free article] [PubMed] [Google Scholar]
- Delaye M., Gromiec A. Mutual diffusion of crystallin proteins at finite concentrations: a light-scattering study. Biopolymers. 1983 Apr;22(4):1203–1221. doi: 10.1002/bip.360220413. [DOI] [PubMed] [Google Scholar]
- Garland D., Zigler J. S., Jr, Kinoshita J. Structural changes in bovine lens crystallins induced by ascorbate, metal, and oxygen. Arch Biochem Biophys. 1986 Dec;251(2):771–776. doi: 10.1016/0003-9861(86)90389-9. [DOI] [PubMed] [Google Scholar]
- Guptasarma P., Balasubramanian D., Matsugo S., Saito I. Hydroxyl radical mediated damage to proteins, with special reference to the crystallins. Biochemistry. 1992 May 5;31(17):4296–4303. doi: 10.1021/bi00132a021. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Kinoshita J. H., Yates E. M., Hocker L. O., Benedek G. B. Calcium-induced aggregation of bovine lens alpha crystallins. Invest Ophthalmol. 1972 Nov;11(11):905–915. [PubMed] [Google Scholar]
- Jedziniak J. A., Nicoli D. F., Baram H., Benedek G. B. Quantitative verification of the existence of high molecular weight protein aggregates in the intact normal human lens by light-scattering spectroscopy. Invest Ophthalmol Vis Sci. 1978 Jan;17(1):51–57. [PubMed] [Google Scholar]
- Kramps H. A., Stols A. L., Hoenders H. J. On the quaternary structure of high-molecular-weight proteins from the bovine eye lens. Eur J Biochem. 1975 Jan 15;50(3):503–509. doi: 10.1111/j.1432-1033.1975.tb09889.x. [DOI] [PubMed] [Google Scholar]
- Latina M., Chylack L. T., Jr, Fagerholm P., Nishio I., Tanaka T., Palmquist B. M. Dynamic light scattering in the intact rabbit lens. Its relation to protein concentration. Invest Ophthalmol Vis Sci. 1987 Jan;28(1):175–183. [PubMed] [Google Scholar]
- Liang J. N., Li X. Y. Interaction and aggregation of lens crystallins. Exp Eye Res. 1991 Jul;53(1):61–66. doi: 10.1016/0014-4835(91)90145-5. [DOI] [PubMed] [Google Scholar]
- Lin MY, Lindsay HM, Weitz DA, Ball RC, Klein R, Meakin P. Universal reaction-limited colloid aggregation. Phys Rev A. 1990 Feb 15;41(4):2005–2020. doi: 10.1103/physreva.41.2005. [DOI] [PubMed] [Google Scholar]
- Lindsay HM, Klein R, Weitz DA, Lin MY, Meakin P. Effect of rotational diffusion on quasielastic light scattering from fractal colloid aggregates. Phys Rev A Gen Phys. 1988 Sep 1;38(5):2614–2626. doi: 10.1103/physreva.38.2614. [DOI] [PubMed] [Google Scholar]
- Luthra M., Balasubramanian D. Nonenzymatic glycation alters protein structure and stability. A study of two eye lens crystallins. J Biol Chem. 1993 Aug 25;268(24):18119–18127. [PubMed] [Google Scholar]
- Miesbauer L. R., Zhou X., Yang Z., Yang Z., Sun Y., Smith D. L., Smith J. B. Post-translational modifications of water-soluble human lens crystallins from young adults. J Biol Chem. 1994 Apr 29;269(17):12494–12502. [PubMed] [Google Scholar]
- Santini S. A., Mordente A., Meucci E., Miggiano G. A., Martorana G. E. Conformational stability of bovine alpha-crystallin. Evidence for a destabilizing effect of ascorbate. Biochem J. 1992 Oct 1;287(Pt 1):107–112. doi: 10.1042/bj2870107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurtenberger P., Augusteyn R. C. Structural properties of polydisperse biopolymer solutions: a light scattering study of bovine alpha-crystallin. Biopolymers. 1991 Sep;31(10):1229–1240. doi: 10.1002/bip.360311011. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Bindels J. G., Hoenders H. J. The interrelationship between monomeric, oligomeric and polymeric alpha-crystallin in the calf lens nucleus. Exp Eye Res. 1979 May;28(5):551–567. doi: 10.1016/0014-4835(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Srivastava O. P. Age-related increase in concentration and aggregation of degraded polypeptides in human lenses. Exp Eye Res. 1988 Oct;47(4):525–543. doi: 10.1016/0014-4835(88)90092-9. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Laporte D., Licinio P., Krop B., Delaye M. Calf lens alpha-crystallin quaternary structure. A three-layer tetrahedral model. J Mol Biol. 1986 Dec 20;192(4):711–724. doi: 10.1016/0022-2836(86)90023-9. [DOI] [PubMed] [Google Scholar]
- Walsh M. T., Sen A. C., Chakrabarti B. Micellar subunit assembly in a three-layer model of oligomeric alpha-crystallin. J Biol Chem. 1991 Oct 25;266(30):20079–20084. [PubMed] [Google Scholar]
- Wistow G. Possible tetramer-based quaternary structure for alpha-crystallins and small heat shock proteins. Exp Eye Res. 1993 Jun;56(6):729–732. doi: 10.1006/exer.1993.1090. [DOI] [PubMed] [Google Scholar]
- Xia J. Z., Aerts T., Donceel K., Clauwaert J. Light scattering by bovine alpha-crystallin proteins in solution: hydrodynamic structure and interparticle interaction. Biophys J. 1994 Mar;66(3 Pt 1):861–872. doi: 10.1016/s0006-3495(94)80862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


