Abstract
Although standard anticancer chemotherapeutic drugs have been designed to inhibit the survival or growth of rapidly dividing tumor cells, it is possible to enhance the efficacy of such drugs by targeting the proliferating host endothelial cells (ECs) of the tumor vasculature. A theoretical advantage of this strategy lies in the possibility of circumventing, or significantly delaying, acquired drug resistance driven by the genetic instability of tumor cells. Here, we show that both vascular endothelial growth factor (VEGF) and basic fibroblast growth factor significantly reduce the pro-apoptotic potency of chemotherapy on both micro- and macrovascular ECs. This cytoprotection to drug toxicity was found to be phosphatidylinositol 3-kinase-dependent and could be recapitulated in the absence of VEGF by overexpressing the dominant-active form of the serine/threonine kinase protein kinase B/Akt. Downstream of phosphatidylinositol 3-kinase, we also show that survivin plays a pivotal role in VEGF-mediated EC protection by preserving the microtubule network. In this respect, its induction effectively protects ECs against chemotherapeutic damage, whereas overexpression of its dominant-interfering mutant (C84A) abrogates the protective effects of VEGF. Accordingly, the potency of VEGF as a chemoprotectant was more pronounced with drugs that interfere with microtubule dynamics than those that damage DNA. These studies implicate a role for survivin up-regulation as a novel mechanism of EC drug “resistance” and support the notion that angiogenic factors that induce the expression of survivin may act to shield tumor ECs from the apoptotic effects of chemotherapy. Thus, exploiting chemotherapeutic drugs as antiangiogenics is likely to be compromised by the high concentrations of proangiogenic survival/growth factors present in the tumor microenvironment; targeting EC survival pathways should improve the antiangiogenic efficacy of antineoplastic agents, particularly microtubule-inhibitor drugs.
In addition to specific antiangiogenic agents, virtually every conventional cytotoxic anticancer drug has been “accidentally” discovered to have antiangiogenic effects in various in vivo models (1, 2). Recently, Miller et al. (2) have discussed the notion of “redefining the target” of such drugs to the activated, proliferating endothelial cell (EC) of a tumor's newly forming vasculature. Interest in exploiting chemotherapeutics as antiangiogenics has been stimulated particularly by reports showing that frequent administration of low doses of various chemotherapeutic drugs (3, 4) called “metronomic dosing” (5) or “antiangiogenic chemotherapy” (3) can target the tumor vasculature with limited host toxicity. This theory is based on the rationale that damaging side effects to tumor ECs associated with standard maximum tolerated doses are likely to be repaired during rest periods between successive cycles of such therapy (3). Furthermore, tumor-associated ECs should lack the extensive genetic instabilities of tumor cells that are responsible for the emergence of heritable drug-resistant mutants (6). As such, targeting the tumor vasculature might circumvent or at least delay acquired drug resistance.
However, in addition to the possibility of tumor cell genetic mutations that might lead to gradual development of resistance to antiangiogenic drugs (7), the tumor microenvironment may induce alternative, epigenetic-based protective mechanisms, which may lead to a gradual loss of response of activated ECs to chemotherapy (2, 4, 8). In this regard, various angiogenic growth factors such as vascular endothelial growth factor (VEGF) (9), basic fibroblast growth factor (bFGF) (10), and angiopoietin 1 (11) promote survival of ECs, thereby lowering their threshold levels of susceptibility to apoptosis. Given the constant high concentrations of VEGF frequently found in tumors, VEGF could function in vivo as a potent antagonist of activated EC death induced by chemotherapy (8). It thus is not surprising that the antiangiogenic and antitumor effects of low-dose metronomic vinblastine therapy are lost eventually but can be enhanced significantly and sustained by concurrent administration of a VEGF receptor (VEGF-R) 2 (flk-1/KDR/VEGF-R2) neutralizing antibody, in a human tumor xenograft model (4).
The role of VEGF as an EC-specific survival factor raises questions regarding the intracellular mechanisms involved. In this regard, VEGF binding to VEGF-R2 activates the phosphatidylinositol 3-kinase (PI3K) survival pathway, resulting in phosphorylation and activation of the serine/threonine kinase protein kinase B (PKB/Akt) (12). VEGF also induces the expression of several anti-apoptotic effector molecules in ECs, including bcl-2 (13, 14), A1 (13), and two members of the inhibitor of apoptosis (IAP) family, X-linked IAP (XIAP), and survivin (15–17).
The purpose of the present report was to determine whether angiogenic growth factors, such as VEGF, can activate and consequently protect ECs against chemotherapeutic injury and, if so, to dissect the molecular mechanisms underlying this cytoprotection. We show that VEGF, particularly in combination with bFGF, acts as a potent EC chemoprotectant after the activation of PI3K and PKB and subsequent induction in survivin expression. We demonstrate further that both VEGF addition and survivin overexpression can rescue ECs from drug-induced cell death by preserving the microtubule network. As such, survivin induction by VEGF may ensure the integrity of microtubule dynamics, which culminates in chemoprotection of ECs. These results strengthen the rationale for targeting EC survival pathways to enhance the antiangiogenic potency of standard chemotherapeutic drugs.
Materials and Methods
Cell Lines.
Human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HDMECs) (Clonetics) were used between passages 2 and 6 and were maintained as described (15). Phoenix cells (a gift from Gary Nolan, Stanford University, Stanford, CA) were grown in DMEM with 10% FBS. WM1341B and the VEGF165-transfected WM1341B (165–24) have been described (18). Conditioned medium from both cell lines was collected after 24 h of incubation.
Retroviral Infection.
Human survivin (a gift from Eric Lacasse, Aegera Oncology, Ottawa) and its dominant-interfering mutant (C84A) (a gift from Dario Altieri, Yale University, New Haven, CT) in the retroviral vector pLCN3 and the dominant-active mutant of PKB/Akt (DA PKB) in the retroviral vector pBABE (a gift from J. Woodgett, Ontario Cancer Institute, Toronto) each were transfected into Phoenix cells with Lipofectamine 2000 (GIBCO) per the manufacturer's instructions. Medium from transfected Phoenix cells containing infectious retrovirus was collected after 48 h, filtered, and used to infect HUVECs for 16 h in the presence of Polybrene (Sigma). Infected HUVECs were grown for an additional 24 h in standard medium and treated as described below.
EC Stimulations.
ECs were stimulated with or without 50 ng/ml VEGF (R&D Systems) or 1 ng/ml bFGF with 10 units/ml heparin for 24 h, with or without 10 μM LY294002 (Sigma) and either one of the following drugs: 1 ng/ml adriamycin (ADR) (Amersham Pharmacia), 20 μM cisplatinum (CDDP) (Faulding, Montreal), 10 nM Taxol (Abbott), or 1 ng/ml vinblastine (VBL) sulfate (Calbiochem).
Apoptosis Measurements.
Cell Death Detection ELISA Plus Kit (Roche, Gipf-Oberfrick, Switzerland) was used to quantify apoptosis as described (15). All raw values were plotted as a percentage of apoptosis relative to untreated cells (no VEGF, no drug), which were labeled as 100% ± SD. Student's t test was used to test the difference in means, and the statistical significance was determined with a 95% confidence interval. All experiments were repeated three times with at least two replicates per sample. All graphs shown are representative of a single experiment, with all experiments displaying similar values.
Antibodies.
Antibodies used were as follows: rabbit α-survivin antibody (Alpha Diagnostics, San Antonio, TX), rabbit α-phosphoserine 473 Akt (NEB, Beverly, MA), α-panAkt (NEB), and anti-β-tubulin (Sigma).
Immunohistochemical Staining.
HUVECs grown on slides were permeabilized in methanol, fixed in acetone, and incubated with anti-β-tubulin for 1 h followed by a secondary α-mouse FITC-labeled antibody incubation (The Jackson Laboratory). Images were captured at ×630 magnification by confocal microscopy (Zeiss).
Results
VEGF and bFGF Rescue ECs from Cell Death Induced by Chemotherapy.
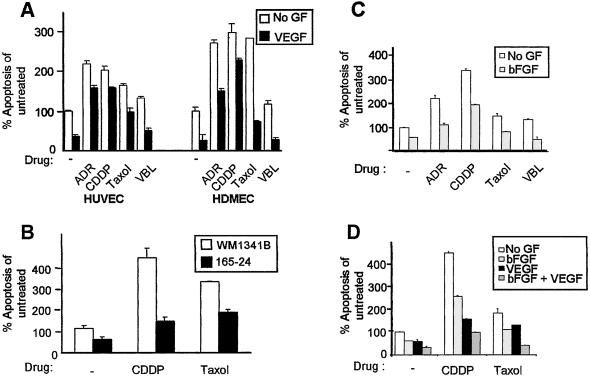
Because the majority of chemotherapeutic drugs cause apoptosis, we set out to determine whether the angiogenic growth factor VEGF could reduce EC drug toxicity induced by chemotherapy. Primary macrovascular HUVECs were treated with one of four chemotherapeutic agents—ADR, CDDP, Taxol, or VBL—in the presence or absence of VEGF. Whereas ADR and CDDP are DNA-damaging agents, Taxol and VBL interfere with microtubule dynamics. As shown previously (15), the addition of VEGF significantly lowered apoptotic levels by 60% as compared with ECs grown in growth factor-deprived conditions (Fig. 1A). The addition of various drugs in growth factor-deprived conditions increased apoptosis in HUVECs whereas addition of VEGF significantly lowered apoptosis (50–160%) at 24 (Fig. 1A) and 48 h (data not shown). Microvascular HDMECs behaved similarly to HUVECs and were less refractory to chemotherapy-induced cell death in the presence of VEGF (Fig. 1A). In addition, we have confirmed our results by staining with Annexin V for at least two of the chemotherapeutic drugs (data not shown). It is interesting to note that chemoprotection by VEGF was more dramatic in ECs treated with microtubule-interfering drugs (Taxol and VBL) than those that damage DNA (ADR and CDDP). These results demonstrate that the presence of VEGF can compromise the cytotoxic potency of chemotherapy on both macro- and microvascular ECs.
Figure 1.
VEGF and bFGF rescue ECs from chemotherapeutic drug-induced apoptosis. (A) HUVECs and HDMECs in the absence of drug or VEGF (untreated) were normalized to 100% apoptosis. The addition of VEGF lowered apoptosis of ECs by 60% relative to untreated cells. Treatment with ADR, CDDP, Taxol, or VBL significantly increased percentage of apoptosis of ECs above untreated samples, which is reversed by the addition of VEGF by 50–75%. (B) HUVECs were grown in conditioned medium collected from either the parental WM1341B or WM1341B expressing VEGF165 (165–24) either with or without CDDP or Taxol. Conditioned medium from 165–24 significantly reduced cell death of HUVECs treated with either CDDP or Taxol as compared with cells grown in WM1341B-conditioned medium. (C) HUVECs were stimulated with or without bFGF either in the presence of absence of drug. Stimulation of cells with bFGF decreased apoptosis caused by growth factor deprivation by 50%. Cells stimulated with bFGF in the presence of drug also lowered apoptosis levels. (D) HUVECs were stimulated with or without bFGF and/or VEGF either in the presence or absence of CDDP or Taxol. Stimulation of cells with bFGF decreased apoptosis caused by growth factor deprivation or by drug treatment, and the combination of bFGF and VEGF displayed an enhanced chemoprotective effect superior to treatments with either growth factor alone.
We have shown previously that the nontumorigenic human melanoma cell line WM1341B acquires the ability to form tumors in vivo after transfection with VEGF165 (referred to as 165–24) (24). HUVECs grown in conditioned medium collected from WM1341B displayed more cell death than those grown in 165–24 (Fig. 1B). Similar to recombinant VEGF, conditioned medium from 165–24 dramatically reduced cell death of HUVECs treated with CDDP or Taxol, as compared with cells treated in condition medium of the parental line WM1341B. As such, secretion of VEGF, and possibly other growth factors, from an in vivo tumorigenic cell line can rescue ECs from chemotherapy-induced death.
To test whether other angiogenic growth factors such as bFGF also can protect ECs from chemotherapy, HUVECs were treated with or without drug and/or bFGF. bFGF reduced cytotoxicity of HUVECs to all drugs (Fig. 1C), and, moreover, treatment of HUVECs with both bFGF and VEGF resulted in an additive protection against chemotherapy (Fig. 1D). These results suggest that angiogenic survival factors such as VEGF and bFGF can solely and additively protect ECs from chemotherapy by inducing drug resistance epigenetically.
VEGF-Mediated Chemoprotection Requires Activation of PI3K and PKB.
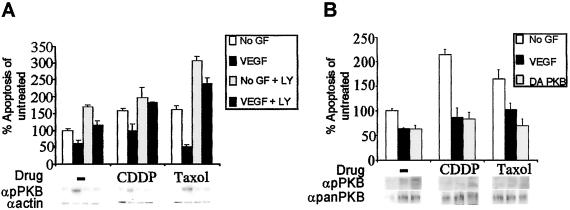
To dissect the molecular mechanism underlying EC chemoprotection, we chose VEGF as a prototypical survival factor, and two drugs with two different modes of action (Taxol and CDDP) were used. Activation of PI3K and PKB has been shown previously to mediate cell survival downstream of many growth factor receptors (19). To assess whether VEGF-dependent EC chemoprotection depends on this pathway, HUVECs were treated with or without the PI3-kinase pharmacological inhibitor LY294002 in the absence/presence of VEGF and/or drug. Inhibition of PI3K by LY294002 treatment abrogated VEGF-mediated cytoprotection from growth factor deprivation or chemotherapy (Fig. 2A). The reduction of VEGF-mediated chemoprotection because of the lack of PI3K activity was correlated by a substantial reduction in serine phosphorylation of PKB (Fig. 2A). To ensure that PKB activation was required for VEGF-induced chemoresistance, we infected HUVECs with a retrovirus expressing DA PKB, which resulted in constitutive serine phosphorylation (Fig. 2B). In the absence of VEGF, overexpression of DA PKB rescued ECs from CDDP- and Taxol-induced apoptosis to levels comparable to HUVECs treated with drug in the presence of VEGF (Fig. 2B). Therefore, activation of PI3K and PKB is critical for VEGF to mediate EC chemoprotection.
Figure 2.
Activation of PI3K and PKB is required for VEGF-mediated chemoprotection. (A) HUVECs cultured in the presence/absence of VEGF and with or without drug were treated with LY294002. In the absence LY294002, VEGF rescued cell death as indicated above; however, addition of LY294002 abolished VEGF-mediated survival of HUVECs in growth factor-deprived conditions or in conditions in which chemotherapeutic drugs were added. Protein lysates prepared from HUVECs treated above were assessed for serine-473 phosphorylation on activated PKB (αpPKB). HUVECs treated with LY294002 demonstrated a dramatic reduction in serine phosphorylation irrespective of VEGF addition. Reprobing with α-actin antibodies revealed equal loading of cell lysates. (B) HUVECs retrovirally infected with DA PKB were grown with or without CDDP or Taxol in the absence of VEGF. Levels of apoptosis in each of these treatments were compared with corresponding treatments of cells infected with control vector virus. Overexpression of the DA PKB in the absence of VEGF was able to fully rescue ECs from apoptosis in the presence of either drug to levels of cells infected with empty vector with VEGF. Immunoblotting with αpPKB antibodies showed that HUVECs treated with LY294002 exhibit a dramatic reduction in serine-473 phosphorylation regardless of the addition of VEGF. Reprobing with α-panPKB antibodies revealed equal loading of cell lysates.
VEGF-Driven Chemoprotection of ECs Requires PI3K-Dependent Induction of Survivin.
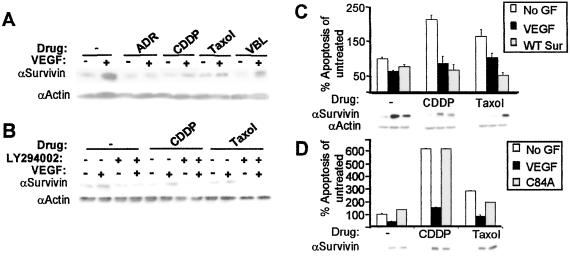
We and others have demonstrated previously that VEGF can protect ECs from apoptosis caused by growth factor deprivation through an induction in the anti-apoptotic protein, survivin (15–17). To demonstrate that VEGF can protect ECs from chemotherapy through survivin induction, we stimulated HUVECs with or without VEGF and/or drug. As shown previously, survivin protein levels were up-regulated 10- to 20-fold in cells stimulated with VEGF (15, 16), and here we show that these levels were maintained further despite the addition of chemotherapy, particularly in the presence of the microtubule-interfering drugs Taxol and VBL (Fig. 3A). Similarly, the addition of bFGF also induced the expression of survivin protein levels, which was maintained upon the addition of chemotherapeutic drugs (data not shown).
Figure 3.
VEGF-mediated induction of PI3-kinase is required for survivin induction and chemoprotection of ECs. (A) Lysates of HUVECs stimulated with or without VEGF either in the presence or absence of drugs were subjected to Western blot analysis by using α-survivin antibodies. A 10- to 20-fold increase in survivin protein resulted from VEGF-treated HUVECs as compared with untreated HUVECs in the absence of drug. Induction in survivin protein levels was maintained by VEGF treatment of HUVEC in the presence of all four drugs. Relative protein loading was determined by immunoblotting with α-actin antibodies to ensure equal loading of lysates. (B) HUVECs were stimulated with/without VEGF, ±LY294002 in the presence or absence of drug. The addition of LY294002 resulted in a decrease in VEGF-mediated survivin protein expression in the absence of drug. The addition of CDDP or Taxol further decreased VEGF-mediated survivin expression in the presence of LY294002. Reprobing the immunoblot with α-actin antibodies revealed equal loading of lysates. (C) Retroviral-mediated overexpression of wild-type human survivin (WT Sur) in HUVECs rescued cells from growth factor-deprived apoptosis in the absence of drug or exogenous VEGF. Survivin overexpression in the absence of VEGF also protected ECs from apoptosis induced by CDDP or Taxol. Levels of survivin protein expression were determined by Western blot analysis by using α-survivin antibodies, which revealed an increase in survivin protein levels by the addition of VEGF or by retroviral infection in HUVEC. (D) Overexpression of the survivin dominant-interfering mutant (C84A) that has no anti-apoptotic properties reversed VEGF-mediated protection of HUVECs to CDDP and Taxol despite elevated levels of survivin protein expression.
To determine whether VEGF-mediated survivin up-regulation requires PI3K activation, we stimulated HUVECs with/without VEGF in the presence/absence of LY294002. PI3K inhibition resulted in the concomitant down-regulation of VEGF-mediated survivin protein expression despite the addition of chemotherapy, demonstrating that PI3K activity is required for VEGF-mediated induction of survivin (Fig. 3B).
To ensure that survivin induction is required to protect ECs from drug-induced cytotoxicity, we overexpressed wild-type human survivin in HUVECs by retroviral infection. Although survivin overexpression induced chemoresistance of ECs in the absence of VEGF (Fig. 3C), overexpression of a dominant-interfering survivin with a point mutation in the BIR domain (C84A), which is devoid of anti-apoptotic properties (20), dramatically reduced VEGF-mediated chemoprotection to both CDDP and Taxol (Fig. 3D). In light of these results, survivin seems to be a rate-limiting regulator of the apoptotic threshold of ECs, thereby increasing their resilience to chemotherapy-induced cell death in the presence of VEGF.
Microtubule Structural Integrity Is Preserved by VEGF-Mediated Survivin Expression.
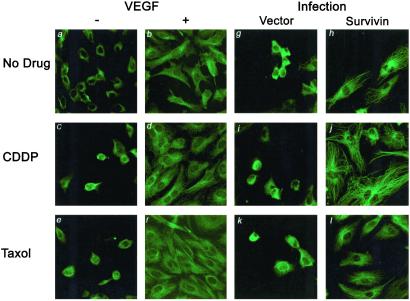
Survivin has been shown previously to colocalize to microtubules at the G2/M checkpoint presumably to stabilize the mitotic spindle (20). To test whether VEGF-mediated cytoprotection through survivin required the maintenance of microtubule integrity, HUVECs treated with chemotherapy were immunostained for β-tubulin. In the absence of both VEGF and drug, cells appeared rounded without any obvious organization of tubulin structures (Fig. 4A). However, in the presence of VEGF and the absence of drug, ECs appeared elongated and spread out (Fig. 4B). Similar to cells growing in the absence of VEGF, HUVECs grown in the presence of drugs also displayed a lack of microtubule organization reflective of cell death (Fig. 4 C and E). In contrast, ECs treated with VEGF in the presence of CDDP and Taxol resumed their healthy-looking appearance with extended and organized tubulin filaments (Fig. 4 D and F). We also show that overexpression of wild-type survivin in the absence of growth factors could preserve the microtubule integrity of HUVECs treated with either drug. Vector-infected HUVECs treated with either CDDP or Taxol displayed disorganized arrangement of the microtubule network (Fig. 4 I and K). Similar to VEGF, overexpression of survivin in HUVECs treated with both CDDP and Taxol maintained their microtubule integrity (Fig. 4 J and L). These results suggest that VEGF signaling through survivin preserves cellular integrity in the presence of chemotherapy by stabilizing the microtubule network.
Figure 4.
Survivin induction by VEGF maintains the integrity of the microtubule network of HUVECs. HUVECs were grown in the presence or absence of VEGF and with or without drug. Immunostaining with anti-β-tubulin antibodies displayed a loss of microtubule organization in VEGF-untreated cells (−) either in the absence of drug (a) or with CDDP (c) and Taxol (e). However, VEGF (+) induces a healthy morphology with HUVECs, appearing elongated and displaying extensive tubulin structures (b, d, and f). HUVECs retrovirally infected with empty vector or wild-type survivin were grown on slides and treated with both CDDP and Taxol in the absence of growth factors. Similar to VEGF, survivin overexpression maintains the organization of microtubule network (h–j), which is not seen in vector-infected cells (g, i, and k).
Our results demonstrate that the presence of angiogenic growth factors such as VEGF and/or bFGF can significantly hamper the efficiency of conventional chemotherapeutic drugs as potent inducers of EC apoptosis. Because the induction of survivin by VEGF-mediated PI3K/PKB activation correlated with a preservation of microtubules, VEGF presumably could protect ECs from chemotherapeutic damage by maintaining their cytoskeletal integrity.
Discussion
Our results implicate VEGF-induced survivin expression as a major drug-resistance mechanism downstream of the PI3K/PKB pathway; activation of this pathway may significantly hamper the ability of chemotherapeutic drugs to damage or kill activated ECs. Furthermore, our results suggest that VEGF signaling through survivin allows for the maintenance of the microtubule network in ECs treated with chemotherapy to preserve cellular integrity. This may explain the limited efficacy of chemotherapeutic drugs as potent vascular targeting agents and the finding that pharmacological blockade of VEGF signaling can significantly enhance the efficacy of chemotherapeutic regimens (4) and radiation (21).
Our results demonstrating that VEGF significantly decreases the sensitivity of micro- and macrovascular ECs to chemotherapy-induced apoptosis support a previous study by Sweeney et al. (8), who reported that the ability of Taxotere to induce EC antiproliferative effects was diminished in vitro by VEGF—an effect that could be reduced by an anti-VEGF neutralizing antibody (8). Because PI3K/PKB activation seems critical for both VEGF- and angiopoietin 1-mediated survival (22, 23), the importance of this pathway in tumor angiogenesis is highlighted further by its pivotal role in inducing chemoresistance of ECs. In our experimental system, VEGF-induced chemoprotection of ECs was associated further with an induction of survivin downstream of PI3K/PKB activation. Our results showing that disruption of survivin is sufficient to counteract the protective effects of VEGF would implicate survivin as a major and critical regulator of EC chemoprotection despite the induction of a number of other survival genes by VEGF. Because survivin is up-regulated similarly in ECs by a number of other angiogenic ligands such as bFGF and angiopoietin 1, it is likely that such factors also could shield ECs from chemotherapeutic damage.
In addition, we show that VEGF or overexpression of survivin results in the organization of tubulin into discrete fibers in ECs despite chemotherapy treatment. In this respect survivin has previously been shown to bind polymerized microtubules in vitro (20), and in fact, analysis of its structure has revealed a putative tubulin-binding domain (24). Furthermore, it has recently been reported that approximately 80% of endogenous survivin exists in the cytosol bound to microtubule structures and that intracellular targeting of survivin with a polyclonal antibody caused significant microtubule perturbations (25). Our results therefore support previous work demonstrating a role for survivin in the maintenance of microtubules within the mitotic spindle (20). Interestingly, recent work has shown that microtubules and microfilaments cooperate to promote EC cell survival in a process dependent on the phosphorylation of PKB (26). In this respect, survivin induction by VEGF after PKB activation and the subsequent maintenance of the microtubule network may be a prerequisite for EC chemoresistance (26). As a result, the role of VEGF as a stabilizer of microtubule dynamics may explain why VEGF is a better antagonist of EC apoptosis caused by microtubule-interfering agents than DNA-damaging drugs (Fig. 1). Indeed, we have shown recently in vivo that tumor regression was more dramatic by combining a VEGF-R2-neutralizing antibody (DC101) with microtubule-interfering drugs than with DNA-damaging drugs (4). Overall, our data seem to suggest that cell survival and cytoskeletal integrity may be more intimately related than previously thought.
In light of these results, the prosurvival role of survivin may be extended to a function in drug resistance in both tumor cells and EC tumor-associated blood vessels. The preferential tumor cell overexpression of survivin raises the intriguing possibility that therapeutically targeting survivin may result in suppression of both the tumor (27) and vascular compartments in the relative absence of host toxicity. Because survivin overexpression seems to elevate the apoptotic threshold to chemotherapy, abrogation of its function likely would sensitize both tumor and ECs to drug-induced cytotoxicity. Whether it would be more therapeutically advantageous to target survivin rather than VEGF receptor function remains to be determined.
The possibility of targeting survivin function in vivo as an anticancer strategy has been evaluated recently by studies in which it was shown that inhibition of survivin could effectively inhibit de novo tumor formation and tumor progression (17, 28, 29). Altogether, these observations would imply that targeting survivin expression therapeutically not only would compromise survival of the tumor cell population but could specifically affect activated ECs of the tumor vasculature as well.
Our results showing that EC chemoprotection can be induced by bFGF, similar to VEGF, and that this effect is enhanced by the combination of both factors suggest that a number of angiogenic factors may act cooperatively, or perhaps even in synergy, to prevent EC apoptosis caused by cytotoxic agents. As such, therapeutic agents that simultaneously abrogate signaling of several EC-survival pathways may be more effective in eradicating the tumor vasculature to chemotherapy. The possibility that tumors, particularly when faced with selective pressures, may switch their angiogenic dependence between a number of growth factors further strengthens the rationale for developing such inhibitors (4).
In summary, we propose a mechanism of tumor drug resistance in which tumor-associated ECs do not develop resistance through heritable genetic mutations but, rather, as a result of their exposure to growth factors present in the tumor microenvironment, become less sensitive or refractory to chemotherapy. Because this protective effect presumably is epigenetic and transient, it should be possible to reverse this chemoprotective effect by using specific growth factor or growth factor receptor antagonists. Because our results further implicate survivin as a novel rate-limiting regulator of drug resistance in ECs, targeting its expression similarly may optimize vascular damage. Ultimately, therapeutically targeting EC survival mechanisms may significantly sensitize the tumor vasculature to various standard anticancer modalities, including radiation and other antiangiogenic agents capable of inducing EC apoptosis, such as thrombospondin-1 (TSP-1) or TSP-1 peptides (30–32).
Acknowledgments
We thank Cassandra Cheng and Lynda Woodcock for their secretarial assistance. We also acknowledge Dr. Dario Altieri and Dr. Eric Lacasse for their expertise and generosity in providing important reagents. J.T. is supported by a National Cancer Institute of Canada studentship. Z.M. and J.L.Y. are supported by studentships from the Canadian Institutes for Health Research. This work was supported by grants to R.S.K. from the National Institutes of Health (CA-41233) and the Canadian Institutes of Health Research. D.J.D. is supported by grants from the Canadian Institutes for Health Research and National Cancer Institute of Canada.
Abbreviations
- EC
endothelial cell
- VEGF
vascular endothelial growth factor
- bFGF
basic fibroblast growth factor
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- DA PKB
dominant-active form of PKB
- ADR
adriamycin
- CDDP
cisplatinum
- VBL
vinblastine
- HUVEC
human umbilical vein endothelial cell
- HDMEC
human dermal microvascular endothelial cell
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kerbel R S, Viloria-Petit A, Klement G, Rak J. Eur J Cancer. 2000;36:1248–1257. doi: 10.1016/s0959-8049(00)00092-7. [DOI] [PubMed] [Google Scholar]
- 2.Miller K D, Sweeney C J, Sledge G W. J Clin Oncol. 2001;19:1195–1206. doi: 10.1200/JCO.2001.19.4.1195. [DOI] [PubMed] [Google Scholar]
- 3.Browder T, Butterfield C E, Kraling B M, Marshall B, O'Reilly M S, Folkman J. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 4.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin D, Bohlen P, Kerbel R S. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Bergers G, Bergsland E. J Clin Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerbel R S. BioEssays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 7.Yu J L, Rak J, Coomber B L, Hicklin D J, Kerbel R S. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney C J, Miller K D, Sissons S E, Nozaki S, Heilman D K, Sledge G W. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- 9.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 10.Karsan A, Yee E, Poirier G G, Zhou P, Craig R, Harlan J M. Am J Pathol. 1997;151:1775–1784. [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak H J, So J N, Lee S J, Kim I, Koh G Y. FEBS Lett. 1999;448:249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 12.Gerber H P, McMurtrey A, Kowalski J, Yan M, Keyt B A, Dixit V, Ferrara N. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 13.Gerber H P, Dixit V, Ferrara N. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 14.Nor J E, Christensen J, Liu J, Peters M, Mooney D J, Strieter R M, Polverini P J. Cancer Res. 2001;61:2183–2188. [PubMed] [Google Scholar]
- 15.Tran J, Rak J, Sheehan C, Saibil S D, LaCasse E, Korneluk R G, Kerbel R S. Biochem Biophys Res Commun. 1999;264:781–788. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor D S, Schechner J S, Adida C, Mesri M, Rothermel A L, Li F, Nath A K, Pober J S, Altieri D C. Am J Pathol. 2000;156:393–398. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesri M, Morales-Ruiz M, Ackermann E J, Bennett C F, Pober J S, Sessa W C, Altieri D C. Am J Pathol. 2001;158:1757–1765. doi: 10.1016/S0002-9440(10)64131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu, J. L., Rak, J. W., Klement, G. & Kerbel, R. S. (2001) Cancer Res., in press. [PubMed]
- 19.Downward J. Curr Opin Oncol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Ambrosini G, Chu E Y, Plescia J, Tognin S, Marchisio P C, Altieri D C. Nature (London) 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 21.Geng L, Donnelly E, McMahon G, Lin P C, Sierra-Rivera E, Oshinka H, Hallahan D E. Cancer Res. 2001;61:2413–2419. [PubMed] [Google Scholar]
- 22.Kim I, Kim H G, So J N, Kim J H, Kwak H J, Koh G Y. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 23.Papapetropoulos A, Fulton D, Mahboubi K, Kalb R G, O'Connor D S, Li F, Altieri D C, Sessa W C. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 24.Verdecia M A, Huang H, Dutil E, Kaiser D A, Hunter T, Noel J P. Nat Struct Biol. 2000;7:602–608. doi: 10.1038/76838. [DOI] [PubMed] [Google Scholar]
- 25.Fortugno P, Wall N R, Giodini A, O'Connor D S, Plescia J, Padgett K M, Tagnin S, Marchisio P C, Altieri D C. J Cell Sci. 2002;115:575–585. doi: 10.1242/jcs.115.3.575. [DOI] [PubMed] [Google Scholar]
- 26.Flusberg D A, Nomaguchi Y, Ingber D E. Mol Biol Cell. 2001;12:3087–3094. doi: 10.1091/mbc.12.10.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamm I, Wang Y, Sausville E, Scudiero D A, Vigna N, Oltersdorf T, Reed J C. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 28.Kanwar J R, Shen W P, Kanwar R K, Berg R W, Krissansen G W. J Natl Cancer Inst. 2001;93:1541–1552. doi: 10.1093/jnci/93.20.1541. [DOI] [PubMed] [Google Scholar]
- 29.Mesri M, Wall N R, Li J, Kim R W, Altieri D C. J Clin Invest. 2001;108:981–990. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauceri H J, Hanna N N, Beckett M A, Gorski D H, Staba M J, Stellato K A, Bigelow K, Heimann R, Gately S, Dhanabal M, et al. Nature (London) 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 31.Guo N, Krutzsch H C, Inman J K, Roberts D D. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- 32.Jiminez B, Volpert O V, Crawford S E, Febbraio M, Silverstein R L, Bouck N. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]