Abstract
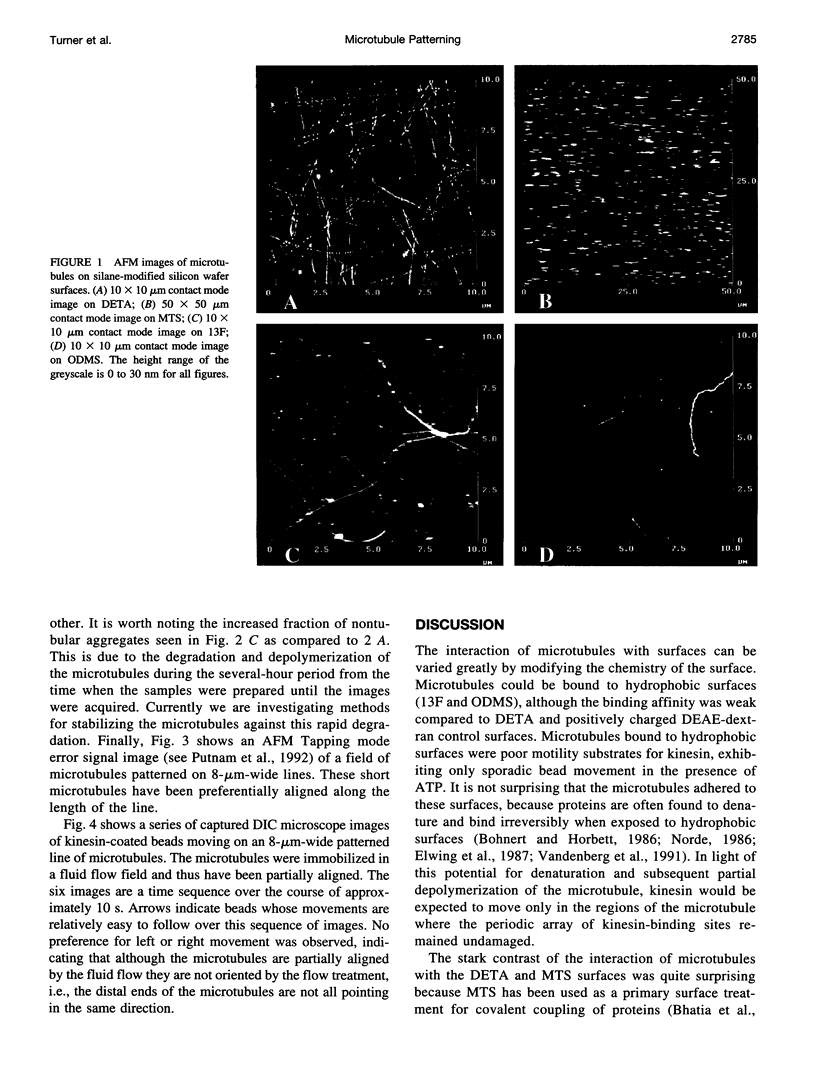
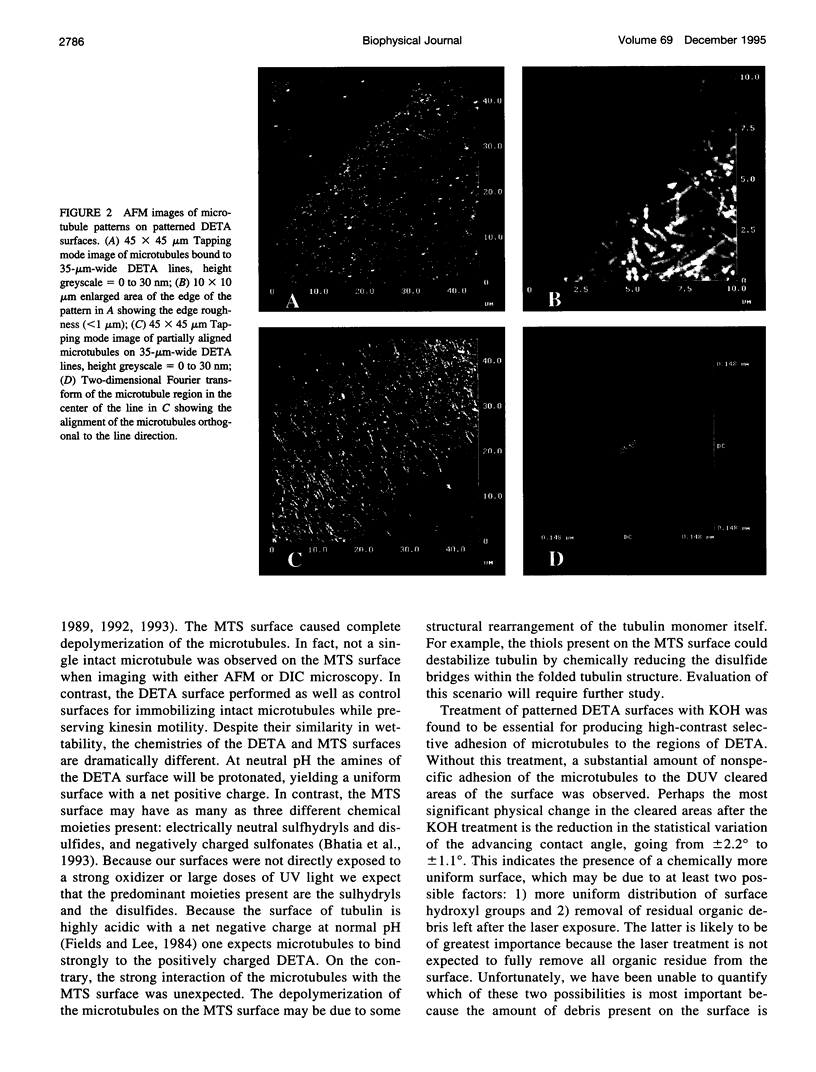
We show that microtubule polymers can be immobilized selectively on lithographically patterned silane surfaces while retaining their native properties. Silane films were chemisorbed on polished silicon wafers or glass coverslips and patterned using a deep UV lithographic process developed at the Naval Research Laboratory. Hydrocarbon and fluorocarbon alkyl silanes, as well as amino and thiol terminal alkyl silanes, were investigated as substrates for microtubule adhesion with retention of biological activity. Microtubules were found to adhere strongly to amine terminal silanes while retaining the ability to act as substrates for the molecular motor protein kinesin. Aminosilane patterns with linewidths varying from 1 to 50 microns were produced lithographically and used to produce patterns of selectively adhered microtubules. Microtubules were partially aligned on the patterned lines by performing the immobilization in a fluid flow field. Patterns were imaged with atomic force microscopy and differential interference contrast microscopy. Motility assays were carried out using kinesin-coated beads and observed with differential interference contrast microscopy. Kinesin bead movement on the patterned microtubules was comparable to movement on microtubule control surfaces.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatia S. K., Shriver-Lake L. C., Prior K. J., Georger J. H., Calvert J. M., Bredehorst R., Ligler F. S. Use of thiol-terminal silanes and heterobifunctional crosslinkers for immobilization of antibodies on silica surfaces. Anal Biochem. 1989 May 1;178(2):408–413. doi: 10.1016/0003-2697(89)90662-3. [DOI] [PubMed] [Google Scholar]
- Bhatia S. K., Teixeira J. L., Anderson M., Shriver-Lake L. C., Calvert J. M., Georger J. H., Hickman J. J., Dulcey C. S., Schoen P. E., Ligler F. S. Fabrication of surfaces resistant to protein adsorption and application to two-dimensional protein patterning. Anal Biochem. 1993 Jan;208(1):197–205. doi: 10.1006/abio.1993.1027. [DOI] [PubMed] [Google Scholar]
- Britland S., Perez-Arnaud E., Clark P., McGinn B., Connolly P., Moores G. Micropatterning proteins and synthetic peptides on solid supports: a novel application for microelectronics fabrication technology. Biotechnol Prog. 1992 Mar-Apr;8(2):155–160. doi: 10.1021/bp00014a010. [DOI] [PubMed] [Google Scholar]
- Clark P., Connolly P., Curtis A. S., Dow J. A., Wilkinson C. D. Cell guidance by ultrafine topography in vitro. J Cell Sci. 1991 May;99(Pt 1):73–77. doi: 10.1242/jcs.99.1.73. [DOI] [PubMed] [Google Scholar]
- Dulcey C. S., Georger J. H., Jr, Krauthamer V., Stenger D. A., Fare T. L., Calvert J. M. Deep UV photochemistry of chemisorbed monolayers: patterned coplanar molecular assemblies. Science. 1991 Apr 26;252(5005):551–554. doi: 10.1126/science.2020853. [DOI] [PubMed] [Google Scholar]
- Field D. J., Collins R. A., Lee J. C. Heterogeneity of vertebrate brain tubulins. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4041–4045. doi: 10.1073/pnas.81.13.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R. Dynein ATPases as microtubule motors. J Biol Chem. 1988 Nov 5;263(31):15837–15840. [PubMed] [Google Scholar]
- Hunt A. J., Gittes F., Howard J. The force exerted by a single kinesin molecule against a viscous load. Biophys J. 1994 Aug;67(2):766–781. doi: 10.1016/S0006-3495(94)80537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D., Kahler K. H., Hockberger P. E. Controlled outgrowth of dissociated neurons on patterned substrates. J Neurosci. 1988 Nov;8(11):4098–4120. doi: 10.1523/JNEUROSCI.08-11-04098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E. Role of microtubules in the organisation of the Golgi apparatus. Cell Motil Cytoskeleton. 1990;15(2):67–70. doi: 10.1002/cm.970150202. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Force of single kinesin molecules measured with optical tweezers. Science. 1993 Apr 9;260(5105):232–234. doi: 10.1126/science.8469975. [DOI] [PubMed] [Google Scholar]
- Lee C., Chen L. B. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988 Jul 1;54(1):37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- Lom B., Healy K. E., Hockberger P. E. A versatile technique for patterning biomolecules onto glass coverslips. J Neurosci Methods. 1993 Dec;50(3):385–397. doi: 10.1016/0165-0270(93)90044-r. [DOI] [PubMed] [Google Scholar]
- Matthies H. J., Miller R. J., Palfrey H. C. Calmodulin binding to and cAMP-dependent phosphorylation of kinesin light chains modulate kinesin ATPase activity. J Biol Chem. 1993 May 25;268(15):11176–11187. [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T. Erythrocyte microtubule assembly in vitro. Tubulin oligomers limit the rate of microtubule self-assembly. J Biol Chem. 1986 Feb 15;261(5):2319–2324. [PubMed] [Google Scholar]
- Norde W. Adsorption of proteins from solution at the solid-liquid interface. Adv Colloid Interface Sci. 1986 Sep;25(4):267–340. doi: 10.1016/0001-8686(86)80012-4. [DOI] [PubMed] [Google Scholar]
- Sale W. S., Fox L. A. Isolated beta-heavy chain subunit of dynein translocates microtubules in vitro. J Cell Biol. 1988 Nov;107(5):1793–1797. doi: 10.1083/jcb.107.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J. Viewing single microtubules by video light microscopy. Methods Enzymol. 1986;134:561–573. doi: 10.1016/0076-6879(86)34121-1. [DOI] [PubMed] [Google Scholar]
- Schroer T. A., Schnapp B. J., Reese T. S., Sheetz M. P. The role of kinesin and other soluble factors in organelle movement along microtubules. J Cell Biol. 1988 Nov;107(5):1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A., Steuer E. R., Sheetz M. P. Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell. 1989 Mar 24;56(6):937–946. doi: 10.1016/0092-8674(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Singhvi R., Kumar A., Lopez G. P., Stephanopoulos G. N., Wang D. I., Whitesides G. M., Ingber D. E. Engineering cell shape and function. Science. 1994 Apr 29;264(5159):696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Spargo B. J., Testoff M. A., Nielsen T. B., Stenger D. A., Hickman J. J., Rudolph A. S. Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11070–11074. doi: 10.1073/pnas.91.23.11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]






