Abstract
The mechanisms involving neuronal death after ischemic/hypoxic insult are complex, involving both rapid (excitotoxic) and delayed (apoptotic-like) processes. Recent evidence suggests that cell cycle regulators such as cyclin-dependent kinases are abnormally activated in neuropathological conditions, including stroke. However, the function of this activation is unclear. Here, we provide evidence that inhibition of the cell cycle regulator, Cdk4, and its activator, cyclinD1, plays critical roles in the delayed death component of ischemic/hypoxic stress by regulating the tumor suppressor retinoblastoma protein. In contrast, the excitotoxic component of ischemia/hypoxia is predominately regulated by Cdk5 and its activator p35, components of a cyclin-dependent kinase complex associated with neuronal development. Hence, our data both characterize the functional significance of the cell cycle Cdk4 and neuronal Cdk5 signals as well as define the pathways and circumstances by which they act to control ischemic/hypoxic damage.
Keywords: hypoxia, stroke
The mechanisms involved in ischemic neuronal death are complex and depend upon multiple factors, including severity and duration of insult. In the core of the infarct, a relatively rapid excitatory death occurs within minutes to a few hours (1). This type of neuronal death occurs after energy failure and Ca2+ overload. Numerous Ca2+-mediated enzymes such as calpains are activated and participate in the neuronal loss. The region surrounding this core infarct area, the penumbra, experiences less intense ischemia and displays a more delayed type of cell death with characteristics of apoptosis (1). The signaling pathways that regulate both rapid and delayed ischemic are not fully defined.
Cyclin-dependent kinases (CDKs) are a large group of Ser/Thr kinases that are best characterized for their role in cell cycle progression. In this regard, distinct kinase members, along with their cognate cyclin-activating partners, regulate different phases of the cell cycle. Of relevance to the present work, cyclin D/Cdk4 and cyclin E/Cdk2 complexes regulate G1/S transition, partly by phosphorylating and inactivating the tumor suppressor retinoblastoma protein (Rb). Consequently, Rb is released from the transcription factor, E2F. E2F then activates genes required for S phase progression (2).
In addition to this crucial role in cell cycle regulation, CDK members have also been implicated in other fundamental biological processes, including transcription and neuronal function (3). As an example of the latter, Cdk5 is selectively active in neurons and, together with its noncyclin activators, p35 and p39, regulates numerous neuronal processes (4).
Growing evidence suggests that multiple CDK members may also participate in neuronal death. In general, two important hypotheses have emerged. The first describes a paradoxical situation by which inappropriate activation of cell cycle-related CDKs in terminally differentiated neurons leads to death instead of proliferation (5). In support of this, correlative evidence demonstrating activation/up-regulation of cell cycle components has been reported in a number of neuronal death paradigms, including stroke. For instance, increased cyclin D1 expression, down-regulation of p16ink4, and phosphorylation of Rb have been reported in multiple in vivo stroke paradigms (6–9). However, no studies have yet conclusively shown that cell cycle CDKs are critical functionally for neuronal death in adult models of injury. The question of whether inappropriate cell cycle signals are required for death in neuronal injury or whether they may be an epiphenomenon of diseased neurons remains unresolved.
A second hypothesis proposes that deregulated Cdk5 activity can also induce neuronal damage. In this case, one model states that calpain proteases cleave the p35 to a smaller more stable and mislocalized p25 form. This, in turn, converts Cdk5 into a death inducer. Such inappropriate activation of Cdk5 has been reported in neuronal death induced by a variety of insults, including stroke. Pertinent to the latter, Wang et al. (10) showed that accumulation of p25 after transient forebrain ischemia activates Cdk5 and induces CA1 cell death.
Interestingly, we have shown that administration of flavopiridol, a general CDK inhibitor, is protective in both focal (8) and global (9) ischemia. However, flavopiridol inhibits both cell cycle CDKs and Cdk5 (11, 12), as well as non-CDK-related kinases such as GSK-3β (13). Accordingly, the role of specific CDKs in stroke-induced damage remains unknown.
Taken together, the above observations highlight the following questions: (i) Are specific cell cycle CDKs important in an adult in vivo model of neuronal death such as stroke? (ii) If so, how might these CDKs regulate death after ischemic/hypoxic/excitotoxic insult? and (iii) Under what conditions do cell cycle CDKs or Cdk5 participate in neuronal death after ischemia/hypoxia/excitotoxicity? To answer these questions, we have examined the role of cell cycle CDKs and Cdk5 in ischemic/hypoxic models of delayed and excitotoxic death both in vitro and in vivo.
Methods
Viral Construction. Recombinant adeno-associated virus (rAAV1) vectors were constructed by subcloning cDNA sequences (XbaI fragment) of DNCdk2, 4 (14, 15) and 5 (12, 16) into the SpeI sites of the AM/CBA-pl-WPRE-bGH plasmid. The virus was then generated and purified as described (17). For adenovirus (AV) construction, the same sequences were subcloned into the pAdTrack vector under a cytomegalovirus (CMV) promoter. The construct also contains a second CMV promoter that separately controls expression of GFP. The construct was then used to generate recombinant AV, as described (18). The AV containing the ΔK11 Rb mutant was generated, as described (19).
Transgenic Mice/Knockouts. All animal experiments conformed to the guidelines set forth by the Canadian Council for the Use and Care of Animals in Research and the Canadian Institutes for Health Research.
Dominant Negative Cdk4 Transgenic Mice. Mice expressing DNCdk4 were generated by using a fusion construct composed of a full length human Cdk4 harboring a D158R mutation (see Supporting Text, which is published as supporting information on the PNAS web site).
Cyclin D1 Null Mice. Cyclin D1 heterozygous breeding pairs were commercially obtained from The Jackson Laboratory on a mixed C57BL/6 × 129S2 background.
P35 Null Mice. P35 null mice have been characterized by Hallows et al. (20). Pups from heterozygous breedings were screened by PCR as described.
Cell Culture. Cerebellar granule neuron (CGN) cultures were prepared from 7- to 9-day postnatal mice, as described (21).
Hypoxia. Hypoxia was induced by using a humidified environmental chamber (Coy Laboratory Products, Ann Arbor, MI) set at 37°C, 1% O2, and 5% CO2. Five-day plated CGNs were infected with recombinant AV expressing DNCdk2/4/5, ΔK11 Rb mutant, or GFP by itself as control with a multiplicity of infection of 40. For a more delayed model of death, cultures were incubated in the chamber on day 7 for 16–18 h in the presence of the NMDA blocker, MK801 (10 μM, Research Biochemicals, Natick, MA) and then reoxygenated at 37°C. Control plates contained MK801 but were not exposed to hypoxia. All cultures were fixed (4% paraformaldehyde) at times 12 and 24 h after reoxygenation, then stained with Hoescht 33342 (Sigma), and GFP-positive cells were evaluated for nuclear integrity [analyses of dominant negative CDKs (DNCDKs)]. Nuclei from dying neurons showed severe condensation or fragmentation. For analyses of the effects of the ΔK11 Rb mutant, cultures were first fixed and analyzed for Rb overexpression by using anti-Rb Ab (BD PharMingen). Because this vector did not express GFP, Rb-positive neurons were evaluated for survival as above. Random fields of infected neurons were evaluated for live vs. dead neurons. Data are presented as percentage live/dead ± SEM.
For a more excitotoxic death paradigm, infected cultures were incubated in the hypoxic chamber in the absence of MK801 for 5 h and then reoxygenated for 1 h. Cultures were then fixed as above and stained for Hoechst. The total number of live GFP-positive neurons per well was evaluated and compared with the number of GFP-positive live neurons in control nonhypoxia-induced wells. This analysis was performed for each virus. Expression of the DNCDKs was confirmed by anti-Cdk2/4/5 Abs (Santa Cruz Biotechnology).
Alternatively, neurons from transgenic mice (see above) were used instead of viruses. Both delayed and excitotoxic models were performed, as described above, and cultures were evaluated by lysing the neurons in each well with a lysis buffer that disrupts cells but leaves healthy nuclei intact. Nuclei that displayed characteristics of blebbing and disruption of nuclear membrane were excluded (21). Data are expressed relative to untreated controls ± SEM.
Glutamate Model of Neuronal Death. Five-day plated CGNs were infected with AV, carrying DNCdk2/4/5 or GFP by itself as control, as described above. On day 7, glutamate was added to the wells to a final concentration of 50 μM for 70 min and then washed off with conditioned medium and incubated for 2 h. This was performed in the presence or absence of MK801 (10 μM). Survival was evaluated as described above for the hypoxia (-MK801) death model.
Viral Injection in Vivo. All in vivo studies were performed in male Wistar rats weighing 80–100 g. DNCDKs or GFP control were unilaterally (survival studies) or bilaterally (behavioral studies) delivered by injecting rAAV1 vector 2 weeks before induction of global ischemia or injection of endothelin. rAAV1 was diluted by mixing 2 μl of virus stock (1010 genomes per microliter) with 1 μl of 20% mannitol in PBS and was administered by a pump (Harvard infusion pump, Harvard Apparatus) into the hippocampus (from bregma: -3.6 mm anterioposterior, ± 2.1 mm lateral, -2.75 mm deep) or striatum (from bregma: +0.9 mm anterioposterior, +2.8 mm lateral, -5.8 mm deep) over a 30-min period, as described (9).
Global Ischemia Model. Hippocampal rAAV1-injected rats, weighing 180–220 g, were induced via transient global ischemia [four-vessel occlusion (4VO)], as described (9). Brains were collected 4 days after 4VO surgery, sectioned, stained for hematoxylin/eosin (18), and quantified, as described (9).
Focal Ischemia Model (Endothelin Injection). Striatal rAAV1-injected rats were subjected to endothelin injection. Endothelin-1 (400 pM; Calbiochem) was dissolved in 1 μl of H2O and injected over a period of 3 min into the viral-injected striatal region, as described (22). Brains were collected 4 days after injection, and coronal sections of the striatum were collected as described (12) and stained with cresyl violet. The infarct volume was measured on each slice by a microcomputer-based image display system (Imaging Research, St. Catherine's, ON, Canada) by using the method described by Swanson et al. (23).
Immunohistochemistry. Coronal sections (14 μm) were obtained at the level of middorsal hippocampus or striatum from global or focal ischemia-induced brains, respectively (12). Expression of GFP was shown by using GFP fluorescence, and expression of DNCdk2 was analyzed by using anti-Cdk2 Ab (Santa Cruz Biotechnology).
Western Blot Analyses. For analyses of DNCDKs expression in the hippocampus or striatum, a 2-mm punch was obtained and analyzed by Western blot, as described (12). Membranes were probed with anti-Cdk2/4/5 (Santa Cruz Biotechnology) or anti-flag (Sigma) Abs. Actin was used as loading control (Sigma). Rb phosphorylation was determined in vivo from nuclear proteins extracted from hippocampal extracts, as described (9), by using antiphospho Rb-Ser-795 or –Ser-807/811 Abs (Cell Signaling Technology, Beverly, MA), or anti-Rb Abs (BD PharMingen). For Western blot analyses using cultured neurons, CGNs were harvested at the appropriate times by methods previously described (21).
Morris Water Maze Test. In this test, animals are screened for their ability to find a hidden platform in a pool of milky water by using fixed visual clues, as described (9) (see Supporting Text).
Results
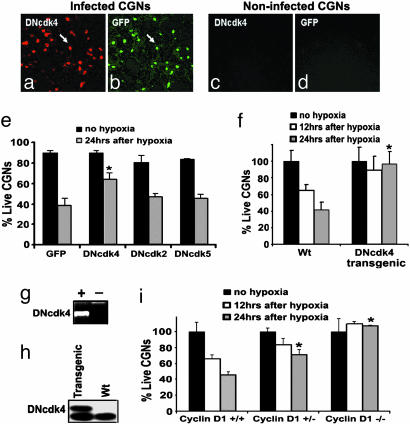
Cdk4 and CyclinD1 as Mediators of Delayed Neuronal Death Induced by Nonexcitotoxic Hypoxic Insult in Vitro. To test the importance of individual CDKs in delayed models of ischemic death, we used an in vitro model of death where neuronal loss occurs in the presence of the NMDA blocker MK801 (Fig. 1). CGNs were infected with a GFP-containing AV expressing kinase-dead dominant negative mutants of the G1-related CDKs (DNCdk2/4), the neuronal CDK, DNCdk5, or an empty viral control. Cultures were then subjected to hypoxia in the presence of MK801, and GFP-expressing neurons were assessed for nuclear integrity. Dead cells displayed condensed and/or fragmented nuclei, whereas healthy nuclei were intact and did not show any signs of condensation. As shown in Fig. 1e, neurons exposed to hypoxia expressing DNCdk4 showed 65% survival vs. 39% survival in GFP-expressing controls. Expression of DNCdk2 and DNCdk5, however, did not show any significant protection when compared with GFP expression alone. These data indicate that Cdk4 plays an important role in delayed ischemic death, whereas the role of Cdk2 or -5 is less central.
Fig. 1.
Delayed ischemic neuronal death in vitro is mediated by Cdk4 and cyclin D1. (a–d) Expression of DNCDKs in CGN cultures. Coexpression of (a) DNCdk4 was detected by using an anti-Cdk4 Ab, whereas expression of (b) GFP was detected by using fluorescence in the same culture. DNCdk2/5-infected cells showed similar results (data not shown). (c and d) Immunofluorescence of noninfected cultures visualized as in a and b as negative controls. (e) Quantitation of survival after 16–18 h of hypoxia followed by 24 h of reoxygenation, in the presence of MK801 (n = 3). (f) CGNs from transgenic mice expressing DNCdk4 are resistant to hypoxia in the presence of MK801. Experiments were performed as in e (n = 3). (g) PCR for the presence of the DNCdk4 transgene in (+) transgenic mice and (-) littermate controls. (h) Western blot showing expression of DNCdk4 in CGNs from transgenic mice compared with WT controls using an anti-Cdk4 Ab. (i) CGNs from cyclin D1-deficient mice are resistant to hypoxia in the presence of MK801, as described in e (n = 3). The data are mean ± SEM. * denotes significance (P < 0.05, t test)
To confirm that Cdk4 may be a critical mediator of delayed death, we generated transgenic mice expressing flag-tagged DNCdk4 under a neuron-specific enolase promoter. PCR analyses showed incorporation of the transgene and expression the DNCdk4 construct in a variety of regions, including in CGNs (Fig. 1 g and h). These mice were grossly normal and did not display any major identifiable abnormalities in brain development (data not shown). Consistent with the viral data described above, CGNs from DNcdk4 transgenic mice were more resistant to hypoxia and showed 96% survival vs. 41% survival in WT controls, in the presence of MK801, after 24 h of reoxygenation (Fig. 1f). The expression of DNCdk4 was confirmed in these neuronal cultures by Western blot. Taken together, these results suggest the importance of Cdk4 in hypoxia-induced delayed death and suggest that the protective effects observed were not due to a viral delivery artifact.
Cyclin D proteins are required activators of Cdk4 (24). To further confirm that Cdk4 plays an important role in delayed hypoxic death, we cultured CGNs from cyclin D1-deficient mice and littermate controls. As shown in Fig. 1i, cyclin D1-deficient mice were much more resistant to hypoxia in the presence of MK801 than controls. In contrast, and consistent with the lack of protective effects of DNcdk5 in this model, neurons cultured from p35-deficient animals were not resistant to delayed death (+MK801) induced by hypoxia (Fig. 6, which is published as supporting information on the PNAS web site). Taken together, the above results suggest that Cdk4 activity is functionally important in delayed death, because inhibition of Cdk4 or deletion of its activator cyclin D1 is protective. In contrast, Cdk2 or -5 appears to play a minimal functional role under these conditions.
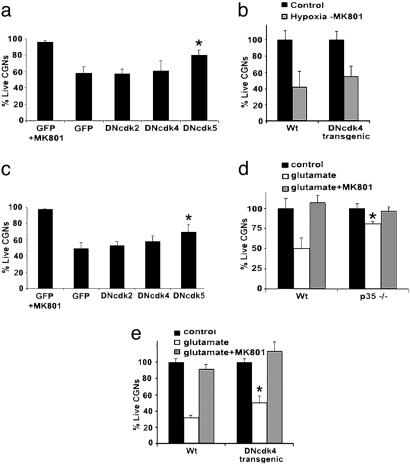
Cdk5 as Mediator of Excitotoxic Neuronal Death in Vitro. We next determined whether cell cycle CDKs and/or Cdk5 participate in more rapid excitotoxic death. To test this, we examined whether DNCdk2/4/5 is protective in models of hypoxia where death is induced in the absence of MK801 (Fig. 2 a and b). Alternatively, we also examined whether direct glutamate-induced death depends upon these CDKs (Fig. 2 c–e). In contrast to the delayed hypoxic model described above, Cdk5 appears to play a predominant role in excitotoxic death when compared with Cdk4 and Cdk2. As shown in Fig. 2a, viral-mediated DNCdk5 expression blocked death induced by hypoxia (-MK801). DNcdk5-expressing cells showed significantly more survival compared with GFP-expressing controls in this model. The lack of protection by DNCdk4 was also confirmed by using CGNs from DNCdk4 transgenic mice (Fig. 2b). Similar results were obtained by using a direct model of excitotoxicity by glutamate exposure (Fig. 2c), where DNCdk5 was more efficient in promoting survival than DNcdk4 (70% survival in DNCdk5 expressing neurons vs. 49% survival in GFP-expressing controls). Because p35 is an important activator of Cdk5, we also asked whether CGNs from p35-deficient mice were resistant to excitotoxic death. As shown in Fig. 2d, CGNs cultured from p35-deficient mice were significantly more resistant to glutamate-induced death when compared with littermate controls (81% vs. 50%). Similar results were obtained with p35 heterozygous neurons after hypoxia (-MK801) when compared with WT controls (data not shown). Moreover, consistent with the weak protective effects of DNcdk4, cyclin D1 deficiency was not protective after glutamate exposure when compared with littermate controls (data not shown). Interestingly, neurons from DNCdk4 transgenic mice were slightly resistant to glutamate exposure when compared with littermate controls (Fig. 2e), suggesting that in select cases of excitotoxicity, Cdk4 may also have a role. However, this role is minor in comparison with that of Cdk5. Taken together, these results suggest that in excitotoxic death, Cdk5 plays a central role, whereas the cell cycle CDK, Cdk4, is more significant in delayed modes of death.
Fig. 2.
Cdk5/p35 is more involved in excitotoxic ischemia than Cdk4/cyclin D1. (a) AV-infected CGNs expressing DNCdk2/4/5 or GFP alone were subjected to 5-h hypoxia and 1- to 2-h reoxygenation in the absence of MK801 (n = 4). (b) CGNs from DNcdk4 transgenic mice or WT littermate controls subjected to hypoxia as in a (n = 3). (c) AV-infected CGNs expressing DNCdk2/4/5 or GFP alone subjected to glutamate (50 μM; n = 4). (d) CGNs from p35-deficient mice are resistant to glutamate-induced death (n = 3). (e) CGNs from DNCdk4 transgenic mice subjected to glutamate (n = 3). * denotes significance (P < 0.05, t test). The data are mean ± SEM
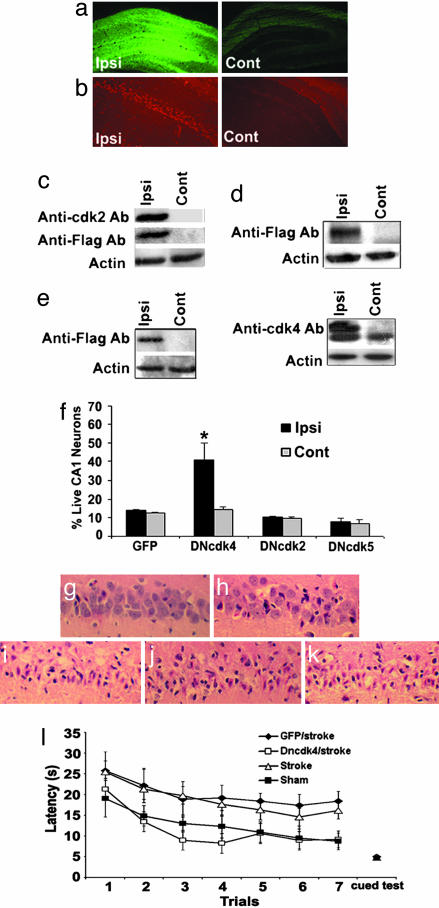
Cdk4 as Mediator of Delayed Death in a Global Model of Stroke. We next asked whether Cdk4 played a role in ischemic delayed death in adult models of injury. This question is significant, because no clear indication of a functional role of Cdk4 in adult injury has previously been demonstrated. To examine this question, we used a 10-min transient forebrain 4VO model of delayed ischemia where CA1 neurons die with a protracted (>24 h) time course after reperfusion. We have shown (9) that the Cdk4/Rb pathway is activated in this model. Recombinant rAAV1 vectors expressing DNCdk2/4/5 or GFP were injected unilaterally into the hippocampus 2 weeks before 10-min 4VO insult to allow for expression of the constructs. Expression of the constructs was confirmed by immunohistochemistry and Western blot (Fig. 3 a–e). Survival of CA1 neurons was assessed 4 days after induction of 4VO. Normal CA1 neurons are characterized by round soma and clear intact nuclei by hematoxylin/eosin analyses (Fig. 3g), whereas dying neurons appeared shrunken with pyknotic nuclei (Fig. 3i). Neuronal counts of CA1 region showed a dramatic increase in survival in the DNCdk4-injected hemisphere compared with noninjected hemisphere (Fig. 3f). In comparison, DNCdk2- and -5-injected rats did not result in any significant survival in the CA1 region. No changes in neuronal numbers in the CA1 region were detected with GFP-treated animals (Fig. 3f). These results are consistent with the previously described in vitro data and indicate that Cdk4 and neither Cdk2 nor -5 plays a critical role in delayed death in vivo.
Fig. 3.
DNCdk4 expression but not DNCdk2 or -5 provides significant protection from 10-min 4VO-induced delayed neuronal death in vivo. (a–e) Expressions of flag-tagged-DNCDKs constructs, as well as GFP (Ipsi, ipsilateral injected hemisphere; Cont, contralateral noninjected hemisphere). Expression of (a) GFP and (b) DNCdk2 shown using GFP fluorescence and anti-Cdk2 Ab, respectively. (c–e) Western blot of hippocampi injected with virus expressing (c) DNCdk2, (d) DNCdk5, and (e) DNCdk4 using anti-flag or -CDK Abs; actin loading control. (f) Quantitation of surviving CA1 neurons expressing GFP (n = 3), DNCdk4 (n = 7), DNcdk2 (n = 8), or DNCdk5 (n = 4). Survival assessments were performed 4 days after 4VO. Data are presented as mean ± SEM. * denotes significance (P < 0.05, t test Ipsi vs. Contra). (g–k) Representative sections from the CA1 region from animals treated with (g) sham, (h) DNCdk4-injected + 4VO, (i) GFP-injected + 4VO, (j) DNCdk2-injected + 4VO, and (k) DNCdk5-injected + 4VO. Sections are stained for hematoxylin/eosin. (l) Improved escape latency in the Morris water maze test (MWM) test in rats expressing DNCdk4 in hippocampus and subjected to 10-min 4VO. Rats injected bilaterally with DNCdk4 and stroked (n = 8), GFP and stroked (n = 7), noninjected and stroked (n = 6), and sham (n = 7) rats were subjected to the MWM test. The data are presented as mean ± SEM. There was a significant difference (P < 0.01, ANOVA) between DNCdk4- and GFP-expressing stroked animals during the testing periods but not with the cued test
We next asked whether protection by DNCdk4 might lead to improved behavioral outcomes. It has been shown that damage to the CA1 region results in impaired spatial learning and memory (25). Accordingly, we used the Morris water maze test to test whether DNCdk4-injected rats had improved memory function. Animals were injected bilaterally with DNCdk4 or GFP control and were subjected to either sham or 4VO surgery. As shown in Fig. 3l, GFP-injected/stroked rats consistently spent almost twice as much time finding the platform (escape latency) when compared with DNcdk4-expressing animals. To ensure that any differences in latency time were not due to motor or visual deficits, a cued test was performed at the end of the test, and no difference was observed between groups.
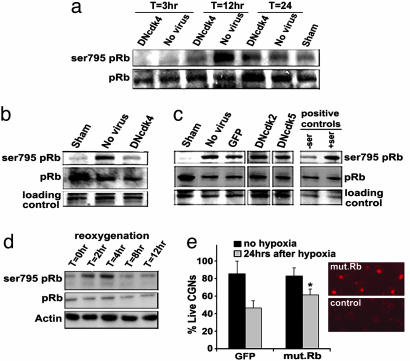
The Role of Rb in Delayed Ischemic Death. What are the mechanisms by which Cdk4 may signal death? Previous reports have indicated that Rb is phosphorylated efficiently on Ser-795 by Cdk4 (26). Accordingly, we examined whether Rb may act as a downstream mediator of Cdk4 after 10 min of 4VO. As shown in Fig. 4a, a dramatic increase in Ser-795 Rb phosphorylation was observed 12 h after reperfusion. No phosphorylation of Rb on Ser-807/-811 sites was observed (data not shown), suggesting some selectivity in Rb phosphorylation. As shown in Fig. 4 a–c, the increase in Ser-795 Rb phosphorylation after ischemia depends upon Cdk4 activity. DNCdk4-expressing animals showed reduced Rb phosphorylation, as determined by Western blot analyses of CA1 extracts. In contrast, DNCdk5 or -2 expression failed to attenuate the increased Ser-795 phospho Rb signal. This indicates that Cdk4 is a mediator of Ser-795 phosphorylation.
Fig. 4.
Phosphorylation of Rb on Ser-795 is diminished by DNCdk4 expression. (a) Time course of phosphorylation of Rb on Ser-795 and effects of DNCdk4 expression. Animals were injected unilaterally with DNCdk4-expressing virus. At the indicated times after 4VO, ipsilateral (with virus) or contralateral (no virus) nuclear hippocampi proteins were extracted and analyzed for Ser-795 phosphorylation by Western blot. Total Rb was used as loading control. (b and c) Comparison of the effects of DNCdk2/4/5 and GFP on Ser-795 Rb phosphorylation 12 h after ischemia. Positive control refers to nuclear hippocampal proteins extracted from noninjected animal O(-Ser) and 12 h (+Ser) after reperfusion. All lanes in c are from the same Western blot; Coomassie blue staining as loading control. (d) Time course of phosphorylation of Rb on Ser-795 in vitro. CGNs were subjected to 16 h of hypoxia, in the presence of MK801, followed by up to 12 h of reoxygenation; actin was used as loading control. (e) Expression of mutant Rb provides significant protection from hypoxia-induced delayed neuronal death in vitro. Quantitation of survival after 16–18 h of hypoxia followed by 24-h reoxygenation, in the presence of MK801. The data are mean ± SEM (n = 3). * denotes significance (P < 0.05, t test hypoxia GFP vs. hypoxia mut.Rb)
Rb phosphorylation could activate a number of potentially proapoptotic responses such as E2F, JNKs, and NF-κB (27). Accordingly, we examined whether expression of a mutant Rb with several phosphorylation sites removed (including Ser-795) might be protective in ischemic injury. Because multiple phosphorylation sites are removed, it might be expected to act as a constitutively active form of Rb. Unfortunately, we could not obtain Rb expression in rAAV1, perhaps due to size limitations of the constructs used. However, we could express the active Rb by using AV for testing in vitro. As shown in Fig. 4d and similar to our in vivo results, Rb becomes phosphorylated in vitro after reoxygenation in our hypoxia (+MK801) model of delayed injury. As shown in Fig. 4e, expression of constitutively active Rb was significantly protective after hypoxic insult when compared with the GFP control. CGNs exposed to hypoxia (+MK801) expressing mutant Rb showed 74% survival vs. 51% survival in GFP-expressing controls after 24 h of reoxygenation. Taken together with the results above, we propose that Cdk4 may transduce the delayed hypoxic death signal, at least in part through phosphorylation of Rb.
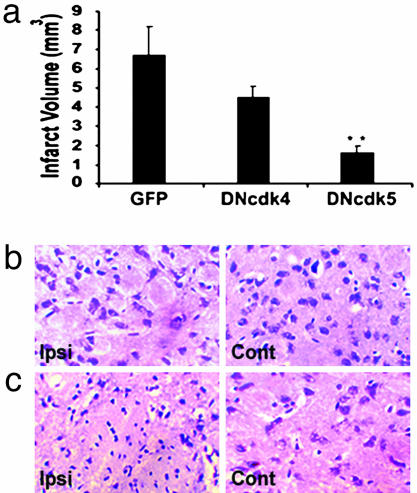
Cdk5 as Mediator of Focal Stroke in Vivo. Although the above results indicate that Cdk4 may be more important in delayed ischemic death present in the global model of stroke, we next asked whether, then, cdk5 may be more effective in a focal model of stroke where more rapid excitotoxic forms of death may predominate. Endothelin-1 is a powerful and long-lasting vasoconstrictive peptide that has been used to induce focal stroke (22). Accordingly, we injected endothelin-1 directly into the striatum. Recombinant rAAV1 vectors expressing DNCdk4/5 or GFP were injected unilaterally into the striatum, where robust expression was observed (data not shown). Regions of striatum with infarct could be distinguished from nondamaged normal regions by cresyl violet staining. Damaged regions displayed readily detectable shrunken compacted dying neurons 4 days after endothelin treatment. Measurement of infarct volume showed a very significant decrease in the damaged region in DNCdk5-expressing brains, compared with GFP or DNCdk4-expressing brains (Fig. 5a). In contrast, inhibition of Cdk4 was less protective against endothelin-1-induced lesion than DNCdk5 (Fig. 5a). Animals injected with virus (GFP, DNCdk4/5) did not show damage in the absence of endothelin (data not shown). This indicates that Cdk5 and not Cdk4 plays a critical role in the excitotoxic type of death in a focal model of ischemia in vivo and is consistent with the in vitro results.
Fig. 5.
DNCdk5 but not DNCdk4 expression provides significant protection from endothelin-induced excitotoxic neuronal death in vivo. (a) Infarct volume of focal ischemic brains expressing GFP (n = 4), DNCdk4 (n = 4), or DNCdk5 (n = 4) measured 4 days after endothelin injection. Data are presented as mean ± SEM. * denotes significance (P < 0.01, DNCdk5- vs. GFP-expressing brains). There was also a significant difference (P < 0.05) between DNCdk4- and DNCdk5- but not between GFP- and DNCdk4-expressing brains (P > 0.05; ANOVA, Newman–Keuls Multiple Comparison Test). (b and c) Representative sections of the striatum from animals treated with (b) DNCdk5 or (c) GFP followed by endothelin injection. Sections are stained with cresyl violet
Discussion
Although the potential role of the cell cycle in neuronal death has been hypothesized, clear functional data indicating the relevance of such a signal in neuronal injury in vivo have not been reported. In the present investigation, we used both in vitro and in vivo paradigms of ischemia/hypoxia to explore the role of cell cycle signaling in neuronal death. Our results are significant, because they (i) provide clear evidence of the importance of cell cycle CDKs and (ii) define the conditions under which distinct CDK members participate in stroke-induced death signaling. Our results serve to resolve an outstanding question of whether cell cycle CDKs or neuronal CDKs such as Cdk5 are important in neuronal death.
Because both rapid/excitotoxic as well as delayed mechanisms of death (and spectra in between) appear in any single brain exposed to loss of blood flow, we explored whether Cdk5 and/or Cdk4 may (i) participate in ischemic processes and (ii) act differentially in models of excitotoxic vs. more delayed apoptotic-like death. Our data point to a model by which Cdk5 acts preferentially to regulate excitotoxic damage, whereas Cdk4 is involved in pathways of ischemic/hypoxic injury where excitotoxic mechanisms are not the primary mode of death. The evidence for this can be summarized as follows. First, in ischemic models of NMDA receptor-independent delayed death in vitro, DNCdk4 expression as well as cyclin D1 deficiency robustly blocks death, whereas DNCdk5 expression or p35 deficiency is not protective. In contrast, DNCdk5 expression and/or p35 deficiency are protective in in vitro models of excitotoxic damage. In these latter excitotoxic paradigms, DNCk4 is less protective when compared with DNCdk5, and cyclin D1-deficient neurons fail to show resistance to death. These results also help to resolve a persistent controversy over which CDKs (cell cycle or Cdk5) may be important in neuronal death. We provide evidence that both pathways are of importance, but their significance likely depends upon the type of death insult and the differential initiating death pathways. These in vitro results are also consistent with our data in vivo. For example, in a global model of stroke, where more delayed modes of death are thought to predominate, Cdk4 appears to play a significant role. In contrast, in more severe focal models of stroke, where death is thought to be more rapid and excitotoxic, Cdk5 appears to play a more important role.
How might Cdk4 mediate a death signal? In both mild focal ischemia and a global model of stroke, Rb is phosphorylated on a known Cdk4 site, Ser-795. Our data show that ischemia-induced elevated Rb phosphorylation depends upon Cdk4. Moreover, it is likely that Rb plays a functional role, because expression of a constitutively active Rb, which cannot be phosphorylated on Ser-795 is protective, at least in vitro. However, it must be stressed that definitive evidence that Cdk4 acts solely through Rb has yet to be presented. The downstream effectors of Rb-mediated death in stroke are not completely clear. However, recent reports have indicated that E2F1, a well characterized Rb target, is important in neuronal death (28). For example, E2F1 expression kills neurons in vitro, and E2F1-deficient neurons are resistant to potassium deprivation (21) and β-amyloid exposure (29). Interestingly, E2F1-deficient mice are also resistant to mild focal ischemia (30), again suggesting that Cdk4/Rb pathway may be more significant in situations with more delayed ischemic death. Finally, it is important to mention that, because Cdk4 and Cdk6 have potentially overlapping functions, the role of Cdk6 cannot be excluded.
Unlike with Cdk4, we propose that Cdk5 is more relevant in acute excitotoxic death than delayed ischemic injury. Numerous reports have indicated that increased intracellular Ca2+ is a critical proximal event in excitotoxicity (31). Reports using in vitro systems have also established that Ca2+-activated proteases, calpains, cleave p35 to a more stable and mislocalized p25, from which mediate the pathogenic effects of Cdk5 (4). Accordingly, the participation of Cdk5 in excitotoxicity is concordant with the deregulated Ca2+, the prime effectors of excitotoxic damage. The link between excitotoxicity, calpains, and Cdk5 is strengthened by reports that calpain inhibition is also protective in focal models of stroke (32). Numerous substrates of Cdk5 have been reported. Of these candidates, two are particularly intriguing with regard to neuronal death. A recent report has indicated that MEF2 is phosphorylated and inactivated by a p25/Cdk5 complex, which is mislocalized from the cytoplasm to the nucleus (16). The importance of this mechanism in adult models of ischemic injury is presently unknown and should be clarified in future studies. An alternative potential mechanism involves Cdk5-mediated phosphorylation of the NMDA receptor 2A subunit at Ser-1232 (10). This phosphorylation is although to potentiate the activity of the NMDA receptor. The description of the NMDA receptor subunit as a Cdk5 substrate is consistent with our hypothesis that Cdk5 is functionally more relevant in excitotoxic mechanisms of death. Similar to Wang et al. (10), we have also shown that DNCdk5 expression is protective, with a shorter 5-min ischemic global insult (data not shown). This is likely due to the fact that in the global model, shorter insult times lead to more MK801-responsive death pathways, as has been reported (33).
Conclusion
We have shown that cell cycle CDKs and Cdk5 modulate distinct ischemic death pathways. Because both excitotoxic and delayed pathways are critical in mediating stroke damage, strategies designed to inhibit multiple CDK members may be an important and effective therapeutic strategy.
Supplementary Material
Acknowledgments
We thank Dr. Barbara Vanderhyden for production of transgenic mice, Dr. Jean-Pierre Julien (Laval University, Quebec) for providing the neuron-specific enolase promoter, and Michael O'Hare for reading of the manuscript. This work was supported by grants from the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Stroke Network, the Centre for Brain Recovery (D.S.P.), and the National Institutes of Health (Grant AG12721, to I.V.).
Author contributions: J.R., G.I., and D.S.P. designed research; J.R., G.I., H.A., and M.R. performed research; H.A., I.V., S.C., R.J.B., R.S.S., M.J.D., and D.S.P. contributed new reagents/analytic tools; J.R., G.I., R.S.S., and D.S.P. analyzed data; and J.R., G.I., and D.S.P. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CDK, cyclin-dependent kinase; rAAV1, recombinant adeno-associated virus 1; AV, adenovirus; CGN, cerebellar granule neuron; DNCDK, dominant negative CDK; 4VO, four-vessel occulsion; Rb, retinoblastoma protein.
References
- 1.Dirnagl, U., Iadecola, C. & Moskowitz, M. (1999) Trends Neurosci. 22, 391-397. [DOI] [PubMed] [Google Scholar]
- 2.Ekholm, S. & Reed, S. (2000) Curr. Opin. Cell Biol. 12, 676-684. [DOI] [PubMed] [Google Scholar]
- 3.Gold, M. & Rice, A. (1998) Nucleic Acids Res. 26, 3784-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhavan, R. & Tsai, L. (2001) Nat. Rev. Mol. Cell. Biol. 2, 749-759. [DOI] [PubMed] [Google Scholar]
- 5.Copani, A., Uberti, D., Sortino, M., Bruno, V., Nicoletti, F. & Memo, M. (2001) Trends Neurosci. 24, 25-31. [DOI] [PubMed] [Google Scholar]
- 6.Timsit, S., Rivera, S., Ouaghi, P., Guischard, F., Tremblay, E., Ben-Ari, Y. & Khrestchatisky, M. (1999) Eur. J. Neurosci. 11, 263-278. [DOI] [PubMed] [Google Scholar]
- 7.Katchanov, J., Harms, C., Gertz, K., Hauck, L., Waeber, C., Hirt, L., Priller, J., von Harsdorf, R., Bruck, W., Hortnagl, H., et al. (2001) J. Neurosci. 21, 5045-5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osuga, H., Osuga, S., Wang, F., Fetni, R., Hogan, M., Slack, R., Hakim, A., Ikeda, J. & Park, D. (2000) Proc. Natl. Acad. Sci. USA 97, 10254-10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, F., Corbett, D., Osuga, H., Osuga, S., Ikeda, J., Slack, R., Hogan, M., Hakim, A. & Park, D. (2002) J. Cereb. Blood Flow Metab. 22, 171-182. [DOI] [PubMed] [Google Scholar]
- 10.Wang, J., Liu, S., Fu, Y., Wang, J. & Lu, Y. (2003) Nat. Neurosci. 6, 1039-1047. [DOI] [PubMed] [Google Scholar]
- 11.De Azevedo, W., Jr., Mueller-Dieckmann, H., Schulze-Gahmen, U., Worland, P., Sausville, E. & Kim, S. (1996) Proc. Natl. Acad. Sci. USA 93, 2735-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith, P., Crocker, S., Jackson-Lewis, V., Jordan-Sciutto, K., Hayley, S., Mount, M., O'Hare, M. J., Callaghan, S., Slack, R., Przedborski, S., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 13650-13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclerc, S., Garnier, M., Hoessel, R., Marko, D., Bibb, J., Snyder, G., Greengard, P., Biernat, J., Wu, Y., Mandelkow, E., et al. (2001) J. Biol. Chem. 276, 251-260. [DOI] [PubMed] [Google Scholar]
- 14.Park, D., Levine, B., Ferrari, G. & Greene, L. (1997) J. Neurosci. 17, 8975-8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Heuvel, S. & Harlow, E. (1993) Science 262, 2050-2054. [DOI] [PubMed] [Google Scholar]
- 16.Gong, X., Tang, X., Wiedmann, M., Wang, X., Peng, J., Zheng, D., Blair, L., Marshall, J. & Mao, Z. (2003) Neuron 38, 33-46. [DOI] [PubMed] [Google Scholar]
- 17.Zolotukhin, S., Potter, M., Zolotukhin, I., Sakai, Y., Loiler, S., Fraites, T., Jr., Chiodo, V., Phillipsberg, T., Muzyczka, N., Hauswirth, W., et al. (2002) Methods 28, 158-167. [DOI] [PubMed] [Google Scholar]
- 18.He, T., Zhou, S., da Costa, L., Yu, J., Kinzler, K. & Vogelstein, B. (1998) Proc. Natl. Acad. Sci. USA 95, 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park, D., Morris, E., Bremner, R., Keramaris, E., Padmanabhan, J., Rosenbaum, M., Shelanski, M., Geller, H. & Greene, L. (2000) J. Neurosci. 20, 3104-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallows, J., Chen, K., DePinho, R. & Vincent, I. (2003) J. Neurosci. 23, 10633-10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Hare, M., Hou, S., Morris, E., Cregan, S., Xu, Q., Slack, R. & Park, D. (2000) J. Biol. Chem. 275, 25358-25364. [DOI] [PubMed] [Google Scholar]
- 22.Biernaskie, J. & Corbett, D. (2001) J. Neurosci. 21, 5272-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson, R., Morton, M., Tsao-Wu, G., Savalos, R., Davidson, C. & Sharp, F. (1990) J. Cereb. Blood Flow Metab. 10, 290-293. [DOI] [PubMed] [Google Scholar]
- 24.Pines, J. (1993) Biochem. Soc. Trans. 21, 921-925. [DOI] [PubMed] [Google Scholar]
- 25.Briones, T. & Therrien, B. (2000) Biol. Res. Nurs. 1, 276-286. [DOI] [PubMed] [Google Scholar]
- 26.Connell-Crowley, L., Harper, J. & Goodrich, D. (1997) Mol. Biol. Cell 8, 287-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris, E. & Dyson, N. (2001) Adv. Cancer Res. 82, 1-54. [DOI] [PubMed] [Google Scholar]
- 28.Liu, D. & Greene, L. (2001) Neuron 32, 425-438. [DOI] [PubMed] [Google Scholar]
- 29.Giovanni, A., Keramaris, E., Morris, E., Hou, S., O'Hare, M., Dyson, N., Robertson, G., Slack, R. & Park, D. (2000) J. Biol. Chem. 275, 11553-11560. [DOI] [PubMed] [Google Scholar]
- 30.MacManus, J., Jian, M., Preston, E., Rasquinha, I., Webster, J. & Zurakowski, B. (2003) J. Cereb. Blood Flow Metab. 23, 1020-1028. [DOI] [PubMed] [Google Scholar]
- 31.Arundine, M. & Tymianski, M. (2003) Cell Calcium 34, 325-337. [DOI] [PubMed] [Google Scholar]
- 32.Markgraf, C., Velayo, N., Johnson, M., McCarty, D., Medhi, S., Koehl, J., Chmielewski, P. & Linnik, M. (1998) Stroke 29, 152-158. [DOI] [PubMed] [Google Scholar]
- 33.Murase, K., Kato, H. & Kogure, K. (1993) Neurosci. Lett. 149, 229-232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.