Abstract
Progesterone induces G2-arrested Xenopus oocytes to develop into fertilizable eggs in a process called meiotic maturation. Protein kinase A (PKA), the cAMP-dependent protein kinase, has long been known to be a potent inhibitor of meiotic maturation, but little information is available on how PKA functions. We have cloned two Xenopus PKA catalytic subunit isoforms, XPKAα and XPKAβ. These proteins are 89% identical and both inhibit progesterone-induced meiotic maturation when overexpressed at low levels, suggesting that PKA activity is tightly regulated in the oocyte. Unexpectedly, catalytically inactive XPKA mutants are able to block progesterone-induced maturation as efficiently as the wild-type active XPKA. These mutants also block meiotic maturation induced by Mos, but are less efficient at inhibiting Cdc25C-induced maturation. Our results indicate that PKA can inhibit meiotic maturation by a novel mechanism, which does not require its kinase activity and is also independent of binding to the PKA regulatory subunits.
Oocytes from Xenopus laevis are arrested at the first meiotic prophase and can be induced to develop into fertilizable eggs by the steroid hormone progesterone in a process called meiotic maturation. Progesterone triggers various signal transduction pathways in the oocyte (1, 2), which lead to the activation of Cdc2/cyclin B and entry into M phase of meiosis.
The cAMP-dependent protein kinase (PKA) plays a crucial role in meiotic maturation. Inactive PKA is a tetrameric holoenzyme composed of two functionally distinct subunits, a dimeric regulatory subunit (PKA-R) and two monomeric catalytic subunits (PKAc) (3). Four PKA-R subunits (RIα, RIβ, RIIα, and RIIβ) and three PKAc subunits (α, β, and γ) have been identified in mammals as distinct gene products (4, 5). The PKA-R dimer acts in part as a pseudosubstrate to inhibit the phosphotransferase activity of the PKAc subunit and can bind four cAMP molecules in a cooperative manner, resulting in the release of active PKAc monomers. A second class of physiological PKA inhibitors is the heat-stable protein kinase inhibitors PKIs (6), which bind with high specificity and affinity to PKAα and PKAβ, whereas PKAγ is insensitive to inhibition by PKI (7).
It was reported 25 years ago that injection of PKAc purified from rabbit skeletal muscle blocks progesterone-induced oocyte maturation, whereas injection of purified PKA-R type II or PKI induced meiotic maturation in the absence of progesterone (8). These results suggested that PKA activity was necessary and sufficient to maintain the meiotic G2 block of oocytes. Consistent with this idea, a transient (and modest) decrease in the level of the second messenger cAMP was measured within minutes after progesterone stimulation, which correlated with a 50% reduction in the membrane-bound adenylate cyclase activity (9, 10). Inhibitors of phosphodiesterases, such as 3-isobutyl-1-methylxanthine, that increase intracellular cAMP levels also blocked progesterone-induced meiotic maturation (11).
Initial studies concluded that early protein synthesis-dependent steps were affected by PKAc during oocyte maturation (11). More recently, PKAc has also been shown to inhibit meiotic maturation induced by a variety of downstream effectors of the maturation signal transduction pathways (12–14). For example, purified bovine PKAc can inhibit the maturation induced by injection of Mos protein kinase (13) and also blocks Mos accumulation in response to progesterone (14). However, PKAc does not block the synthesis of endogenous Mos when mitogen-activated protein kinase (MAPK) is previously activated by injection of recombinant Mos protein (15), consistent with the observation that MAPK activity is required for full stimulation of Mos translation (16, 17). Elevation of endogenous PKA activity by treatment of oocytes with 3-isobutyl-1-methylxanthine can also delay Cdc2/cyclin B activation and germinal vesicle breakdown (GVBD) induced by injection of recombinant cyclin B or Cdc25A proteins (12). Moreover, in progesterone- or Mos-stimulated oocytes, PKAc prevents the electrophoretic mobility shift of Cdc25C, which normally correlates with Cdc25C hyperphosphorylation and activation (14), indicating that PKA may act on a signaling pathway that regulates Cdc25C activation.
Here we report the cloning of two Xenopus PKAc isoforms, XPKAα and XPKAβ, which are both able to inhibit meiotic maturation. Unexpectedly, we found that the catalytic activity of XPKAc is not necessary to block meiotic maturation induced by progesterone or Mos, whereas it is important for the inhibition of Cdc25C-induced maturation.
Materials and Methods
Cloning of Two Xenopus PKAc Isoforms and Generation of Mutants.
A Xenopus oocyte cDNA library (gift from J. Shuttleworth, University of Birmingham, Birmingham, U.K.) was screened with the full-length murine PKAα cDNA as a probe (18). Screening of 106 plaque-forming units resulted in around 200 positive clones. We analyzed 10 phages and found that eight of them corresponded to the same cDNA encoding a protein of 351 aa that was most related to human PKAα. Another clone encoded a protein of 350 aa that was most similar to human PKAβ.
All mutations were introduced into XPKAα in the FTX5 expression vector (provided by C. Hill, Imperial Cancer Research Fund, London) by using the QuikChange site-directed mutagenesis kit (Stratagene) and were confirmed by DNA sequencing. The frameshift-6 mutation was generated by introducing an A between codons 6 and 7 (ACC A ACA) of the XPKAα ORF. The stop-36 mutation was generated by changing codon 36 (CAG) to a stop codon (TAG).
Isolation of Xenopus Oocytes and Induction of Meiotic Maturation.
Oocytes were isolated from ovaries of X. laevis frogs as described (19). For the induction of meiotic maturation stage VI oocytes were incubated with 5 μg/ml progesterone (Sigma) or injected with 50 nl of purified recombinant proteins (19). For the generation of recombinant glutathione S-transferase (GST)-PKI, a human PKI cDNA (20) was amplified by PCR and cloned into the bacterial expression vector pGEX-KG. Maturation was scored by the appearance of a white spot on the animal pole of the oocytes. GVBD was confirmed by dissection of oocytes that had been fixed in 3% trichloroacetic acid for 10 min.
Preparation of mRNAs for Injection into Oocytes.
Capped mRNAs were obtained from FTX5 constructs by using the MEGAscript in vitro transcription kit (Ambion, Austin, TX) according to the manufacturer's instructions (19). Oocytes were injected with 50 nl of mRNA solutions and left for 8–16 h at 18°C to allow protein expression.
Preparation of Oocyte Lysates and Immunoblotting.
Oocyte lysates were prepared in histone H1 kinase (H1K) buffer (10 μl per oocyte) as described (19). For membrane preparation, oocyte lysates were centrifuged again at 100,000 × g in a table ultracentrifuge (Beckman TL-100) for 1 h at 4°C. The membrane pellets were washed twice, resuspended in H1K buffer (1 μl per oocyte), and analyzed by SDS/PAGE (equivalent to 10 oocytes per lane).
Immunoblotting was performed as described (19, 21) by using the following antibodies: PKAα, PKAβ, PKAγ, PKA-RIIα, myc, p90Rsk1, and p90Rsk2 (Santa Cruz Biotechnology), PKA-RI (Transduction Laboratories, Lexington, KY), phospho-PKA substrate (Cell Signaling Technology, Beverly, MA), Plx1 (provided by D. Glover, University of Cambridge, Cambridge, U.K.), Cdc2 (3E1, provided by J. Gannon and T. Hunt, Imperial Cancer Research Fund, South Mimms, U.K.), p42 MAPK XMpk1 and Myt1 (21), and XCdc25C (affinity-purified rabbit antiserum prepared against the catalytic domain of Xenopus Cdc25C fused to GST).
Purification and Kinase Activity of Recombinant GST-XPKAc.
Expression constructs encoding wild-type and K72R XPKAα were prepared in the pGEX-KG vector and transformed into Escherichia coli BL21-CodonPlus (DE-3) (Stratagene). The GST-fusion proteins were purified by using standard protocols (21).
Purified GST-XPKA wild-type and GST-XPKA K72R (100 ng) were incubated with histone H1 (4 μg) or kemptide (2 μg) in 12 μl of 50 mM Tris⋅HCl, pH 7.5 containing 10–50 μM cold ATP, 10 mM MgCl2, 2 μM microcystin, 2 μM DTT, and 2 μCi [γ-32P]ATP (3,000 Ci/mmol, Amersham Pharmacia) for 30 min at 30°C. The reactions were analyzed by SDS/PAGE and autoradiography.
PKA Activity in Total Oocyte Lysates and Immunoprecipitates.
Oocytes were lysed in H1K buffer (10 μl per oocyte) and 2.5 μl of the oocyte lysate was incubated in 15 μl H1K buffer containing 100 μM ATP, 2 μCi [γ-32P]ATP (3,000 Ci/mmol, Amersham Pharmacia), 2 μM microcystin, and 150 μM kemptide for 30 min at 30°C followed by SDS/PAGE on a 18.5% Laemmli gel (22). As a control, the oocyte lysates were preincubated for 15 min with the specific PKA inhibitor PKI (10 μM; fragment 6–22 amide, Sigma) before kemptide kinase activity assay.
To assay PKA activity in immunoprecipitates, 10 oocytes expressing myc-tagged XPKAc mutants were lysed in H1K buffer (10 μl per oocyte), and the cleared supernatants were mixed with 100 μl of immunoprecipitation (IP) buffer (19) containing 5 μl agarose-conjugated myc antibodies (10 μg IgG, Santa Cruz Biotechnology) and incubated for 2 h at 4°C on a rotating wheel. The beads were washed twice with IP buffer and twice with H1K buffer then incubated for 30 min at 30°C in 15 μl of H1K buffer containing 50 μM cold ATP, 2 μCi [γ-32P]ATP (3,000 Ci/mmol, Amersham Pharmacia), 2 μM microcystin, and 4 μg histone H1. The reactions were analyzed by SDS/PAGE and autoradiography.
Kinase Assays.
The kinase activity of Cdc2 was assayed in oocyte lysates containing 50 μM PKI with histone H1 as a substrate as described (19). The kinase activities of p90Rsk and Plx1 were assayed in immunoprecipitates prepared from oocyte lysates by using the substrates GST-Myt1 Ct and α-casein, respectively (21, 23).
Results
Identification and Quantification of Endogenous PKAc in Xenopus Oocytes.
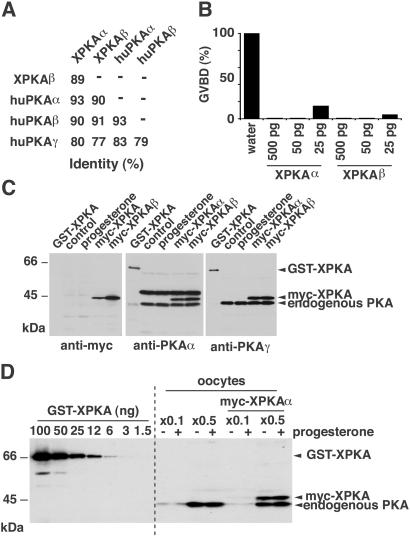
We cloned two different Xenopus PKAc isoforms that were 89% identical in their amino acid sequences and most similar to human PKAα and PKAβ, respectively (Fig. 1A and Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). Both isoforms (XPKAα and XPKAβ) were equally effective in blocking progesterone-induced maturation on overexpression in oocytes (Fig. 1B).
Figure 1.
Cloning of two Xenopus PKAc isoforms that inhibit oocyte maturation. (A) Identity in the amino acid sequences of XPKAα, XPKAβ, and human PKAc isoforms. (B) Oocytes were injected with the indicated concentrations of mRNAs encoding myc-tagged XPKAα or XPKAβ and incubated overnight before stimulation with progesterone for 20 h. (C) Purified GST-XPKAα (10 ng) and lysates from oocytes untreated (control) and treated with progesterone or injected with mRNAs encoding myc-tagged XPKAα and XPKAβ mRNAs were analyzed by immunoblotting by using the indicated antibodies. (D) The indicated amounts of purified GST-XPKAα and lysates from oocytes either uninjected or injected with XPKAα mRNA were analyzed by immunoblotting with the human PKAγ antibody.
Given the high amino acid sequence similarity between Xenopus and human PKAc, we used commercial antibodies raised against human isoforms to identify the endogenous PKAc in Xenopus oocytes. Antibodies directed against huPKAα and huPKAγ recognized with similar efficiency endogenous PKAc in oocytes and the overexpressed (from injected mRNAs) myc-tagged XPKAα and XPKAβ, as well as recombinant GST-XPKAα (Fig. 1C). Thus, these antibodies do not appear to be specific for the α or β isoforms of Xenopus PKAc. When compared with the anti-myc antibody on myc-tagged XPKAα and β, it looked like both antibodies recognize XPKAα slightly better than XPKAβ (Fig. 1C). Antibodies against huPKAβ hardly detected overexpressed myc-tagged XPKAα and XPKAβ or endogenous PKAc in oocytes (not shown).
To quantify the endogenous PKAc in oocytes, we used known amounts of recombinant GST-XPKAα in immunoblots with an antibody that cross-reacted well with both XPKAα and XPKAβ as a reference (Fig. 1D). The total amount of PKAc in the oocyte was estimated to be between 50 and 70 ng per oocyte (1.25–1.75 μM). No changes in the concentration of PKAc could be detected by immunoblot between G2-arrested and mature oocytes, which is in agreement with previous reports (24).
Catalytically Inactive XPKAc Blocks Progesterone-Induced Meiotic Maturation in Xenopus Oocytes.
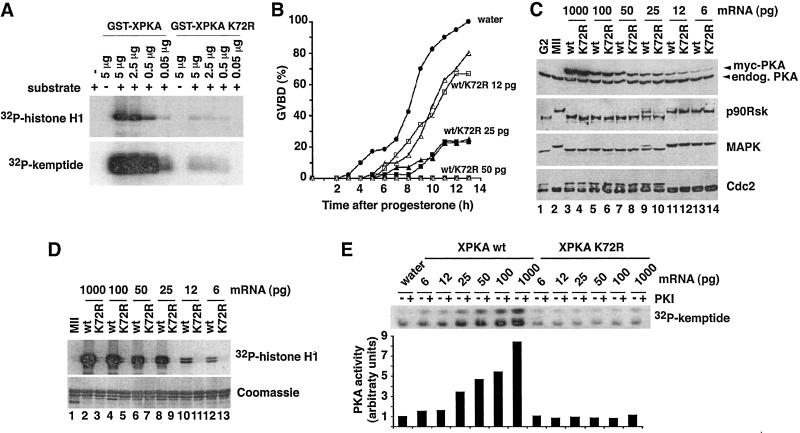
To analyze whether the catalytic activity of PKA was required for inhibition of oocyte maturation, a kinase-inactive version of XPKAα was generated by mutation of lysine 72 to arginine (K72R mutant). This mutation in the ATP-binding pocket has been shown to impair catalytic activity of mammalian PKAc (25, 26). We confirmed that the K72R mutation reduced the kinase activity of recombinant GST-XPKAc approximately 200 times by using either histone H1 or kemptide as in vitro substrates (Fig. 2A).
Figure 2.
Kinase-inactive XPKAc inhibits Xenopus oocyte maturation. (A) The indicated amounts of GST-XPKAα wild-type or the K72R mutant were incubated in a kinase assay with histone H1 (5 μg) or kemptide (2 μg) and analyzed by SDS/PAGE and autoradiography. (B) Oocytes were injected with the indicated amounts of myc-tagged wild-type (squares) and K72R (triangles) XPKAc mRNAs and 14 h later were incubated with progesterone. Injection of more than 25 pg of either wild-type or K72R XPKAc mRNA completely blocked maturation, whereas injection of 6 pg had no effect on the kinetics of meiotic maturation. (C) Oocytes untreated (lane 1) or treated with progesterone for 14 h (lanes 2–14) from the same experiment as in B were analyzed by immunoblotting by using PKA, p90Rsk, p42 MAPK, and Cdc2 antibodies, as indicated. (D) Myc-tagged wild-type or K72R XPKAc were immunoprecipitated from groups of 10 oocytes injected with the indicated amounts of mRNAs as in B and the immunoprecipitates were used for a kinase assay with histone H1. The kinase reactions were analyzed by SDS/PAGE and autoradiography. (E) PKA activity was assayed in total oocyte lysates from the same experiment as in B with kemptide as a substrate in the presence (+) or absence (−) of PKI. The kinase reactions were analyzed by SDS/PAGE. Phosphorylated kemptide was directly quantified on the gels by using a Fuji PhosphorImaging device, and the arbitrary units for PKA-specific kemptide activity were calculated by subtracting the values obtained in the presence of PKI.
Oocytes were injected with different concentrations of mRNAs encoding myc-tagged forms of wild-type and K72R XPKAc and then incubated with progesterone (Fig. 2B). Unexpectedly, kinase-inactive XPKAc blocked progesterone-induced maturation as efficiently as wild-type XPKAc. The expression levels of overexpressed wild-type and K72R PKAc in these oocytes were investigated by anti-PKA immunoblots (Fig. 2C). Injection of 50 pg of XPKAc mRNA led to a 50–100% increase over the endogenous PKAc level and completely blocked oocyte maturation (Fig. 2C, lanes 7 and 8, anti-PKA), whereas overexpression of only 20% by injection of 25 pg of mRNA (Fig. 2C, lanes 9 and 10) significantly delayed meiotic maturation (Fig. 2B). Biochemical analysis confirmed that only progesterone-treated oocytes injected with water (Fig. 2C, lane 2) or low amounts of XPKAc mRNA (Fig. 2C, lanes 11–14) underwent meiotic maturation, as demonstrated by both the appearance of phosphorylated MAPK and p90Rsk and the disappearance of the tyrosine-phosphorylated Cdc2 of slower electrophoretic mobility (Fig. 2C).
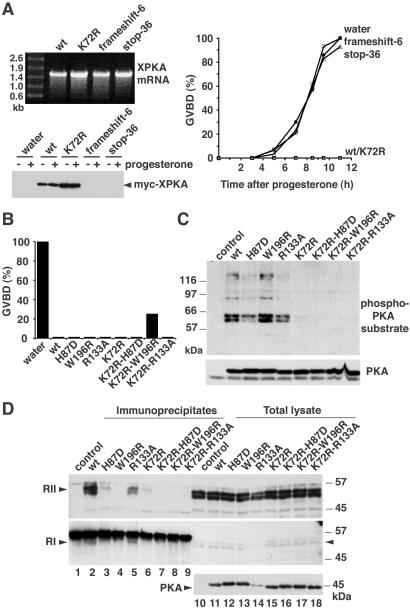
As overexpression of only small amounts of XPKAc had such a strong inhibitory effect, we investigated whether K72R XPKAc had any residual kinase activity in the oocytes. For this purpose, myc-tagged wild-type or kinase-inactive PKA was immunoprecipitated from oocytes, and its kinase activity was assayed by using histone H1 as substrate (Fig. 2D). We could not detect any activity in the K72R XPKAc immunoprecipitates even at the highest concentration of mRNA injected (Fig. 2D, lane 3). Moreover, the activity of wild-type XPKAc was still high in samples in which the maturation was merely delayed (Fig. 2D, lanes 8 and 10) or not affected at all (Fig. 2D, lane 12). These results were confirmed by directly assaying PKA activity in total oocyte lysates with kemptide as substrate (Fig. 2E). Overexpression of active XPKAc substantially increased the kemptide kinase activity of the oocyte lysates, which could be totally inhibited by PKI (Fig. 2E, XPKA wt). In contrast, we could detect no increase in PKA activity when catalytically inactive XPKAc was overexpressed in the oocytes (Fig. 2E, XPKA K72R). Furthermore, another kinase-dead XPKAc mutant, aspartate 184 to alanine (D184A), also blocked progesterone-induced oocyte maturation as efficiently as the wild-type XPKAc (see Fig. 7, which is published as supporting information on the PNAS web site). Mutation of the residue corresponding to D184 in Saccharomyces cerevisiae PKA (D228A) has been proposed to completely abolish kinase activity based on a cell viability assay (27). Taken together, these experiments showed that overexpressed XPKAc does not need to be catalytically active to block progesterone-induced oocyte maturation. To demonstrate that the PKA inhibitory activity was at the protein level and not at the mRNA level, we introduced two nonsense mutations in the PKA ORF: a frameshift mutation at codon 6 and a stop codon after amino acid 35. Injection of either of these two mRNAs had no effect on progesterone-induced oocyte maturation, consistent with the absence of XPKAc overexpression in the oocytes (Fig. 3A). We also confirmed that oocytes injected with high concentrations of mRNAs encoding other myc-tagged proteins, such as tubulin or XCdk8, matured normally in response to progesterone (see Fig. 8, which is published as supporting information on the PNAS web site).
Figure 3.
Inhibition of progesterone-induced meiotic maturation by XPKAc mutants impaired in binding to PKA-R or PKI but not by XPKA mRNAs encoding frameshift mutants. (A) The indicated in vitro transcribed XPKA mRNAs (500 ng) were analyzed by electrophoresis on a 1% agarose gel (Upper Left). Oocytes were injected with 2 ng of the mRNAs and 14 h later were incubated with progesterone. Meiotic maturation was monitored by the appearance of a white spot on the animal pole of the oocytes and GBVD was confirmed by dissection of the oocytes (Right). Oocyte lysates were prepared 12 h after progesterone addition and analyzed by immunoblotting with a myc antibody (Lower Left). (B) Oocytes were injected with 300 pg of mRNA encoding the indicated XPKAc mutants and expression was allowed for 14 h before treatment with progesterone. Meiotic maturation was scored 8 h later as indicated in A. (C) Lysates corresponding to one oocyte expressing the indicated XPKAc mutants were analyzed by SDS/PAGE and immunoblotting by using a phospho-PKA substrate antibody. (D) Myc-tagged PKA mutants were expressed in oocytes by injection of 1 ng of the corresponding mRNAs and the oocytes were incubated for 16 h. PKA immunoprecipitates were prepared from 10 oocytes by using myc antibodies (lanes 1–9) and together with an aliquot of the total lysate equivalent to one oocyte (lanes 10–18) were analyzed by SDS/PAGE followed by immunoblotting. The blots were probed with antibodies against RII (Top) and RI (Middle). Expression of the PKA mutants was confirmed with anti-PKA antibodies (Bottom).
Inhibition of Progesterone-Induced Meiotic Maturation by XPKAc Mutants Impaired in Binding to the PKA-R Subunits or PKI.
In the absence of cAMP, PKA-RI and PKA-RII subunits bind to PKAc and block its catalytic activity. Thus, overexpressed K72R XPKAc could compete with endogenous PKAc for binding to the regulatory subunits, thereby releasing active endogenous PKAc and blocking oocyte maturation. Although the results shown above (Fig. 2E) suggested that endogenous PKA activity was not affected by overexpressed kinase-inactive PKA, the assay might not have been sensitive enough to detect subtle changes in PKA activity. Thus, we introduced both into the active and inactive XPKAc the mutations histidine 87 to aspartate (H87D) and tryptophan 196 to arginine (W196R), which have been reported to specifically impair binding of PKAc to its regulatory subunits (28, 29). We confirmed that on overexpression in oocytes, the H87D mutation did not affect the ability of either active or K72R XPKAc to block progesterone-induced maturation, whereas the W196R mutation slightly reduced the inhibitory activity of K72R PKA (Fig. 3B).
The K72R XPKAc could also inhibit meiotic maturation by binding to endogenous PKI, thereby releasing and activating endogenous PKAc. Although there is no evidence for the existence of PKIs in Xenopus oocytes, their presence cannot be ruled out. High-affinity binding of PKI to the catalytic subunit of PKA can be selectively abolished by the mutation arginine 133 to alanine (R133A) (30). When R133A active or kinase-inactive PKA mutants were expressed in oocytes, they completely blocked progesterone-induced meiotic maturation (Fig. 3B), demonstrating that kinase-inactive PKA was unlikely to inhibit meiotic maturation by binding to endogenous PKI.
To confirm the lack of binding to the PKA-R subunits, the H87D and W196R XPKAc mutants were expressed in oocytes, immunoprecipitated, and then analyzed by immunoblotting by using antibodies specific for PKA-RI and RII, respectively (Fig. 3D). The PKA-RII antibody recognized a doublet of 50–55 kDa in oocytes (Fig. 3D Top, lanes 10–18), which was efficiently coimmunoprecipitated with wild-type PKA (Fig. 3D, lane 2) and to a certain extent with the PKI binding-deficient R133A mutant (Fig. 3D, lane 5). However, the mutants H87D and K72R both coimmunoprecipitated only very minor amounts of PKA-RII (Fig. 3D, lanes 3 and 6), whereas W196R and the K72R double mutants did not coimmunoprecipitate detectable PKA-RII (Fig. 3D, lanes 4 and 7–9). The RI antibody recognized a faint band of approximately 52 kDa in oocytes (Fig. 3D Middle, lanes 10–18, arrowhead), which runs slightly faster than the myc antibody used for the immunoprecipitation (Fig. 3D Middle, lanes 1–9). Both wild-type and R133A XPKAc seemed to coimmunoprecipitate the PKA-RI subunit (Fig. 3D Middle, lanes 2 and 5) whereas none of the other mutants did. The small amount of PKA-RI detected in immunoblots is consistent with previous reports indicating that PKA holoenzymes of type II are predominant in oocytes (24). Our results demonstrate that the kinase-inactivating mutation K72R interferes with the binding of PKAc to PKA-R subunits. It seems therefore unlikely that these mutants can influence endogenous PKA activity by binding to the regulatory subunits.
To investigate the kinase activity of XPKAc mutants on endogenous oocyte proteins, we used a phospho-PKA substrate antibody that recognizes proteins containing phospho-threonine and phospho-serine in the motifs RxxT and RRxS, respectively. Overexpression of wild-type PKA in G2-arrested oocytes led to the detection of several bands by the phospho-PKA substrate antibody whereas overexpression of kinase-inactive XPKAc mutants did not change the phospho-PKA substrate pattern observed in uninjected oocytes (Fig. 3C). This finding is consistent with the kinase activity measured in PKA immunoprecipitates prepared from the same oocytes (see Fig. 9, which is published as supporting information on the PNAS web site) and further supports that the kinase-inactive PKAc mutants neither phosphorylate endogenous substrates themselves nor activate endogenous PKAc.
Effects of Wild-Type and K72R XPKAc on Meiotic Maturation Induced by Different Activators.
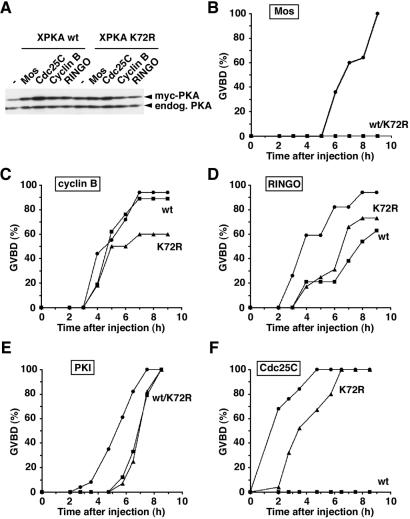
Meiotic maturation can be induced by injection of signaling molecules and cell cycle regulators that participate in the signaling pathways normally activated by progesterone. To investigate the effect of kinase-inactive PKA on different components of the meiotic maturation pathways, oocytes were first injected with low levels of wild-type or K72R XPKAc mRNA and then with the purified recombinant Mos, RINGO, PKI, cyclin B, and Cdc25C proteins (Fig. 4).
Figure 4.
Induction of meiotic maturation by different activators in the presence of wild-type or kinase-inactive XPKAc. Oocytes were injected with 50 pg of wild-type or K72R XPKAc mRNAs and 14 h later were injected again with 25 ng of MalE-Mos (B), His-6-cyclin B (C), MalE-RINGO (D), GST-Cdc25 (E), and GST-PKI (F) recombinant proteins. Lysates corresponding to one oocyte were analyzed by SDS/PAGE and immunoblotted with PKA antibodies to compare the amounts of overexpressed and endogenous PKA (A). Meiotic maturation was monitored by the occurrence of GVBD. Water-, wild-type PKA-, and K72R PKA-injected oocytes are shown as circles, squares, and triangles, respectively.
Mos-induced oocyte maturation, as monitored by GVBD, was completely blocked by low amounts (about the same level as the endogenous PKAc) of either wild-type or K72R XPKAc (Fig. 4 A and B), exactly as progesterone-induced maturation. In contrast, meiotic maturation induced by injection of cyclin B or RINGO, two direct activators of Cdc2 (reviewed by ref. 2), was delayed only by the XPKAc concentration that completely blocked Mos-induced maturation (Fig. 4 C and D). Both wild-type and K72R XPKAc also delayed to similar extents PKI-induced maturation (Fig. 4E). Interestingly, maturation induced by injection of GST-Cdc25C was completely blocked by active XPKAc but only slightly delayed by kinase-inactive XPKAc (Fig. 4F). This finding indicates a differential requirement for the catalytic activity of PKA depending on the step along the oocyte maturation pathway.
Kinase-Inactive XPKAc Blocks Mos-Induced Meiotic Maturation Downstream of p90Rsk.
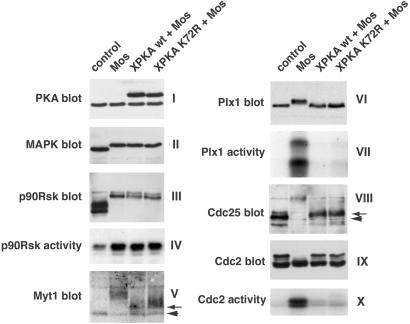
As Mos-induced maturation was completely blocked in all oocytes injected with low levels of either wild-type or K72R XPKAc even after 24 h, we investigated which steps in the Mos-triggered signal transduction pathway were inhibited. We found that the phosphorylation of MAPK and p90Rsk (a MAPK substrate) was not affected in oocytes overexpressing wild-type or K72R XPKAc (Fig. 5 II and III). Accordingly, normal levels of p90Rsk activity were measured in these oocytes (Fig. 5IV). In contrast, the Cdc2-inhibitory kinase Myt1 was only partially phosphorylated (Fig. 5V), consistent with the activation of p90Rsk but not Cdc2.
Figure 5.
Kinase-inactive XPKAc blocks Mos-induced meiotic maturation downstream of p90Rsk. Oocytes were injected with 50 pg of wild-type or K72R XPKAc mRNAs and 14 h later were injected again with 35 ng of MalE (control) or MalE-Mos (Mos) recombinant proteins. After another 24 h, oocyte lysates were prepared and analyzed by SDS/PAGE and immunoblotting by using antibodies against PKA, p42 MAPK, p90Rsk, Myt1, Plx1, Cdc25C, and Cdc2, as indicated. For Myt1 immunoblots, membrane fractions corresponding to 10 oocytes were used. In V and VIII, the arrowheads and arrows indicate the unphosphorylated and partially phosphorylated forms of Myt1 and Cdc25C, respectively. The activities of p90Rsk and Plx1 were measured in immunoprecipitates by using GST-Myt1-Ct and α-casein as substrates, respectively. The activity of Cdc2 was measured in oocyte lysates by using histone H1 as substrate.
Interestingly, the polo-like kinase Plx1, which can phosphorylate and activate Cdc25C (reviewed by ref. 2), was neither phosphorylated nor active in oocytes expressing wild-type or K72R XPKAc (Fig. 5 VI and VII). Likewise, Cdc25C was not hyperphosphorylated to the level observed in mature oocytes. However, a small mobility shift of Cdc25C could be detected in oocytes injected with either wild-type or kinase-inactive PKA (Fig. 5VIII), indicating that an as yet unidentified kinase activity toward Cdc25C was active in these oocytes but not in G2-arrested oocytes. In Mos-injected oocytes overexpressing wild-type or K72R XPKAc, Cdc2 was not tyrosine-dephosphorylated, as demonstrated by the presence of a band of slower electrophoretic mobility (Fig. 5IX). This result correlated with the absence of detectable H1K activity in the oocyte lysates (Fig. 5X). Our results demonstrate that the kinase activity of PKAc is not required to block Mos-induced meiotic maturation.
Discussion
PKAc has long been known to be a potent inhibitor of progesterone-induced meiotic maturation, but little is known about the endogenous PKA in oocytes. Here we have cloned Xenopus PKAc and shown that progesterone-induced oocyte maturation can be blocked by XPKAc mutants that neither have kinase activity nor can activate endogenous PKA.
Using as a reference XPKAα and XPKAβ proteins, we estimated that the concentration of endogenous PKAc in oocytes is about 1.5 μM (about 60 ng PKAc per oocyte). These numbers are in good agreement with indirect estimations based on the injection of rabbit muscle PKAc (8) or PKI (31). Because PKI can induce meiotic maturation (8) and PKAγ is not inhibited by PKI (7), the PKA isoform responsible for the inhibition of meiotic maturation is most likely PKAα or PKAβ. As the available anti-PKA antibodies cross-reacted with both XPKAα and XPKAβ, it was not possible to determine which one was the main isoform in oocytes. It should be noted, however, that overexpression of either XPKAα and XPKAβ can block oocyte maturation and only subtle differences have been reported between mammalian PKAα and PKAβ in substrate specificity and binding to the regulatory subunits (32).
Inhibiton of Meiotic Maturation by Catalytically Inactive PKA.
We found that small increases in the amount of PKAc can block or strongly delay progesterone-induced maturation, suggesting that PKAc is tightly regulated in the oocyte. Unexpectedly, overexpression of either of two catalytically inactive XPKAc mutants (K72R and D184A) blocked progesterone-induced oocyte maturation as efficiently as wild-type active XPKAc. More importantly, the activity of overexpressed wild-type XPKAc was significantly high in oocytes in which the maturation was only delayed or not affected at all. Thus, it seems that the critical factor for the PKA-dependent block of oocyte maturation is the amount of overexpressed XPKAc rather that its kinase activity.
Using XPKAc mutants defective in binding to PKA-R subunits or PKI, we ruled out the possibility that the overexpressed catalytically inactive XPKAc might compete with endogenous PKAc for binding to either PKA-R or PKI, allowing the release of active PKAc. We also found that the mutant K72R itself was impaired in binding to PKA-R, making it unlikely that endogenous PKA was activated in the oocytes on overexpression of the mutants. Moreover, the pattern of potential PKA substrates in oocytes was not changed by overexpression of kinase-inactive XPKAc, further implying that the K72R XPKAc neither phosphorylates oocyte proteins nor activates endogenous PKAc in the oocytes. Finally, no increase in kemptide kinase activity was detected in total oocyte lysates when 5–10 times more K72R XPKAc than endogenous PKAc was expressed in the oocytes. In contrast, overexpression of wild-type XPKAc always led to a detectable increase in kemptide kinase activity in this assay, even when the amount of overexpressed XPKAc was not sufficient to inhibit oocyte maturation.
Taken together, these results demonstrate that overexpression of kinase-inactive XPKAc does not activate endogenous PKA in the oocytes. Thus, PKAc can block meiotic maturation by a novel mechanism, which does not require its kinase activity. Catalytically inactive mutants of other kinases have also been reported to have biological effects. For example, kinase-dead KSR mutants can complement ksr1 loss-of-function alleles in Caenorhabditis elegans (33), and the kinase activity of Bub1 is not necessary to trigger the spindle checkpoint signal from the kinetochore (34). The yeast MAPK Kss1 has also a kinase-independent inhibitory function in the filamentation pathway (35), which is caused by its direct association with the transcription factor Ste12 (36).
PKAc has been shown to interact directly with several proteins including the NF-κB inhibitory proteins IκB-α and IκB-β (37), the transcription factor serum amyloid A-activating factor-1 (38), and 3-phosphoinositide-dependent kinase-1 (PDK1) (39). The interaction with PDK1 is intriguing because it is mediated by a hydrophobic motif at the C terminus of PKAc, which is homologous to the PDK1-interacting motif of several PDK1 substrates (39). PDK1 can also activate PKA by phosphorylation of Thr-197 in the activation loop (40), but stable association between PDK1 and full-length PKAc has not been demonstrated (only the C-terminal 223 aa of PKA were used in ref. 39). Interestingly, PDK1 can phosphorylate and activate the protein kinase PKB/Akt (reviewed by ref. 41) and injection of mRNA encoding a constitutively active PKB/Akt mutant induces oocyte maturation in the absence of progesterone (42). PKB/Akt has recently been shown to phosphorylate and down-regulate the Cdc2 inhibitory kinase Myt1 in starfish oocytes, leading to meiotic maturation (43). There is no evidence, however, for the implication of endogenous PKB/Akt in the progesterone-induced maturation of Xenopus oocytes. It will be very interesting to elucidate whether binding of catalytically inactive XPKAc to PDK1 could interfere with PDK1 signaling in Xenopus oocytes.
Kinase-Dependent and Independent Roles of PKA in Meiotic Maturation.
Our findings indicate that the early block of meiotic maturation by PKA does not require its kinase activity. Mos-induced maturation was also completely blocked by low amounts of K72R XPKAc. In these oocytes, the MAPK/p90Rsk pathway was normally activated, but the Cdc25C-activating kinase Plx1 was not active and this correlated with the absence of Cdc25C hyperphosphorylation. Cdc25C, however, was partially phosphorylated as indicated by a small electrophoretic mobility shift, suggesting that a kinase activity toward Cdc25C was active in these oocytes injected with PKAc plus Mos, which was not active in G2-arrested oocytes. This Cdc25C kinase appears to be different from Plx1 and Cdc2, because both are inactive in these oocytes. A small electrophoretic mobility shift of Cdc25C, which is independent of Plx1 and does not appear to result in Cdc25C activation, has also been reported in an oocyte cell-free system (44). The activation of Plx1 in Mos-injected oocytes has been shown to require protein synthesis (23), suggesting that PKAc blocks a Mos/MAPK-initiated positive feedback loop that requires mRNA translation and regulates Plx1 activity.
The catalytic activity of XPKAc was not required for any of the above effects on Mos-triggered maturation. However, kinase-inactive XPKAc was significantly less efficient to inhibit meiotic maturation induced by recombinant Cdc25C protein than active XPKAc. Cdc25C can directly dephosphorylate the inhibitory Tyr-15 residue of Cdc2 in preformed Cdc2/cyclinB complexes (pre-maturation-promoting factor) and thereby activate the kinase activity of Cdc2. Our results indicate that the kinase activity of PKA is required to inhibit pre-maturation-promoting factor activation by Cdc25C. This might involve direct inhibition of the Cdc25C phosphatase activity. For example, PKA has been proposed to stimulate an okadaic acid-sensitive serine/threonine phosphatase, probably PP2A, that down-regulates Cdc25C in Xenopus oocyte extracts (22). Alternatively, PKA might interfere with the accessibility of Cdc25C to the Tyr-phosphorylated Cdc2/cyclin B substrate.
It therefore seems that PKA can block at least two steps in oocyte maturation and only one of them requires PKA catalytic activity. The kinase-independent step is sufficient to block oocyte maturation induced by progesterone and Mos, probably by inhibiting a step upstream of Plx1 activation that requires mRNA translation and might be part of the positive feedback loops. In contrast, wild-type, but not catalytically inactive, PKAc interferes with Cdc25C-induced maturation. This kinase-dependent PKA effect might ensure the G2 arrest of the oocytes by negatively regulating Cdc25C activity, which acts late in the maturation process.
Our results suggest that PKAc may maintain the G2 arrest of Xenopus oocytes by binding to and sequestration of a protein or proteins rather than phosphorylating them. We also found that a small increase in the amount of PKAc potently blocks meiotic maturation, suggesting that the PKAc-interacting protein is present in a limited amount. Dissociation of the complex between PKAc and this protein might be an early step in the maturation process, perhaps triggered by the reduction in cAMP levels, which would allow PKA-R to compete for PKAc binding.
Supplementary Material
Abbreviations
- GST
glutathione S-transferase
- GVBD
germinal vesicle breakdown
- H1K
histone H1 kinase
- MAPK
mitogen-activated protein kinase
- PDK1
3-phosphoinositide-dependent kinase-1
- PKA
protein kinase A
- PKAc
PKA catalytic subunit
- PKA-R
PKA regulatory subunit
- PKI
PKA inhibitor
Footnotes
References
- 1.Ferrell J E., Jr BioEssays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Nebreda A R, Ferby I. Curr Opin Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 3.Taylor S S, Buechler J A, Yonemoto W. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 4.Beebe S J. Semin Cancer Biol. 1994;5:285–294. [PubMed] [Google Scholar]
- 5.Tasken K, Skalhegg B S, Tasken K A, Solberg R, Knutsen H K, Levy F O, Sandberg M, Orstavik S, Larsen T, Johansen A K, et al. Adv Second Messenger Phosphoprotein Res. 1997;31:191–204. doi: 10.1016/s1040-7952(97)80019-5. [DOI] [PubMed] [Google Scholar]
- 6.Walsh D A, Ashby C D, Gonzalez C, Calkins D, Fischer E H. J Biol Chem. 1971;246:1977–1985. [PubMed] [Google Scholar]
- 7.Beebe S J, Salomonsky P, Jahnsen T, Li Y. J Biol Chem. 1992;267:25505–25512. [PubMed] [Google Scholar]
- 8.Maller J L, Krebs E G. J Biol Chem. 1977;252:1712–1718. [PubMed] [Google Scholar]
- 9.Mulner O, Huchon D, Thibier C, Ozon R. Biochim Biophys Acta. 1979;582:179–184. doi: 10.1016/0304-4165(79)90301-5. [DOI] [PubMed] [Google Scholar]
- 10.Sadler S E, Maller J L. J Biol Chem. 1981;256:6368–6373. [PubMed] [Google Scholar]
- 11.Maller J L, Krebs E G. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- 12.Rime H, Haccard O, Ozon R. Dev Biol. 1992;151:105–110. doi: 10.1016/0012-1606(92)90217-5. [DOI] [PubMed] [Google Scholar]
- 13.Daar I, Yew N, Vande Woude G F. J Cell Biol. 1993;120:1197–1202. doi: 10.1083/jcb.120.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matten W, Daar I, Vande Woude G F. Mol Cell Biol. 1994;14:4419–4426. doi: 10.1128/mcb.14.7.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faure S, Morin N, Doree M. Oncogenes. 1998;17:1215–1221. doi: 10.1038/sj.onc.1202056. [DOI] [PubMed] [Google Scholar]
- 16.Matten W T, Copeland T D, Ahn N G, Vande Woude G F. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 17.Howard E L, Charlesworth A, Welk J, MacNicol A M. Mol Cell Biol. 1999;19:1990–1999. doi: 10.1128/mcb.19.3.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slice L W, Taylor S S. J Biol Chem. 1989;264:20940–20946. [PubMed] [Google Scholar]
- 19.Ferby I, Blazquez M, Palmer A, Eritja R, Nebreda A R. Genes Dev. 1999;13:2177–2189. doi: 10.1101/gad.13.16.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen S R, Uhler M D. J Biol Chem. 1991;266:11158–11162. [PubMed] [Google Scholar]
- 21.Palmer A, Gavin A-C, Nebreda A R. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grieco D, Avvedimento E V, Gottesman M E. Proc Natl Acad Sci USA. 1994;91:9896–9900. doi: 10.1073/pnas.91.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavin A C, Ni Ainle A, Chierici E, Jones M, Nebreda A R. Mol Biol Cell. 1999;10:2971–2986. doi: 10.1091/mbc.10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masaracchia R A, Maller J L, Walsh D A. Arch Biochem Biophys. 1979;194:1–12. doi: 10.1016/0003-9861(79)90589-7. [DOI] [PubMed] [Google Scholar]
- 25.Zoller M J, Nelson N C, Taylor S S. J Biol Chem. 1981;256:10837–10842. [PubMed] [Google Scholar]
- 26.Taylor S S, Knighton D R, Zheng J, Sowadski J M, Gibbs C S, Zoller M J. Trends Biochem Sci. 1993;18:84–89. doi: 10.1016/0968-0004(93)80001-r. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs C S, Zoller M J. J Biol Chem. 1991;266:8923–8931. [PubMed] [Google Scholar]
- 28.Cox S, Taylor S S. J Biol Chem. 1994;269:22614–22622. [PubMed] [Google Scholar]
- 29.Gibson R M, Taylor S S. J Biol Chem. 1997;272:31998–2005. doi: 10.1074/jbc.272.51.31998. [DOI] [PubMed] [Google Scholar]
- 30.Wen W, Taylor S S. J Biol Chem. 1994;269:8423–8430. [PubMed] [Google Scholar]
- 31.Cicirelli M F, Pelech S L, Krebs E G. J Biol Chem. 1988;263:2009–2019. [PubMed] [Google Scholar]
- 32.Gamm D M, Baude E J, Uhler M D. J Biol Chem. 1996;271:15736–15742. doi: 10.1074/jbc.271.26.15736. [DOI] [PubMed] [Google Scholar]
- 33.Stewart S, Sundaram M, Zhang Y, Lee J, Han M, Guan K L. Mol Cell Biol. 1999;19:5523–5534. doi: 10.1128/mcb.19.8.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp-Baker H, Chen R H. J Cell Biol. 2001;153:1239–1250. doi: 10.1083/jcb.153.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhani H D, Styles C A, Fink G R. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 36.Bardwell L, Cook J G, Voora D, Baggott D M, Martinez A R, Thorner J. Genes Dev. 1998;12:2887–2898. doi: 10.1101/gad.12.18.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 38.Ray B K, Chen J, Ray A. J Immunol. 2001;167:2343–2348. doi: 10.4049/jimmunol.167.4.2343. [DOI] [PubMed] [Google Scholar]
- 39.Biondi R M, Cheung P C, Casamayor A, Deak M, Currie R A, Alessi D R. EMBO J. 2000;19:979–988. doi: 10.1093/emboj/19.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng X, Ma Y, Moore M, Hemmings B A, Taylor S S. Proc Natl Acad Sci USA. 1998;95:9849–9854. doi: 10.1073/pnas.95.17.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawlor M A, Alessi D R. J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 42.Andersen C B, Roth R A, Conti M. J Biol Chem. 1998;273:18705–18708. doi: 10.1074/jbc.273.30.18705. [DOI] [PubMed] [Google Scholar]
- 43.Okumura E, Fukuhara T, Yoshida H, Hanada S, Kozutsumi R, Mori M, Tachibana K, Kishimoto T. Nat Cell Biol. 2002;4:111–116. doi: 10.1038/ncb741. [DOI] [PubMed] [Google Scholar]
- 44.Karaiskou A, Jessus C, Brassac T, Ozon R. J Cell Sci. 1999;112:3747–3756. doi: 10.1242/jcs.112.21.3747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.