Abstract
VEGF receptor 1 (VEGFR-1/Flt-1) is a high-affinity tyrosine kinase (TK) receptor for VEGF and regulates angiogenesis as well as monocyte/macrophage functions. We previously showed that the osteoclast deficiency in osteopetrotic Csf1op/Csf1op (op/op) mice is gradually restored in an endogenous, VEGF-dependent manner. However, the molecular basis of the recovery is still not clear. To examine which VEGFR is important and to clarify how colony-stimulating factor 1 (CSF-1) and VEGF signals interact in osteoclastogenesis, we introduced a VEGFR-1 signaling deficiency (Flt1TK-/-) into op/op mice. The original Flt1TK-/- mice showed mild osteoclast reduction without bone marrow suppression. The double mutant (op/opFlt1TK-/-) mice, however, exhibited very severe osteoclast deficiency and did not have numbers of osteoclasts sufficient to form the bone marrow cavity. The narrow bone marrow cavity in the op/opFlt1TK-/- mice was gradually replaced with fibrous tissue, resulting in severe marrow hypoplasia and extramedullary hematopoiesis. In addition to osteoclasts, osteoblasts also decreased in number in the op/opFlt1TK-/- mice. These results strongly suggest that the interaction of signals by means of VEGFR-1 and the CSF-1 receptor plays a predominant role not only in osteoclastogenesis but also in the maintenance of bone marrow functions.
Keywords: osteoblast, hematopoiesis, hematopoietic niche, osteopetrosis, op/op mouse
Osteoclasts are terminally differentiated cells derived from the monocyte/macrophage lineage and serve critical functions in bone resorption. The differentiation, activation, and survival of osteoclasts are primarily regulated by colony-stimulating factor 1 (CSF-1)/macrophage colony-stimulating factor, whose biological effects are mediated through a cell surface receptor, CSF-1R/c-Fms (1, 2). The role of CSF-1 in osteoclast biology was first revealed in the osteopetrotic (Csf1op/Csf1op, hereafter abbreviated as op/op) mouse, which has a recessive mutation in the Csf1 gene (3). The op/op mouse exhibits a severe deficiency of osteoclasts, monocytes, and tissue macrophages owing to a lack of CSF-1 function (4). Interestingly, however, the defect is evident only in juvenile mice. Osteoclasts gradually appear in op/op bone and correct the osteopetrosis spontaneously. In addition, a single administration of CSF-1 protein resulted in long-term, active bone resorption in op/op mice (5, 6). These findings suggest that some alternative factor (or factors) support and maintain osteoclastogenesis in the absence of CSF-1. We demonstrated that the administration of VEGF-A ameliorated osteoclastogenesis and bone resorption and that treatment with an antagonist for VEGF-A suppressed the spontaneous recruitment of osteoclasts in op/op mice (7). These results indicate that VEGF is a candidate cytokine to substitute for CSF-1 in the osteoclast development in op/op mice.
VEGF-A is a key regulator of physiological angiogenesis and hematopoiesis (8, 9) and has been implicated in the establishment of epiphyseal vascularization and endochondral bone formation (10, 11). VEGF-A belongs to a gene family of growth factors (the VEGF family) that includes VEGF-A, placenta growth factor (PlGF), VEGF-B, VEGF-C, and VEGF-D (12). Also, an orf virus-derived VEGF, VEGF-E, has been identified (13). VEGF-A has multiple spliced isoforms, including VEGF-A120, VEGF-A164, and VEGF-A188, in mice (12). VEGF-A binds to tyrosine kinase (TK) receptors, VEGF receptor 1 (VEGFR-1/Flt-1) and VEGFR-2 (Flk-1/KDR), subsequently serving as key mediators for angiogenesis (14, 15). PlGF and VEGF-B bind only to VEGFR-1. VEGF-C and VEGF-D bind to VEGFR-3 and regulate lymphatic angiogenesis. VEGF-E is a specific ligand to VEGFR-2 (13-15).
VEGFR-1 is expressed in monocytes and regulates their activation and chemotaxis (16, 17). We also revealed that monocyte/macrophage lineage cells including osteoclasts express VEGFR-1 (7, 18), indicating that, at the very least, VEGFR-1 is involved in osteoclastogenesis. In addition, recent studies suggested that VEGFR-2 is also expressed to some extent in mature osteoclasts (19, 20). To determine the function of the VEGF-VEGFR system in osteoclast development and activity, we introduced a VEGFR-1 TK domain-deficient mutation (Flt1TK-/-) (21) into op/op mice. The double mutant op/opFlt1TK-/- mice showed an extensive osteoclast deficiency compared with op/op mice and could not recruit numbers of osteoclasts sufficient to expand the marrow cavity, resulting in bone marrow fibrosis and extramedullary hematopoiesis.
Materials and Methods
Mice. The Flt1TK-/- mice used in this study are described in ref. 21. Female Flt1TK-/- mice with a C57BL/6 background were mated with male op/op homozygous mice (The Jackson Laboratory) having the B6C3Fe-a/a-Csf1op/Csf1op background. Double heterozygotes (op/+Flt1TK+/-) of the subsequent generation were used for further breeding. The resulting mice, which were deficient in one gene and heterozygote for another, were used for breeding in parallel. Mice with the op/op phenotype were identified by the absence of incisor eruption and/or PCR analysis of tail DNA samples. Mice with the Flt1TK-/- genotype were identified by PCR analysis and/or Southern blot analysis of the same DNA samples as described in ref. 21. The offspring were op/op and double mutant op/opFlt1TK-/- and served as the subjects in this study. All animal experiments were approved by the National Center for Geriatrics and Gerontology's institutional animal experimentation committee.
Histological Analysis. Mice (4-24 wk old) were anesthetized and perfused with a periodate-lysine-paraformaldehyde (PLP) solution (4% paraformaldehyde/0.01 M NaIO4/0.075 M lysine in 0.05 M phosphate buffer, pH 7.4). Bone, spleen, liver, and kidney organ blocks were postfixed for 10 h in the PLP solution. After being rinsed with the buffer, soft tissues were embedded in paraffin. Bones were decalcified in a 10% EDTA solution in 1 mM PBS (pH 7.4) for 2 wk at 4°C and embedded in paraffin. These samples were sectioned (3- to 7-μm thick) and stained with hematoxylin/eosin or toluidine blue for histological and pathological observations. Longitudinal serial sections of the median portion of whole femora were stained for tartrate-resistant acid phosphatase (TRAP) activity and counterstained with hematoxylin as described in ref. 7. TRAP-positive cells on bone surfaces that contained more than two nuclei were counted as osteoclasts. To identify the type of fibers in the myelofibrosis, sections of op/opFlt1TK-/- femora were stained with Azan stain, silver stain, periodic acid-methenamin-silver stain, Masson's trichrom stain, van Gieson stain, and phophotungstic acid-hematoxylin stain to diagnose the changes in op/opFlt1TK-/- bone marrow.
Immunohistochemistry. Sections of several tissues of 4- and 8-wk-old mice were immunohistochemically stained with rat anti-mouse F4/80 Ab for detection of mature macrophages (22) and anti-mouse alkaline phosphatase (ALP) Ab (23). The primary Abs were detected with the streptavidin-biotin complex by using a Vectastain kit (Vector Laboratories) for macrophages and osteoblasts according to the manufacturer's instructions. Normal rabbit IgG was used as a control for the antibodies.
Cytokine Injections. Five micrograms of recombinant human (rh) CSF-1 (provided by Morinaga Milk Industry, Tokyo), recombinant mouse VEGF120, rhPlGF (both from R & D Systems), or orf virus-derived VEGF-E (13) was i.p. injected into 21-d-old op/op and op/opFlt1TK-/- mice. The mice were killed 4 d after the injections. As a control, vehicle (0.1 M PBS) was injected similarly as above.
A group of 7-wk-old op/op mice received three consecutive injections of 5 μg of VEGFR-1/Fc chimeric protein (R & D Systems) under the conditions described above. Finally, three consecutive injections of 5 μg of rhCSF-1 were given to 7-wk-old op/opFlt1TK-/- mice at 24-h intervals. All of these mice were killed 5 d after the last injection.
Osteoclast Formation in Vitro. Spleen cells of 4-wk-old mice were passed through a Sephadex G-10 column (Amersham Pharmacia Biotech) as described in ref. 7. Cells were plated in 96-well plates at a density of 105 cells per well onto confluent OP9 stromal cells, which were established from op/op mouse calvaria (24), and cultured for 6 d in α-MEM supplemented with 10% FBS in the presence of 10 ng/ml rhCSF-1, 50 ng/ml recombinant mouse VEGF120, VEGF-E, and rhPlGF. The cultures were fixed with 4% paraformaldehyde and stained for TRAP. TRAP-positive multinucleated (three or more nuclei) cells were scored as osteoclasts under the microscope.
Statistical Analysis. Values are expressed as the mean ± standard deviation. Significant differences between groups were determined with Student's t test in stat view 5.0 (SAS Institute, Cary, NC).
Results and Discussion
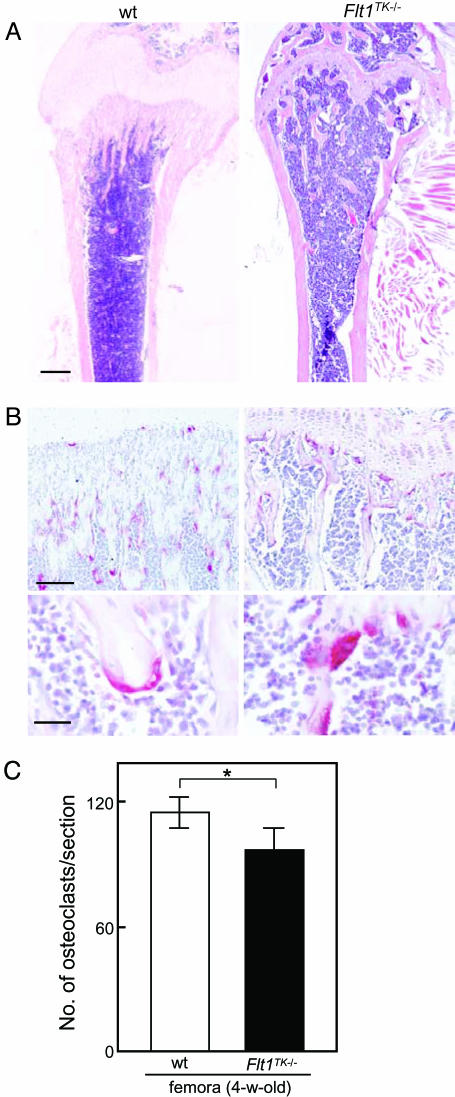
A Mild Reduction of Osteoclasts in Flt1TK-/- Mice. We previously showed that endogenous VEGF-A substituted for CSF-1 in osteoclast development during the adult stage in CSF-1-deficient op/op mice and that osteoclasts expressed VEGFR-1 (7). Furthermore, ovariectomized op/op mice exhibited an increased number of osteoclasts accompanied by up-regulation of VEGF-A and VEGFR-1 mRNA expression (25). Thus, before crossing the op/op mice with Flt1TK-/- mice, which undergo a basically normal development including angiogenesis (21), we examined the effects of VEGFR-1 signaling deficiency on the osteoclast formation in vivo by using Flt1TK-/- mice. These mice displayed a mild reduction in numbers of TRAP-positive multinucleated osteoclasts and bone trabeculae just below the growth plate in long bones compared with that in WT mice (Fig. 1). Although the number of osteoclasts was sufficient for bone morphogenesis, these results suggest that VEGFR-1 signaling is partly implicated in physiological osteoclastogenesis.
Fig. 1.
Bone histology and number of osteoclasts in femora of 4-wk-old WT and Flt1TK-/- mice. (A) Longitudinal sections of femora were stained with hematoxylin/eosin. (Scale bar: 0.5 mm.) (B) Red-stained cells on the bone trabeculae are TRAP-positive osteoclasts. (Lower) High-magnification image of multinucleated osteoclasts. (Scale bars: Upper, 100 μm; Lower,25 μm.) (C) The numbers of osteoclasts in the sections of the median portion of femora were counted. The TK domain deficiency showed a mild reduction in number of osteoclasts (*, P < 0.05)
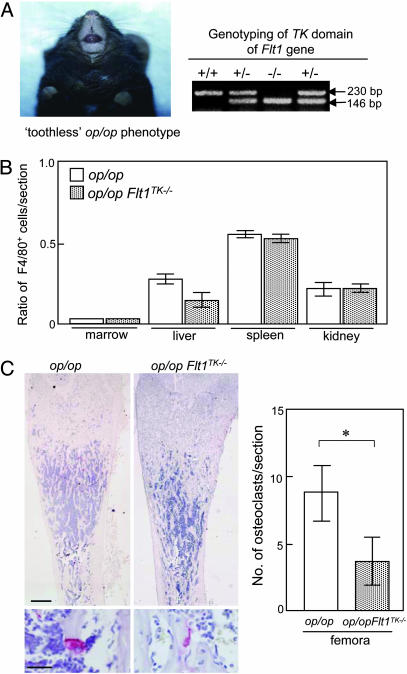
op/op Mice Lacking a VEGFR-1 TK Domain Show Severe Bone Marrow Cavity Occlusion. To clarify the roles of VEGFR-1 in osteoclast formation in more detail, the Flt1TK-/- mice were bred with the op/op mice (Fig. 2A). The op/op mice lacking the VEGFR-1 TK domain (op/opFlt1TK-/-) showed no significant difference in body weight or skeletal size compared with op/op mice (data not shown). F4/80-positive macrophage numbers were similarly reduced in marrow, liver, spleen, and kidney in both op/op and op/opFlt1TK-/- mice (Fig. 2B). However, the limb bones in 2-wk-old op/opFlt1TK-/- mice exhibited a more severe osteopetrosis with a decrease in the number and size of osteoclasts compared with those in op/op mice (Fig. 2C).
Fig. 2.
Phenotype of op/opFlt1TK-/- mice. (A) Double mutant op/opFlt1TK-/- mice exhibit an op/op toothless phenotype with the genotype of Flt1TK-/- (-/-) from a litter resulting from the interbreeding of op/+Flt1TK+/- and op/+Flt1TK+/- mice. Tail DNA was isolated and analyzed by PCR according to our previous study (21). (B) The ratio of numbers of macrophages with F4/80 immunoreaction in various tissues of op/opFlt1TK-/- mice to WT mice. (C) The op/opFlt1TK-/- femur exhibits a more severe osteopetrosis. The small red spots are osteoclasts. TRAP-positive cells containing nuclei, as shown in the high-magnification image, were counted as osteoclasts. The number of osteoclasts in the sections of 2-wk-old op/opFlt1TK-/- mice femora significantly (*, P < 0.01) decreased compared with that in age-matched op/op mice. (Scale bars: Upper, 0.5 mm; Lower, 25 μm.)
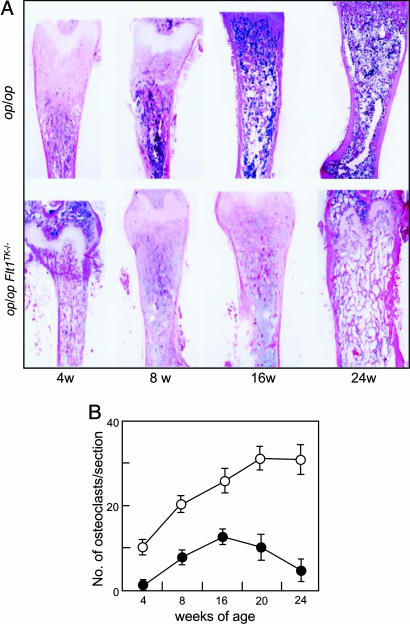
Next, we examined histological changes of femora in mice aged 4-24 wk. In op/op mice, the original osteopetrosis gradually ameliorated and marrow cellularity increased between the ages of 8 and 24 wk (Fig. 3A), as shown previously (7). In contrast, in op/opFlt1TK-/- mice, the osteoclastic bone resorption did not recover throughout the observation period (6 mo). The osteopetrotic phenotype remained in op/opFlt1TK-/- mice even at the 24-wk-old stage, although the thickened growth plate that is one of the features of osteopetrotic mice had been replaced with bone trabeculae (Fig. 3A). These results suggest that the VEGF-dependent osteoclastic bone resorption system does not function sufficiently in op/opFlt1TK-/- mice.
Fig. 3.
op/opFlt1TK-/- mice cannot recruit enough osteoclasts to form bone marrow. (A) Growth changes of femora in op/op and op/opFlt1TK-/- mice. Longitudinal sections of femora were stained with hematoxylin/eosin. Bone trabeculae in op/op mice decreased with aging, whereas those of op/opFlt1TK-/- mice remained until 24 wk of age. (B) Changes in numbers of TRAP+ osteoclasts in op/op (open circles) and op/opFlt1TK-/- (filled circles) mice
Then, we compared the number of osteoclasts in femora of op/op and op/opFlt1TK-/- mice. Small numbers of TRAP-positive osteoclasts were observed in 4-wk-old op/op femora, and numbers gradually increased during the observation period (Fig. 3B). Although osteoclasts were hardly detectable in 4-wk-old op/opFlt1TK-/- femora, a small number appeared in 8-wk-old bone. The number of osteoclasts transiently increased to a lesser extent at 16 wk, returning to a barely detectable level again at 24 wk (Fig. 3B). In age-matched WT mice, 100 or 200 osteoclasts were observed during the observation period. These results clearly indicate that the TK domain in VEGFR-1 plays a pivotal role in the recruitment of osteoclasts in mice with a CSF-1-deficient background. The transient appearance of osteoclasts in the op/opFlt1TK-/- bones, however, might suggest other signaling pathway(s).
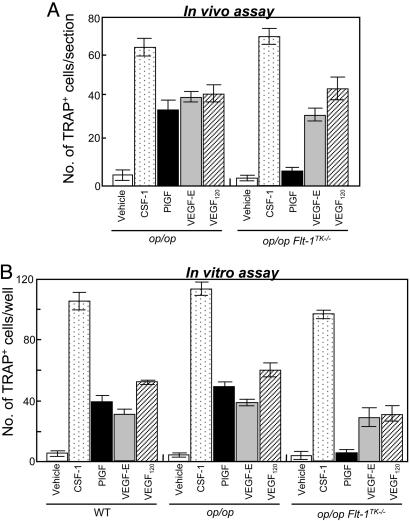
Exogenous VEGFs Rescue Osteopetrosis in op/opFlt1TK-/- Mice. Given our previous observation that the administration of neutralized Ab against VEGF-A completely inhibited osteoclast development in op/op mice (7), VEGFR-2 may be responsible for the mild and transient recruitment of osteoclasts in op/opFlt1TK-/- mice. To test this hypothesis, we used ligands specific for VEGFR-1 and VEGFR-2 in op/op and op/opFlt1TK-/- mice. rhCSF-1 was used as a control. The administration of rhPlGF, a VEGFR-1-specific ligand (26, 27), efficiently restored osteoclast formation in op/op mice but not in op/opFlt1TK-/- mice (Fig. 4A), although the osteoclasts were mostly small, and the degree was half that with rhCSF-1 (Fig. 4A). These results support our basic idea that VEGFR-1 is an important mediator for osteoclast formation. We next examined whether VEGF-E, a VEGFR-2-specific ligand, leads to osteoclastogenesis in op/opFlt1TK-/- mice. The VEGF-E administrations induced small osteoclasts in both op/op and op/opFlt1TK-/- mice. Similar results were obtained from the administration of VEGF120, which binds both receptors (Fig. 4A).
Fig. 4.
Osteoclast-induction activity of rhCSF-1, recombinant mouse VEGF120, rhPlGF, and VEGF-E in both mutant mice. (A) rhCSF-1 and VEGFs were injected in a single dosage of 5 μg into <3-wk-old op/op and op/opFlt1TK-/- mice, and mice were killed 4 d after the injection. Osteoclasts in longitudinal sections of the median portion of femora were counted. (B) In vitro assay of osteoclastogenic activity of rhCSF-1 and various VEGFs. Spleen cells of op/op and op/opFlt1TK-/- mice were cultured for 6 d with OP9 stromal cells in the presence of each cytokine in 96-well plates. The cultures were stained for TRAP activity, and TRAP-positive cells were counted
These activities of VEGFs were also observed in an in vitro osteoclast formation assay in which spleen cells were cocultured with OP9 osteoclastogenesis-supportive stromal cells (Fig. 4B). Although PlGF-induced osteoclast-like cells were smaller than those induced by CSF-1 treatment, they have multiple nuclei. The cells induced with VEGF-E were the smallest, mostly with only a few nuclei. These features were essentially the same without OP9 feeder cells (see the supporting information, which is published on the PNAS web site); however, the survival rate of the cells was lower compared with that in the OP9 feeder system. These findings suggest that VEGFR-2 might play some role in the development of osteoclasts in op/op background mice. In our previous study, however, VEGFR-2 was under detectable levels in monocyte/macrophage lineage cells (18). A low level of VEGFR-2 might induce a differentiation signal, or VEGFR-2 could be up-regulated during the culture period, because a recent study showed that VEGFR-2-expression in monocytes/macrophages, initially undetectable, was induced by VEGF stimulation (28).
Our findings indicate that exogenous PlGF has an osteoclastogenic activity, raising the question of whether endogenous PlGF (or VEGF-B) plays a role in the activation of VEGFR-1 and up-regulates osteoclastogenesis in vivo. However, recent studies provided evidence that PlGF-deficient mice are normally developed and healthy without clear abnormality (29). VEGF-B-deficient mice are also basically healthy, with normal morphology except in the heart. Some VEGF-B-/- mice showed an enlarged heart (30, 31). These findings suggest that endogenous PlGF and VEGF-B have only a minor effect, if any, on the osteoclastogenesis by means of activation of VEGFR-1 and that the major signal is generated by means of endogenous VEGF-A.
Another question is why the endogenous VEGF-A cannot rescue osteoclastogenesis by means of VEGFR-2 in op/opFlt1TK-/- mice. We suggest two reasons. The first is a quantitative point. Concentration of endogenous VEGF-A is known to be significantly low compared with that of exogenous VEGF-A. Furthermore, the affinity of VEGFR-2 to VEGF-A is one order weaker than that of VEGFR-1 (14, 27). Thus, the endogenous VEGF-A may not be sufficient to stimulate VEGFR-2 at high levels. The second is a qualitative point. Our in vitro studies (Fig. 4B) indicate that the VEGFR-2-specific ligand induced osteoclast-like cells with only a few nuclei. Therefore, VEGFR-2 signaling might be qualitatively insufficient to generate a full differentiation signal in osteoclast precursor cells.
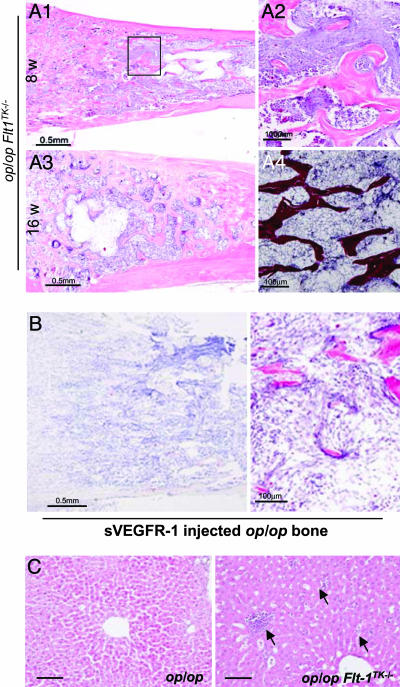
Myelofibrosis in op/opFlt1TK-/- Mice. Another striking feature of op/opFlt1TK-/- mice is the significant increase in fibrous tissues instead of hematopoietic cells in the marrow cavity. In 8-wk-old mice, fibrous tissue was initially observed in the diaphysial regions of the femora, although hematopoietic cells still occupied the intratrabecular spaces in the epiphysial region (Fig. 5 A1 and A2). With aging, the fibrous tissue gradually expanded to the whole marrow cavity, resulting in marked decreases in marrow cellularity, including osteoclasts (Fig. 5A3). Histological analysis indicated that the fibrous tissue consisted of reticular fiber-like fibrils (Fig. 5A4). Furthermore, we found that the phenotype obtained on administration of soluble VEGFR-1 chimeric protein, an efficient VEGF inhibitor, into op/op mice mimicked the marrow phenotype of op/opFlt1TK-/- mice (Fig. 5B).
Fig. 5.
Severe myelofibrosis in op/opFlt1TK-/- mice. (A1 and A2) Myelofibrosis was found in the diaphysial region of the marrow cavity in 8-wk-old mice. A high-magnification image of the boxed area shows the junction of normal marrow and myelofibrosis. (A3) Myelofibrosis filled the bone marrow space in 16-wk-old mice. (A4) A high-magnification image shows silver staining for myelofibrosis. (B) TRAP and histology of the femur of 8-wk-old op/op mice treated with soluble VEGFR-1/Fc chimeric protein, indicating very few osteoclasts. (C) Hematoxylin/eosin-stained livers of op/op and op/opFlt1TK-/- mice. Small hematopoietic foci were observed in op/opFlt1TK-/- mice (Right, arrows) but not in op/op mice (Left). (Scale bars: 100 μm.)
Recent studies suggest that a portion of hematopoietic stem cells (HSCs) express VEGFR-1 (32) and that HSCs require VEGFR-1 signaling for their recruitment and mobilization in marrow (33). According to these reports, the decreased marrow cellularity in op/opFlt1TK-/- mice might be due to defective HSCs. However, the single-gene mutant Flt1TK-/- mice showed no apparent defect in marrow cellularity (Fig. 1 A). Furthermore, a number of small hematopoietic foci were found in the liver of op/opFlt1TK-/- mice (Fig. 5C). Taken together, these findings indicate that HSCs do exist even in the op/opFlt1TK-/- mice and are functional for extramedullary hematopoiesis.
A Possible Intercommunication Between Osteoclasts and Osteoblasts. Myelopoiesis is supported by marrow stromal cells, including osteoblasts, which produce various osteogenic and hematopoietic growth factors (34). Osteoblast-deficient mice, owing to a lack of the Runx2/Cbfa1 gene, which encodes a transcription factor for osteoblastogenesis, exhibit an absence of marrow cells (35). Osteoblast deficiency induced by different genetic approaches also arrests marrow hematopoiesis and establishes extramedually hematopoiesis (36). Increases in the number of osteoblasts correlate with the establishment of hematopoietic niches (37, 38). Therefore, next we examined the activity of osteoblasts in the op/opFlt1TK-/- mice.
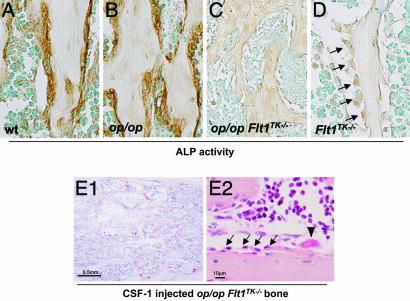
Immunostaining for A LP in the bone sections of op/opFlt1TK-/- mice revealed a remarkable reduction in the immunoreaction compared with that of op/op and WT mice (Fig. 6 A-C). Osteoblasts were significantly decreased on the surface of bone trabeculae, adjacent to myelofibrosis (Fig. 6C). The down-regulation of osteoblast activity may disrupt the hematopoiesis-supportive microenvironment in op/opFlt1TK-/- mice, resulting in a reduction in marrow cellularity and an increase in fibrosis. Because marrow hematopoiesis was weakly initiated in young op/opFlt1TK-/- mice, we tested for ALP activity of osteoblasts in Flt1TK-/- mouse bone. Although many osteoblasts were observed in Flt1TK-/- mice, ALP activity was extremely weak (Fig. 6D). Taken together, our data provide genetic evidence that VEGFR-1 signaling is important for osteoblast activity during bone formation.
Fig. 6.
Immunohistochemistry of ALP activity in four mouse genotypes: WT, op/op, op/opFlt1TK-/-, and Flt1TK-/-. (A-D) Cells stained brown are ALP-positive osteoblasts. The ALP activity of the op/opFlt1TK-/- and Flt1TK-/- mice is extremely weak compared with that of the WT and op/op mice. Although the ALP activity is weak, the osteoblasts in the Flt1TK-/- mice retain the morphology of active-phase cells (D). However, in the op/opFlt1TK-/- mice, most osteoblasts disappeared from the bone trabeculare. (E) TRAP activity (E1) and histology (E2) of the bone marrow in the femur of 8-wk-old op/op mice treated with rhCSF-1. Administration of rhCSF-1 prevented changes in the marrow and osteoblast reduction in the op/opFlt1TK-/- mice. Arrows, osteoblasts; arrowhead, osteoclast
Bone undergoes remodeling through the coordinated process of bone resorption and bone formation to maintain bone mass. It is considered that this harmonious balance is modulated by coupling paracrine signaling between osteoclasts and osteoblasts (for review, see ref. 39). Thus, we hypothesized that the survival of osteoblasts in Flt1TK-/- mice may be supported by the existence of osteoclasts. To test this, we lastly examined whether induction of osteoclasts would rescue the hypoplastic marrow in op/opFlt1TK-/- mice. Administration of rhCSF-1 to 7-wk-old op/opFlt1TK-/- mice restored not only osteoclasts but also osteoblasts (Fig. 6E). Moreover, rhCSF-1 treatment also prevented marrow alterations (Fig. 6E2). These findings suggest that osteoclasts are implicated in the survival of osteoblasts and that osteoblasts are crucial for construction of the marrow hematopoiesis-supportive microenvironment as described in refs. 36 and 37. Simultaneously, our findings demonstrate that CSF-1 plays an important role not only in bone remodeling but also in the organization of marrow structure.
In conclusion, we provided anatomical and genetic findings to show the importance of the interaction of VEGFR-1 signaling and CSF-1 receptor signaling in mice. Lack of these signals induces a severe alteration in bone and marrow structure. These findings may contribute to further understanding of the interaction between bone cells and marrow cells.
Supplementary Material
Acknowledgments
We thank Drs. H. Kodama, S.-I. Nishikawa, and H. Yoshida for valuable advice; Dr. H. Amano for in vitro technical support; Dr. K. Oda (Niigata University Graduate School of Medical and Dental Sciences) for providing of the anti-ALP antibody; and Dr. S. Moriwaki, Dr. T. Sasaki, and Ms. J. Tsurudome for technical contributions and assistance. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology (to S. N. and M.S.), the Grant of Longevity Science and Research on Dementia and Fracture from the Ministry of Health, Labour, and Welfare (to S.N.), the Programs for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan (K. I. and M.S.), and “Research for the Future” of the Japan Society for the Promotion of Science (M.S.).
Author contributions: S.N. designed research; S.N., T.K., S.-I.H., and N.A. performed research; S.-I.H., N.A., and T.N. contributed new reagents/analytic tools; S.N., S.-I.H., K.I., and M.S. analyzed data; and S.N. and M.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CSF-1, colony-stimulating factor 1; VEGFR, VEGF receptor; PlGF, placenta growth factor; TK, tyrosine kinase; TRAP, tartrate-resistant acid phosphatase; ALP, alkaline phosphatase; rh, recombinant human.
References
- 1.Tanaka, S., Takahashi, N., Udagawa, N., Tamura, T., Akatsu, T., Stanley, F. R., Kurokawa, T. & Suda, T. (1993) J. Clin. Invest. 91, 257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotze, M. T. & Hamilton, J. A. (1998) in The Cytokine Handbook, eds. Thomson, A. W. & Lotze, M. T. (Elsevier Science, London), pp. 927-958.
- 3.Yoshida, H., Hayashi, S., Kunisada, T., Ogawa, M., Nishikawa S., Okamura, H., Sudo, T., Shultz, L. D. & S.-I. Nishikawa. (1990) Nature 345, 442-444. [DOI] [PubMed] [Google Scholar]
- 4.Kodama, H., Yamasaki, A., Nose, M., Niida, S., Ohgame, Y., Abe, M., Kumegawa, M. & Suda, T. (1991) J. Exp. Med. 173, 269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodama, H., Yamasaki, A., Abe, M., Niida, S., Hakeda, Y. & Kumegawa, M. (1993) J. Bone Miner. Res. 8, 45-50. [DOI] [PubMed] [Google Scholar]
- 6.Niida, S., Amizuka, N., Hara, F., Ozawa, H. & Kodama, H. (1994) J. Bone Miner. Res. 9, 873-881. [DOI] [PubMed] [Google Scholar]
- 7.Niida, S., Kaku, M., Amano, H., Yoshida, H., Kataoka, H., Nishikawa, S., Tanne, K., Maeda, N., Nishikawa, S.-I. & Kodama, H. (1999) J. Exp. Med. 190, 293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., et al. (1996) Nature 380, 435-439. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O'Shea, K. S., Powell-Braxton, L., Hillan, K. J. & Moore, M. W. (1996) Nature 380, 439-442. [DOI] [PubMed] [Google Scholar]
- 10.Maes, C., Stockmans, I., Moermans, K., Van Looveren, R., Smets, N., Carmeliet, P., Bouillon, R. & Carmeliet, G. (2004) J. Clin. Invest. 113, 188-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber, H.-P., Vu, T. H., Ryan, A. M., Kowalski, J., Werb, Z. & Ferrara, N. (1999) Nat. Med. 5, 623-628. [DOI] [PubMed] [Google Scholar]
- 12.Robinson, C. J. & Stringer, S. E. (2001) J. Cell Sci. 114, 853-865. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa, S., Oku, A., Sawano, A., Yamaguchi, S., Yazaki, Y. & Shibuya, M. (1998) J. Biol. Chem. 273, 31273-31282. [DOI] [PubMed] [Google Scholar]
- 14.Shibuya, M., Ito, N. & Claesson-Welsh, L. (1999) Curr. Top. Microbiol. Immunol. 237, 59-83. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara, N. & Davis-Smyth, T. (1997) Endocr. Rev. 18, 4-25. [DOI] [PubMed] [Google Scholar]
- 16.Barleon, B., Sozzani, S., Zhou, D., Weich, H. A., Mantovani, A. & Marme, D. (1996) Blood 87, 3336-3343. [PubMed] [Google Scholar]
- 17.Clauss, M., Weich, H., Breier, G., Knies, U., Rockl, W., Waltenberger, J. & Risau, W. (1996) J. Biol. Chem. 271, 17629-17634. [DOI] [PubMed] [Google Scholar]
- 18.Sawano, A., Iwai, S., Sakurai, Y., Ito, M., Shitara, K., Nakahara, T. & Shibuya, T. (2001) Blood 97, 785-791. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa, M., Kaneda, T., Arakawa, T., Morita, S., Sato, T., Yomada, T., Hanada, K., Kumegawa, M. & Hakeda, Y. (2000) FEBS Lett. 473, 161-164. [DOI] [PubMed] [Google Scholar]
- 20.Tombran-Tink, J. & Barnstable, C. J. (2004) Biochem. Biophys. Res. Commun. 316, 573-579. [DOI] [PubMed] [Google Scholar]
- 21.Hiratsuka, S., Minowa, O., Kuno, J., Noda, T. & Shibuya, M. (1998) Proc. Natl. Acad. Sci. USA 95, 9349-9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austyn, J. M. & Gordon, S. (1981) Eur. J. Immunol. 11, 805-815. [DOI] [PubMed] [Google Scholar]
- 23.Oda, K., Amaya, Y., Fukushi-Irie, M., Kinameri, Y., Ohsyu, K., Kubota, I., Fujimura, S. & Kobayashi, S. (1999) J. Biochem. 126, 694-699. [DOI] [PubMed] [Google Scholar]
- 24.Kodama, H., Nose, M., Niida, S., Nishikawa, S. & Nishikawa, S. (1994) Exp. Hematol. 22, 979-984. [PubMed] [Google Scholar]
- 25.Kodama, I., Niida, S., Sanada, M., Yoshiko, Y., Tsuda, M., Maeda, N. & Ohama, K. (2004) J. Bone Miner. Res. 19, 200-206. [DOI] [PubMed] [Google Scholar]
- 26.Park, J. E., Chen, H. H., Winer, J., Houck, K. A. & Ferrara, N. (1994) J. Biol. Chem. 269, 25646-25654. [PubMed] [Google Scholar]
- 27.Sawano, A., Takahashi, T., Yamaguchi, S., Aonuma, T. & Shibuya, M. (1996) Cell Growth Differ. 7, 213-221. [PubMed] [Google Scholar]
- 28.Yang, Z. F., Poon, R. T., Luo, Y., Cheung, C. K., Ho, D. W., Lo, C. M. & Fan, S. T. (2004) J. Immunol. 173, 2507-2515. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet, P., Moons, L., Luttun, A., Vincenti, V., Compernolle, V., De Mol, M., Wu, Y., Bono, F., Devy, L., Beck, H., et al. (2001) Nat. Med. 7, 575-583. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson, U. & Alitalo, K. (1999) Curr. Top. Microbiol. Immunol. 237, 41-57. [DOI] [PubMed] [Google Scholar]
- 31.Lagercrantz, J., Farnebo, F., Larsson, C., Tvrdik, T., Weber, G. & Piehl, F. (1998) Biochim. Biophys. Acta 1398, 157-163. [DOI] [PubMed] [Google Scholar]
- 32.Lyden, D., Hattori, K., Dias, S., Costa, C., Blaikie, P., Butros, L., Chadburn, A., Heissig, B., Marks, W., Witte, L., et al. (2001) Nat. Med. 7, 1194-1201. [DOI] [PubMed] [Google Scholar]
- 33.Gerber, H. P., Malik, A. K., Solar, G. P., Sherman, D., Liang, X. H., Meng, G., Hong, K., Marsters, J. C. & Ferrara, N. (2002) Nature 417, 954-958. [DOI] [PubMed] [Google Scholar]
- 34.Taichman, R. S., Reilly, M. J. & Emerson, S. G. (2000) Hematology 4, 421-426. [PubMed] [Google Scholar]
- 35.Komori, T., Yagi, H., Nomura, S., Yamaguchi, A., Sasaki, K., Deguchi, K., Shimizu, Y., Bronson, R. T., Gao, Y. H., Inada, M., et al. (1997) Cell 89, 755-764. [DOI] [PubMed] [Google Scholar]
- 36.Visnjic, D., Kalajzic, Z., Rowe, D. W., Katavic, V., Lorenzo, J. & Aguila, H. L. (2004) Blood 103, 3258-3264. [DOI] [PubMed] [Google Scholar]
- 37.Calvi, L. M., Adams, G. B. & Weibrecht, K. W. (2003) Nature 425, 841-846. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, J., Niu, C., Ye, L., Huang, H., He, Xi., Tong, W.-G., Ross, J., Haug, J., Johnson, T., Feng, J. Q., et al. (2003) Nature 425, 836-841. [DOI] [PubMed] [Google Scholar]
- 39.Martin, T. J. & Sims, N. A. (2005) Trends Mol. Med. 11, 76-81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.