Abstract
Free gangliosides bind fibroblast growth factor 2 (FGF2), thus preventing cell interaction and biological activity of the growth factor in endothelial cells. Here we investigated the role of cell-associated gangliosides in mediating the biological activity of FGF2. Treatment of endothelial cells of different origin with the ganglioside biosynthesis inhibitors fumonisin B1, D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol or D-1-threo-1-phenyl-2-hexa-decanoylamino-3-pyrrolidino-1-propanol-HCl, impairs their capacity to proliferate when exposed to FGF2. Also, the mitogenic activity of FGF2 is inhibited by the GM1-binding cholera toxin B subunit (CTB). Conversely, overloading of endothelial GM 7373 cell membranes with exogenous GM1 causes a 10-fold increase of the mitogenic potency of FGF2. 125I-FGF2 binds to cell membrane GM1 (Kd = 3 nM) in complex ganglioside/heparan sulfate-deficient Chinese hamster ovary (CHO)-K1-pgsA745 cell mutants that were overloaded with exogenous GM1. Moreover, FGF2 competes with FITC-CTB for the binding to cell membrane GM1 in different CHO cell lines independently of their capacity to express heparan sulfate proteoglycans. Conversely, CTB inhibits cell proliferation triggered by FGF2 in CHO cells overexpressing the tyrosine kinase FGF receptor 1. Finally, GM1-overloading confers to FGF receptor 1-transfected, complex ganglioside-deficient CHO-K1 cell mutants the capacity to proliferate when stimulated by FGF2. This proliferation is inhibited by CTB. Cell proliferation triggered by serum or by phorbol 12-myristate 13-acetate is instead independent of the cell membrane ganglioside milieu. In conclusion, cell membrane GM1 binds FGF2 and is required for the mitogenic activity of the growth factor. Our data indicate that cell-associated gangliosides may act as functional FGF2 co-receptors in different cell types.
Gangliosides are neuraminic acid-containing glycosphingolipids and represent characteristic constituents of the plasma membrane of eukaryotic cells. They are shed in the microenvironment and found as free components in plasma (1). In turn, free gangliosides are efficiently incorporated into the plasma cell membrane (2).
Depending on their free or cell-associated status, gangliosides modulate the activity of growth factors and cytokines inducing opposite effects. Indeed, free gangliosides inhibit neurite outgrowth and cell proliferation induced by platelet-derived growth factor (PDGF), insulin, nerve growth factor, and insulin-like growth factor 1 (3, 4). On the contrary, cellular gangliosides promote fibroblast proliferation induced by epidermal growth factor, fibroblast growth factor 2 (FGF2), and PDGF (5). Also, glucosylceramide synthesis is required for axonal growth stimulated by FGF2 (6). The modulating capacity of gangliosides relies, at least in part, on their ability to bind directly the growth factors, as observed for IFN/gangliosides interaction (7). Accordingly, incorporation of gangliosides in the plasma membrane of IFN-insensitive, ganglioside-deficient cells leads to an increase in cell sensitivity to IFN (8).
FGF2 is a Mr 18,000 heparin-binding polypeptide that induces proliferation, migration, and proteases production in cultured endothelial cells and neovascularization in vivo (9). FGF2 interacts with endothelial cells through three distinct classes of receptors: the high-affinity tyrosine-kinase receptors [FGF receptor (FGFR); ref. 10], the low-affinity heparan sulfate proteoglycans (HSPGs; ref. 11), and αvβ3 integrins (12). All these receptors must be engaged by FGF2 to induce a full biological response (11, 12).
Free gangliosides bind FGF2 (13). The interaction occurs between the neuraminic acid (NeuAc) residue(s) of the ganglioside and the C terminus of the growth factor. By sequestering FGF2, free gangliosides prevent its interaction with FGFRs, thus inhibiting FGF2-mediated proliferation in endothelial cells (13). On the other hand, cellular gangliosides promote FGF2-mediated fibroblast proliferation (5). These observations raise the possibility that the alternative binding of FGF2 to free or cell surface-associated gangliosides may result in the inhibition or enhancement of its biological activity, respectively, as observed for FGF2–heparan sulfate interaction (11).
In this paper we demonstrate that cell membrane-associated gangliosides are required for the mitogenic activity of FGF2 and interact directly with the growth factor, thus acting as FGF2 co-receptors.
Methods
Chemicals.
Human recombinant FGF2 was expressed and purified from transformed Escherichia coli cells (14). GM1, asialo-GM1, fumonisin B1 (FB1), phorbol 12-myristate 13-acetate (TPA), and unlabeled and FITC-cholera toxin B subunit (CTB) were from Sigma. D-Threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) and D-1-threo-1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol-HCl (PPPP) were from Matreya (Pleasant Gap, PA).
Cell Culture.
Chinese hamster ovary (CHO)-K1 cells do not synthesize complex gangliosides, including GM1 (15), whereas CHO-K1-pgsA745 cell mutants also lack the ability to synthesize heparan sulfate (16). Wild-type CHO, CHO-K1, and CHO-K1-pgsA745 cells were stably transfected with FGFR1 cDNA (17, 18), thus generating the CHOflg7G, CHO-K1flgXB, and CHO-K1-pgsA745flg1A clones, respectively. Cell surface expression of the different molecules in the various CHO cell lines is summarized in Table 1. Cells were grown in Ham's F-12 medium supplemented with 10% FCS.
Table 1.
Differential expression of cell surface molecules in the CHO cell lines utilized in the present study
| Cell line | FGFRs | HSPGs | GM1 |
|---|---|---|---|
| Wild-type CHO | − | + | + |
| CHO-K1 | − | + | − |
| CHO-K1-pgsA745 | − | − | − |
| CHOflg7G | + | + | + |
| CHO-K1flgXB | + | + | − |
| CHO-K1-pgsA745flg1A | + | − | − |
See text for further details.
Transformed fetal bovine aortic endothelial GM 7373 cells (19) were obtained from the National Institute of General Medical Sciences, Human Genetic Mutant Cell Repository (Institute for Medical Research, Camden, NJ). They were grown in Eagle's MEM containing 10% FCS, vitamins, and essential and nonessential amino acids.
BALB/c mouse aortic endothelial 22106 cells (MAE cells) were grown in Dulbecco's modified Eagle medium containing 10% FCS (13).
Incorporation of Exogenous GM1 in Cell Membranes.
This was performed as described (13). Briefly, GM 7373 or CHO cells were seeded at 2,500 cells per cm2. After 16 h, they were incubated for further 96 h in fresh medium containing 0.4% FCS alone or added with 100 μM GM1 or asialo-GM1. At the end of incubation, cells were washed, trypsinized, and used for further experimentation.
Cell Proliferation and DNA Synthesis Assays.
Cell proliferation assay.
GM 7373 cells were seeded at 60,000 cells per cm2 in 48-well dishes. After overnight incubation, cells were incubated for 24 h in fresh medium containing 0.4% FCS in the absence or in the presence of FGF2. Then, cells were trypsinized and counted. Under these experimental conditions, both CHOflg7G cells and GM 7373 cells treated with 0.4% or 10% FCS undergo 0.1–0.2 and 1.0 cell population doublings, respectively. FGF2 and TPA (both at 10 ng/ml) cause instead 0.7–0.8 cell population doublings.
DNA synthesis assay.
MAE cells were seeded at 25,000 cells per cm2 in 24-well dishes and incubated for 2 days with 0.5% FCS in the absence or presence of 1 μM PPPP. Quiescent cell cultures were then supplemented with FGF2 (10 ng/ml) or 10% FCS in the absence or in the presence of PPPP and incubated for further 16 h at 37°C. At the end of incubation, cells were pulse labeled with 3H-thymidine (1 μCi/ml; 1 Ci = 37 GBq) for 6 h. The amount of radioactivity incorporated into the trichloroacetic acid-precipitable material was then measured.
125I-FGF2 Binding Assay.
FGF2 was iodinated at a specific radioactivity equal to 800 counts per minute (cpm)/fmol (20). Naive and GM1-overloaded CHO-K1-pgsA745 cells were incubated at 4°C in serum-free medium containing 0.15% gelatin, 20 mM Hepes (pH 7.5), and the indicated concentrations of 125I-FGF2. After 2 h, free 125I-FGF2 was removed, and bound radioactivity was extracted by a 5-min incubation with 2.0 M NaCl in 20 mM Hepes (pH 7.5) and measured. Nonspecific binding was measured in the presence of 250 μg/ml suramin and subtracted from all of the values.
CTB Binding Assay.
CHO cells were seeded at 40,000 cells per cm2 on glass coverslips. After 16 h, cells were incubated at 4°C in PBS containing 50 μg/ml FITC-CTB and 1% BSA. After 30 min, cells were fixed in 4% paraformaldehyde in PBS. For competition experiments, cells were incubated for 30 min at 4°C with FGF2 or unlabeled CTB (both at 500 μg/ml in 1% BSA) before FITC-CTB addition. Observations were carry out under an epifluorescence photomicroscope and FITC-CTB binding was quantified by computerized image analysis.
Results
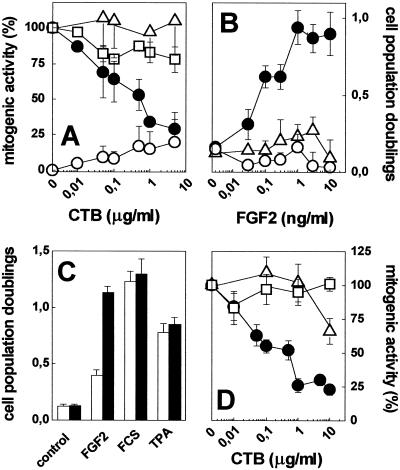
The role of cell surface gangliosides in mediating FGF2 activity was investigated in endothelial cells. In a first set of experiments, we evaluated the capacity of a series of ganglioside biosynthesis inhibitors to affect the mitogenic activity of FGF2 in endothelial GM 7373 cells. The inhibitors were: the ceramide synthase inhibitor FB1 (21); the glucosyl ceramide synthase inhibitor PDMP (22); and the PDMP analogue PPPP that inhibits ganglioside biosynthesis without affecting the intracellular levels of ceramide (23). GM 7373 cells were preincubated at 37°C with 10 μM FB1 for 5 h, with 10 μM PDMP for 72 h, or with 1 μM PPPP for 48 h. Then, cells were incubated a further 24 h at 37°C with FGF2 or TPA (both at 10 ng/ml), or with 10% FCS. At the end of incubation, cells were trypsinized and counted. As shown in Fig. 1A, all of the inhibitors tested caused a significant decrease of the mitogenic activity exerted by FGF2 but not of that exerted by serum or TPA. In agreement with these observations, PPPP pretreatment causes a significant inhibition of DNA synthesis in FGF2-treated MAE cells without affecting 3H-thymidine incorporation triggered by 10% FCS (Fig. 1B). Thus, the impairment of ganglioside biosynthesis leads to a decreased capacity of endothelial cells to respond to FGF2.
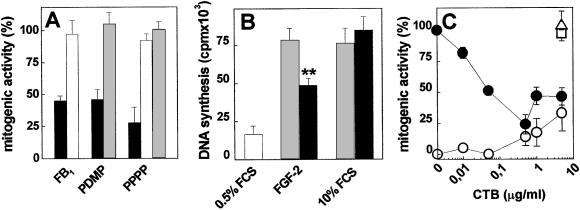
Figure 1.
Role of endogenous gangliosides on the mitogenic activity of FGF2 in endothelial cells. (A) GM 7373 cells were pretreated with FB1, PDMP, or PPPP as described in Methods. Then, cells were further incubated with 10 ng/ml FGF2 (black bars), 10 ng/ml TPA (open bars), or 10% FCS (shaded bars). After 24 h, cells were trypsinized and counted. (B) MAE cells were serum-starved in the absence (shaded bars) or in the presence (black bars) of PPPP. Then, cells were incubated for 24 h with 0.5% FCS, 0.5% FCS plus 10 ng/ml FGF2, or 10% FCS, and pulse labeled with 3H-thymidine during the last 6 h of incubation. The amount of radioactivity incorporated into trichloroacetic acid-precipitable material was then measured. Each point is the mean ± SE of two determinations performed in triplicate. **, P < 0.05 (Student's t test). (C) GM 7373 cells were treated with 10% FCS (□), 0.4% FCS alone (○) or added with 10 ng/ml of FGF2 (●), or TPA (▵) in the presence of increasing concentrations of CTB. After 24 h, cells were trypsinized and counted. Each point is the mean ± SE of two to five experiments performed in duplicate. In A and C, data are expressed as percent of cell proliferation with respect to control cells treated with the corresponding stimulus in the absence of any inhibitor.
Endothelial cells express neuraminic acid-bearing GM3 and GM1 gangliosides (24, 25). GM1 binds FGF2 with a potency significantly higher than GM3 (13). To assess the possibility that the lack of cell surface GM1 was responsible for the observed modulation of FGF2 activity, we evaluated the capacity of the GM1-binding CTB (26) to affect the mitogenic activity of FGF2 in GM 7373 cells. To this purpose, cells were treated with FGF2 or TPA (both at 10 ng/ml) or with 10% FCS in the presence of increasing concentrations of CTB. As shown in Fig. 1C, CTB inhibited the mitogenic activity of FGF2 in a dose-dependent manner without affecting cell proliferation triggered by serum or TPA. In keeping with previous observations (27), CTB alone exerted a slight increase of cell proliferation at the highest doses tested. Thus, the occupancy of cell surface GM1 by CTB specifically hampers the capacity of GM 7373 cells to proliferate when exposed to FGF2, raising the possibility that a direct GM1–FGF2 interaction is required for the biological activity of the growth factor.
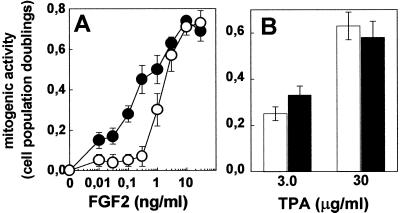
On this basis, GM 7373 cell surface was enriched with GM1 by incorporation of exogenously added ganglioside. To this purpose, cells were incubated for 96 h at 37°C in 0.4% FCS in the absence or in the presence of 100 μM GM1. Under these experimental conditions, ≈2% of the originally added exogenous GM1 is incorporated into the cell membrane (13). Then, GM1-overloaded cells were compared with naive cells for their capacity to proliferate in response to FGF2 or TPA. As shown in Fig. 2A, FGF2 exerted a mitogenic response in GM1-overloaded cells that was approximately 10 times more potent than in naive cultures (ED50 equal to 0.2 and 1.5 ng/ml, respectively). In contrast, GM1-overloading did not affect the ED50 of TPA (Fig. 2B).
Figure 2.
Effect of GM1 overloading on the mitogenic activity of FGF2 in endothelial cells. Naive (open symbols) and GM1-overloaded (black symbols) GM 7373 cells were incubated for 24 h with FGF2 (A) or TPA (B; both at 10 ng/ml). Then, cells were trypsinized and counted. Data are expressed as cell population doublings. Each point is the mean ± SE of five experiments performed in duplicate.
The characterization of a possible interaction of FGF2 with GM1 present on the outer leaflet of the plasma membrane of endothelial cells might be hampered by the presence of various FGF2-binding sites, including FGFRs, HSPGs, and αvβ3 integrin (10–12). In particular, the elevated binding capacity of HSPGs may represent a serious impediment for the study of alternative FGF2-binding sites. Indeed, we did not observe any effect of GM1 overloading (13) or of FB1, PDMP, PPPP, or CTB treatment (data not shown) on the capacity of 125I-FGF2 to bind to GM 7373 cell surface. CHO cell mutants instead, already exploited for investigating the FGF2 co-receptorial function of HSPGs (28), may represent an useful tool to overcome this difficulty. Indeed, different CHO cell clones have been generated that are genetically deficient for the synthesis of complex gangliosides (CHO-K1 cells) or of both complex gangliosides and heparan sulfate (CHO-K1-pgsA745 cells) (refs. 15 and 16; Table 1). Also, CHO cells express very low levels of FGFRs (≤1,000 binding sites per cell, data not shown).
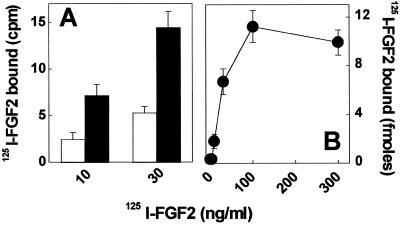
On this basis, we evaluated the capacity of 125I-FGF2 to bind to naive and GM1-overloaded CHO-K1-pgsA745 cells. As shown in Fig. 3A, GM1 overloading increases significantly the 125I-FGF2-binding capacity of CHO-K1-pgsA745 cells. The differential binding between GM1-enriched and naive cells is dose-dependent and saturable (Fig. 3B). Scatchard plot analysis of the differential binding data revealed that the interaction between FGF2 and GM1-overloaded cells occurs with high affinity (Kd = 3 nM). Thus, the data suggest that 125I-FGF2 interacts with exogenous GM1 incorporated into the cell membrane of CHO-K1-pgsA745 cells.
Figure 3.
125I-FGF2 binding to GM1-overloaded CHO-K1-pgsA745 cells. (A) Naive (open bars) and GM1-overloaded (black bars) CHO-K1-pgsA745 cells were incubated for 2 h at 4°C with 125I-FGF2. Then, the amount of radioactivity bound to the cells was evaluated as described in Methods. Each point is the mean ± SE of three experiments performed in duplicate. In B, the values of the binding of 125I-FGF2 to naive cells were subtracted from the corresponding binding values obtained in GM1-overloaded CHO-K1-pgsA745 cells and the differential binding data were expressed as femtomoles of 125I-FGF2 bound per well. The experiment is representative of three independent experiments that gave similar results.
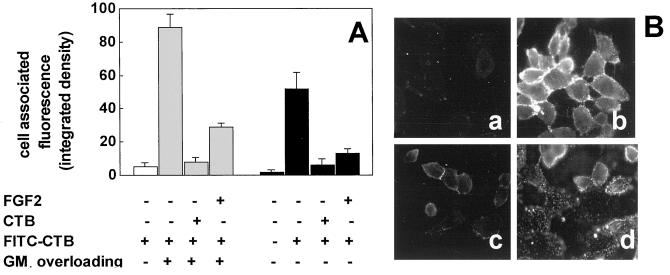
To substantiate this hypothesis, we evaluated the capacity of FGF2 to compete with FITC-labeled CTB for the binding to GM1-enriched CHO-K1 cells and wild-type CHO cells. Naive CHO-K1 cells express HSPGs but not GM1, whereas wild-type CHO cells express both cell surface molecules (Table 1). As shown in Fig. 4, FITC-CTB binds efficiently to the surface of wild-type CHO cells and of GM1-enriched CHO-K1 cells but not of naive CHO-K1 cells. The binding was competed by a molar excess of FGF2 or of unlabeled CTB (Fig. 4). Specificity of the inhibition was supported by the observation that heat inactivated FGF2 does not compete with FITC-CTB for cell binding (data not shown), in keeping with its inability to interact with free gangliosides (13). These data confirm the ability of FGF2 to interact with cell membrane GM1. This interaction occurs also in the presence of cell surface HSPGs.
Figure 4.
Effect of FGF2 on the binding of FITC-CTB to CHO cells. (A) Naive CHO-K1 cells (open bar), GM1-overloaded CHO-K1 cells (shaded bars), and wild-type CHO cells (black bars) were incubated in the absence or in the presence of FITC-CTB alone or added with a molar excess of unlabeled CTB or FGF2. Then, the amount of cell-associated fluorescence was evaluated by computerized image analysis. Each data is the mean ± SE of three to six microscopic fields. The experiments were repeated two to four times with similar results. (B) Representative microphotographs of naive CHO-K1 cells treated with FITC-CTB alone (a) and of GM1-overloaded CHO-K1 cells treated with FITC-CTB in the absence (b) or in the presence of a molar excess of unlabeled CTB (c) or FGF2 (d). (Original magnification, ×200.)
Finally, we evaluated the role of cell membrane GM1 in mediating the biological activity of FGF2 in CHO cells. As stated above, CHO cells express very low levels of FGFRs. Accordingly, wild-type CHO cells and CHO-K1 cells proliferate in response to serum and TPA, but not to FGF2, when tested under the same experimental conditions used to assess the mitogenic activity of FGF2 in endothelial GM 7373 cells (data not shown). On this basis, two FGFR1 transfectants originated from wild-type CHO cells and CHO-K1 cells were used for further experimentation. The clones, named CHOflg7G cells and CHO-K1flgXB cells, respectively, express approximately 30,000 FGFR1 molecules per cell (17). In a first set of experiments, CHOflg7G cells were treated with FGF2, TPA, or 10% FCS. The three mitogenic stimuli exerted a significant increase of cell proliferation, ranging between 0.7–1.0 cell population doublings during the 24 h incubation period, when compared with 0.4% FCS (0.1–0.2 cell population doublings). As observed in endothelial cells, CTB caused a dose-dependent inhibition of the mitogenic activity of FGF2 also in CHOflg7G cells, without affecting cell proliferation triggered by 10% FCS or TPA (Fig. 5A). CTB alone exerted only a slight increase of cell proliferation. Also, PPPP pretreatment abolished the capacity of CHOflg7G cells to proliferate in response to FGF2, without affecting the mitogenic activity exerted by 10% FCS (data not shown).
Figure 5.
Role of cell-associated GM1 on the mitogenic activity of FGF2 in CHO cells. (A) CHOflg7G cells were treated for 24 h with 10% FCS (□), 0.4% FCS alone (○), or added with 10 ng/ml FGF2 (●) or 10 ng/ml TPA (▵) in the presence of increasing concentrations of CTB. (B) Naive (○), GM1-overloaded (●), and asialo-GM1-overloaded (▵) CHO-K1flgXB cells were incubated for 24 h with increasing concentrations of FGF2. (C) Naive (open bars) and GM1-overloaded CHO-K1flgXB cells (black bars) were incubated for 24 h in the absence of mitogenic stimulus (control) or with 10% FCS, 10 ng/ml FGF2, or 10 ng/ml TPA. (D) GM1-overloaded CHO-K1flgXB cells were incubated for 24 h with 10% FCS (□), 10 ng/ml FGF2 (●), or 10 ng/ml TPA (▵) in the presence of increasing concentrations of CTB. In each experiment, cells were trypsinized and counted at the end of incubation. Data are expressed as percent of cell proliferation in respect to control cells treated with the corresponding stimulus in the absence of CTB (A and D) or as cell population doublings (B and C). Each point is the mean ± SE of three to five experiments performed in duplicate.
At variance with CHOflg7G cells, GM1-deficient CHO-K1flgXB cells did not proliferate in response to FGF2 (Fig. 5 B and C), despite the similar levels of FGFR and HSPG expressed by the two cell lines. However, GM1 overloading restored the capacity of CHO-K1flgXB cells to proliferate in response to FGF2 (Fig. 5 B and C). Preloading of CHO-K1flgXB cells with asialo-GM1 was instead ineffective, in keeping with its incapacity to bind FGF2 (13). Also, no significant differences were observed between naive and GM1-enriched CHO-K1flgXB cells in their capacity to proliferate when stimulated by 10% FCS or TPA (Fig. 5C). As observed in CHOflg7G cells, CTB was able to inhibit the mitogenic activity exerted by FGF2 in GM1-overloaded CHO-K1flgXB cells with no significant effects on cell proliferation triggered by 10% FCS or TPA (Fig. 5D).
Taken together, the data indicate that cell membrane GM1 plays a critical role in mediating the mitogenic activity of FGF2 also in CHO cells.
Discussion
In this paper we demonstrate that cell membrane-associated GM1 binds FGF2 and acts as a functional co-receptor for the growth factor in endothelial and CHO cells. These data extend previous observations from our laboratory about the capacity of FGF2 to interact with free gangliosides in cell-free systems and in vitro cell cultures (13).
The cholera toxin subunit CTB is a specific GM1-binding protein (26). Accordingly, FITC-CTB binds to wild-type CHO cells and GM1-overloaded CHO-K1 cells but not to naive CHO-K1 cells that lack complex gangliosides on their cell surface (15). In both wild-type CHO cells and GM1-overloaded CHO-K1 cells, FGF2 efficiently competes with FITC-CTB for cell surface GM1 interaction. Specificity of the competition is confirmed by the inability of heat denatured FGF2 to compete with FITC-CTB, in keeping with its incapacity to bind free gangliosides in vitro (13). These data may have their in vivo counterpart in the observation that CTB binding sites codistribute with FGF2 immunoreactivity within the zona compacta of the substantia nigra in rat brain (29).
In agreement with the FITC-CTB competition experiments, 125I-FGF2 binds exogenous GM1 incorporated into the cell membrane of ganglioside-enriched CHO-K1-pgsA745 cells. These cells do not express significant amounts of GM1 or of HSPGs on their surface (16) and express very low levels of FGFRs, thus representing an almost ideal system to evaluate the interaction of FGF2 with alternative binding sites. Scatchard plot analysis of the differential binding between GM1-enriched and naive CHO-K1-pgsA745 cells indicates that the interaction of 125I-FGF2 with GM1 occurs with an affinity (Kd = 3 nM) similar to that observed for FGF2/HSPG interaction (11) and ≈3–10 times lower than that reported for CTB/GM1 interaction (30, 31).
Several experimental evidences point to a role for the interaction of FGF2 with cell membrane GM1 in mediating the mitogenic activity of the growth factor. (i) Pretreatment of endothelial GM 7373 and MAE cells with inhibitors of ganglioside biosynthesis decreases their responsiveness to FGF2. This is in agreement with previous observations indicating that ganglioside metabolism impairment affects the mitogenic activity of FGF2 in cultured fibroblasts (5). FB1 and PDMP inhibit ganglioside biosynthesis leading to the accumulation of ceramide that may alter the signal transduction generated by several growth factors, including FGF2 (32). However, the novel compound PPPP hampers ganglioside biosynthesis without affecting intracellular levels of ceramide (5, 23). The capacity of these inhibitors, independently of their effect on ceramide turnover, to prevent cell proliferation induced by FGF2 without affecting proliferation induced by serum or TPA strongly supports the hypothesis that cell surface gangliosides are required for the mitogenic activity of FGF2. (ii) GM1-binding CTB inhibits cell proliferation triggered by FGF2 in endothelial GM 7373 cells and in FGFR1-transfected CHO cells (CHOflg7G cells). Again, the effect is specific because CTB does not affect cell proliferation induced by serum or TPA. Control experiments demonstrated that CTB does not interact directly with FGF2 in vitro (M.R., unpublished observations) and with cell surface HSPGs (33). Also, CTB does not compete for the binding of 125I-FGF2 to FGFRs and HSPGs (data not shown). Taken together, the data support the hypothesis that CTB exerts its FGF2 antagonist activity by preventing its interaction with cell surface GM1. (iii) GM1 overloading confers to FGFR1-transfected CHO-K1 cells deficient in complex ganglioside biosynthesis (CHO-K1flgXB cells) the capacity to respond to the mitogenic stimulus exerted by FGF2. Also in this case, CTB suppresses the mitogenic activity of FGF2, thus confirming that exogenous GM1 incorporated into the cell membrane is responsible for the responsiveness of these cells to the growth factor.
Previous observations had shown that membrane-bound gangliosides modulate the biological activity of various growth factors by affecting receptor binding, dimerization, and/or autophosphorylation (refs. 3, 4, and 13, and references therein). GM1 overloading causes a 2-fold increase of the affinity of 125I-FGF2-FGFR interaction in FGFR1-transfected, complex gangliosides/HSPG-deficient CHO-K1-pgsA745flg1A cells (data not shown), thus indicating that cell membrane GM1 may mimic HSPGs for the ability to present FGF2 to its tyrosine kinase receptors (9, 11, 28). However, kinetics and dose–response experiments did not reveal any significant difference between naive and GM1-enriched CHO-K1-pgsA745flg1A cells in the FGF2-dependent phosphorylation of extracellular regulated kinase-1/2 (ERK1/2), a key component of FGFR signal transduction pathway (34). Similarly, ganglioside biosynthesis inhibitors did not affect FGF2-dependent ERK1/2 activation in endothelial GM 7373 cells (data not shown). The possibility exists that FGF2 interaction with membrane-bound GM1 activates signal transduction pathway(s) complementary to those activated by FGFR engagement. Indeed, CTB binding to GM1 triggers intracellular signaling and cell proliferation (27). Accordingly, we observed a slight increase in the proliferation rate of endothelial and CHO cells treated with CTB alone. Relevant to this point, myelin basic protein induces cell proliferation via the engagement of both membrane GM1 and FGFRs (35).
Free and cell-associated sulfated glycosaminoglycans bind FGF2 and play contrasting roles in modulating the activity of the growth factor (9, 11). Similarly, free and membrane-bound gangliosides interact with FGF2 and differently affect its biological activity. Free molecules, via a law of mass action, sequester FGF2 and hamper its interaction with cell surface binding sites. Membrane molecules, in contrast, promote the biological activity of FGF2. We found that GM1 overloading increases endothelial cell responsiveness to subsaturating concentrations of the growth factor (see Fig. 2A). FGF2 levels ranging between 10 pg/ml and 1.0 ng/ml are observed in serum of tumor-bearing patients (36, 37), thus suggesting that a similar effect may occur also in vivo.
Our data demonstrate that cell membrane GM1 acts as a FGF2 co-receptor by interacting with the growth factor and promoting its biological activity. Modulation of the synthesis, shedding, and membrane incorporation of gangliosides, as well as of heparan sulfate glycosaminoglycans, are part of a complex interplay in the regulation of the bioavailability and biological activity of FGF2.
Acknowledgments
We would like to dedicate this article to late Prof. Pietro Gullino. We thank Prof. L. Riboni for helpful discussion. This work was partially supported by grants to M.P. from Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (Centro di Eccellenza “IDET,” Cofin 2000, and ex 60%), Consiglio Nazionale delle Ricerche (Progetto Finalizzato Biotecnologie), Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità (AIDS Project), and Centro per lo Studio del Trattamento dello Scompenso Cardiaco (University of Brescia), and by a grant to M.R. from Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (Cofin 2000).
Abbreviations
- CTB
cholera toxin B subunit
- FGF2
fibroblast growth factor 2
- FGFR
FGF receptor
- FB1
fumonisin B1
- HSPG
heparan sulfate proteoglycan
- PDMP
d-threo-1phenyl-2-decanoylamino-3-morpholino-1-propanol
- PPPP
d-1-threo-1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol-HCl
- TPA
phorbol 12-myristate 13-acetate
- CHO
Chinese hamster ovary
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chang F, Li R, Ladisch S. Exp Cell Res. 1997;234:341–346. doi: 10.1006/excr.1997.3619. [DOI] [PubMed] [Google Scholar]
- 2.Sonderfeld S, Conzelmann E, Schwarzmann G, Burg J, Hinrichs U, Sandhoff K. Eur J Biochem. 1985;149:247–255. doi: 10.1111/j.1432-1033.1985.tb08919.x. [DOI] [PubMed] [Google Scholar]
- 3.Hynds D L, Summers M, Van Brocklyn J, O'Dorisio M S, Yates A J. J Neurochem. 1995;65:2251–2258. doi: 10.1046/j.1471-4159.1995.65052251.x. [DOI] [PubMed] [Google Scholar]
- 4.Hynds D L, Burry R W, Yates A J. J Neurosci Res. 1997;47:617–625. doi: 10.1002/(sici)1097-4547(19970315)47:6<617::aid-jnr7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Li R, Manela J, Kong Y, Ladish S. J Biol Chem. 2000;275:34213–34223. doi: 10.1074/jbc.M906368199. [DOI] [PubMed] [Google Scholar]
- 6.Boldin S A, Futerman A H. J Biol Chem. 2000;275:9905–9909. doi: 10.1074/jbc.275.14.9905. [DOI] [PubMed] [Google Scholar]
- 7.Besancon F, Ankel H. Nature (London) 1974;252:478–480. doi: 10.1038/252478a0. [DOI] [PubMed] [Google Scholar]
- 8.Vengris V E, Reynolds F H, Jr, Hollenberg M D, Pitha P M. Virology. 1976;72:486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- 9.Basilico C, Moscatelli D. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 10.Powers C J, McLeskey S W, Wellstein A. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 11.Rusnati M, Presta M. Int J Clin Lab Res. 1996;26:15–23. doi: 10.1007/BF02644769. [DOI] [PubMed] [Google Scholar]
- 12.Rusnati M, Tanghetti E, Dell'Era P, Gualandris A, Presta M. Mol Biol Cell. 1997;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusnati M, Tanghetti E, Urbinati C, Tulipano G, Marchesini S, Ziche M, Presta M. Mol Biol Cell. 1999;10:313–327. doi: 10.1091/mbc.10.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isacchi A, Statuto M, Chiesa R, Bergonzoni L, Rusnati M, Sarmientos P, Ragnotti G, Presta M. Proc Natl Acad Sci USA. 1991;88:2628–2632. doi: 10.1073/pnas.88.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosales Fritz V M, Daniotti J L, Maccioni H J F. Biochim Biophys Acta. 1997;1354:153–158. doi: 10.1016/s0167-4781(97)00117-6. [DOI] [PubMed] [Google Scholar]
- 16.Rostand K S, Esko J D. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liekens S, Leali D, Neyts J, Esnouf R, Rusnati M, Dell'Era P, Maudgal P C, De Clercq E, Presta M. Mol Pharmacol. 1999;56:204–213. doi: 10.1124/mol.56.1.204. [DOI] [PubMed] [Google Scholar]
- 18.Rusnati M, Dell'Era P, Urbinati C, Tanghetti E, Massardi M L, Nagamine Y, Monti E, Presta M. Mol Biol Cell. 1996;7:369–381. doi: 10.1091/mbc.7.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinspan J B, Stephen N M, Levine E M. J Cell Physiol. 1983;114:328–338. doi: 10.1002/jcp.1041140312. [DOI] [PubMed] [Google Scholar]
- 20.Neufeld G, Gospodarowicz D. J Biol Chem. 1985;260:13860–13868. [PubMed] [Google Scholar]
- 21.Wang E, Norred W P, Bacon C W, Riley R T, Merrill A H., Jr J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 22.Inokuchi J, Radin N S. J Lipid Res. 1987;28:565–571. [PubMed] [Google Scholar]
- 23.Lee L, Abe A, Shayman J A. J Biol Chem. 1999;274:14662–14669. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- 24.Duvar S, Peter-Katalinic J, Hanish F-G, Muthing J. Glycobiology. 1997;7:1109–1109. doi: 10.1093/glycob/7.8.1099. [DOI] [PubMed] [Google Scholar]
- 25.Muthing J, Duvar S, Heitmann D, Hanish F-G, Neumann U, Lochnit G, Geyer R, Peter-Katalinic J. Glycobiology. 1999;9:459–468. doi: 10.1093/glycob/9.5.459. [DOI] [PubMed] [Google Scholar]
- 26.Kuziemko G M, Stroh M, Stevens R C. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- 27.Bukley N E, Su Y, Milstien S, Spigel S. Biochim Biophys Acta. 1995;1256:275–283. doi: 10.1016/0005-2760(95)00030-g. [DOI] [PubMed] [Google Scholar]
- 28.Yayon A, Klagsbrun M, Esko J D, Leder P, Ornitz D M. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 29.Fuxe K, Tinner B, Janson A M, Agnati L F. NeuroReport. 1993;4:857–860. doi: 10.1097/00001756-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Cooper M A, Hansson A, Lofas S, Williams D H. Anal Biochem. 2000;277:196–205. doi: 10.1006/abio.1999.4389. [DOI] [PubMed] [Google Scholar]
- 31.Aman A T, Fraser S, Merritt E A, Rodigherio C, Kenny M, Ahn M, Hol W G, Williams N A, Lencer W I, Hirst T R. Proc Natl Acad Sci USA. 2001;98:8536–8541. doi: 10.1073/pnas.161273098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riboni L, Viani P, Bassi R, Giussani P, Tettamanti G. J Biol Chem. 2001;276:12797–12804. doi: 10.1074/jbc.M011570200. [DOI] [PubMed] [Google Scholar]
- 33.Mugnai G, Lewandowska K, Choi H U, Rosenberg L C, Culp L A. Exp Cell Res. 1988;175:229–247. doi: 10.1016/0014-4827(88)90189-9. [DOI] [PubMed] [Google Scholar]
- 34.Giuliani R, Bastaki M, Coltrini D, Presta M. J Cell Sci. 1999;112:2597–2606. doi: 10.1242/jcs.112.15.2597. [DOI] [PubMed] [Google Scholar]
- 35.Tzeng S-F, Deibler G E, DeVries G H. Neurochem Res. 1999;24:255–260. doi: 10.1023/a:1022514105129. [DOI] [PubMed] [Google Scholar]
- 36.Dirix L Y, Vermeulen P B, Pawinski A, Prove A, Benoy I, De Pooter C, Martin M, Van Oosterom A T. Br J Cancer. 1997;76:238–243. doi: 10.1038/bjc.1997.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguayo A, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O'Brien S, Keating M, Freireich E, Albitar M. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]