Abstract
Antibodies to myelin components are routinely detected in multiple sclerosis patients. However, their presence in some control subjects has made it difficult to determine their contribution to disease pathogenesis. Immunization of C57BL/6 mice with either rat or human myelin oligodendrocyte glycoprotein (MOG) leads to experimental autoimmune encephalomyelitis (EAE) and comparable titers of anti-MOG antibodies as detected by ELISA. However, only immunization with human (but not rat) MOG results in a B cell-dependent EAE. In this study, we demonstrate that these pathogenic and nonpathogenic anti-MOG antibodies have a consistent array of differences in their recognition of antigenic determinants and biological effects. Specifically, substituting proline at position 42 with serine in human MOG (as in rat MOG) eliminates the B cell requirement for EAE. All MOG proteins analyzed induced high titers of anti-MOG (tested by ELISA), but only antisera from mice immunized with unmodified human MOG were encephalitogenic in primed B cell-deficient mice. Nonpathogenic IgGs bound recombinant mouse MOG and deglycosylated MOG in myelin (tested by Western blot), but only pathogenic IgGs bound glycosylated MOG. Only purified IgG to human MOG bound to live rodent oligodendrocytes in culture and, after cross-linking, induced repartitioning of MOG into lipid rafts, followed by dramatic changes in cell morphology. The data provide a strong link between in vivo and in vitro observations regarding demyelinating disease, further indicate a biochemical mechanism for anti-MOG-induced demyelination, and suggest in vitro tools for determining autoimmune antibody pathogenicity in multiple sclerosis patients.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, lipid rafts, B cell-deficient mice, encephalitogenicity
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS) in which both T cells and antibodies against myelin antigens are routinely detected (1, 2). B cell responses in MS pathogenesis are implicated by the presence of Ig deposits and myelin debris in demyelinating lesions (3–8), and the observation that plasma exchange dramatically reduces clinical disease in a subset of patients (9). Of particular interest to the present study, antibodies to myelin oligodendrocyte glycoprotein (MOG) are detected in the sera and plaques of MS patients (10), and thus are possible predictors of disease progression (11). However, because some control subjects can also harbor anti-myelin antibodies (1, 2, 12), their contribution to MS pathogenesis has been controversial and difficult to identify in individual patients. Further complicating the issue, MS may be several diseases of differing etiologies (5), whereby anti-myelin antibodies may be pathogenic in some forms of MS but merely a reflection of tissue damage in others. Thus, an understanding of whether anti-myelin antibodies are in fact pathogenic, and if so, by what mechanisms they operate, could provide important information for novel diagnostic tools and therapeutic interventions.
The mechanism of pathogenesis of experimental autoimmune encephalomyelitis (EAE), a commonly used animal model for MS, can involve either or both T cells and B cells, depending on the antigen used (13). T cells specific for an encephalitogenic MOG peptide can induce clinical signs and CNS inflammation and demyelination in EAE (14–16). A monoclonal antibody to MOG induces demyelination in vitro (17) and exacerbates T cell-mediated disease in mice and rats (18, 19). We have previously demonstrated that immunization of C57BL/6 mice with either rat MOG protein or rat MOG35–55 peptide results in a B cell-independent disease (16); in contrast, immunization with human MOG protein generates a B cell-dependent disease (20, 21), whereas immunization with human MOG 35–55 peptide leads to only minimal clinical signs of EAE (21). In vitro assays have demonstrated that the predominant T cell response in C57BL/6 mice to the extracellular domain of both human and rat MOG proteins is directed to their 35–55 regions (21, 22). Despite the fact that the amino acid at position 42 is neither a T cell receptor nor MHC contact residue (23), it is critical for T cell-mediated disease; the strongly encephalitogenic rodent peptide contains a serine at position 42, whereas the weakly encephalitogenic human analog differs by a proline at that site (24). Consistent with this, substitution of serine with proline at position 42 of rat MOG protein severely attenuates its encephalitogenicity (21).
Attempts have been made to distinguish between pathogenic and nonpathogenic antibodies against MOG antigens in MS and EAE (6). ELISA assays of the antibodies generated by immunization with human or rat MOG do not readily distinguish among different determinants (25). Antibodies generated in H-2s, but not H-2b, mice can bind to MOG cDNA-transfected fibroblasts (26), suggesting a potential method to discriminate between pathogenic and nonpathogenic antibodies. We have previously shown that antibodies generated by immunization of C57BL/6 mice with human or rat MOG demonstrate comparable titers by ELISA, despite the differences in the B cell dependence of the diseases (21). We postulated that these antisera might recognize different determinants, and that these might reflect differential pathogenicity. In addition, we have shown that a demyelinating monoclonal antibody against MOG binds to the surface of live oligodendrocytes (OLs) in culture, and upon cross-linking, rapidly and sequentially induces the repartitioning of MOG into detergent insoluble microdomains characteristic of lipid rafts, alterations in the phosphorylation state of key proteins, and dramatic changes in cell morphology (27, 28). These observations provided a potential mechanism for B cell-driven disease and suggested that these properties might be predictive of antibody encephalitogenicity.
Here we apply our previous observations with regard to differential B cell dependence of disease and in vitro manifestations of one monoclonal antibody to several different preparations of anti-MOG antisera that are of similar titer by ELISA. The effects of these antisera were analyzed on the disease targets, myelin and OLs. We show that antibodies to human and rat MOG differ in their properties both in vivo and in vitro due to differential binding and modifications of physiology of OLs and myelin, apparently related to differences in determinant recognition influenced by position 42 and glycosylation of MOG.
Methods
MOG Antigens. MOG protein was prepared by using bacteria expressing the extracellular domains of MOG from rat, human (C. Linington; University of Aberdeen, Aberdeen, Scotland) or mouse (M. Gardinier, University of Iowa, Iowa City) (21). Human MOG P42S (huP42S) was prepared by site-directed mutagenesis (QuikChange; Stratagene) of the plasmid coding for human MOG to change the proline at position 42 to a serine (21) (forward primer sequence, GTGGGGTGGTACCGCTCCCCCTTCTCTAG; reverse primer sequence, CTAGAGAAGGGGGAGCGGTACCACCCCAC).
Animals and Immunization. For active immunization, female wild-type or B cell-deficient (μMT) C57BL/6 mice (8–12 weeks of age) (The Jackson Laboratories) were immunized and boosted with recombinant MOG proteins and complete Freund's adjuvant (CFA) and treated with pertussis toxin (Pt) at days 0 and 2, and clinical disease was evaluated as described (21). For passive transfer experiments, the protocol of Lyons et al. (29) was used. μMT mice were immunized as above with human MOG and Pt, a manipulation that does not induce EAE in this strain but does prime T cells to human MOG (21). These mice also received four 150-μl injections of pooled antisera at the time of immunization and at 3-day intervals for a total of 600 μl. The donor sera had been obtained from WT mice 14 or 21 days after immunization with recombinant rat, human, or huP42S MOG proteins in CFA.
ELISA. Titers were determined (21) by using dilutions resulting in two standard deviations above the highest reading for a negative control.
IgG Preparation and Purification. IgG from sera pooled from at least 10 mice collected 14 or 21 days after immunization were purified by protein G-Sepharose (Sigma) chromatography; purity and titer were assayed by SDS/PAGE and ELISA. Three independent IgG preparations were used for each experiment.
OL Culture. Mixed primary cultures and enriched populations of mature rat and mouse OLs were prepared and maintained (30, 31). Purified OLs were grown in defined modified N2 medium (32, 33) for 6–7 days to obtain MOG-expressing OLs.
Myelin Purification. Myelin was purified from postnatal day 35 C57BL/6J mouse brains (34). Myelin was deglycosylated by treatment with PNGase F (New England Biolabs).
MOG Crosslinking (27, 28). Mouse mixed primary cultures and mouse and rat OL cultures were incubated for 30 min at 37°C with 100 μg/ml preimmune IgG, anti-MOG mAb 8-18C5 (18) (C. Linington, Aberdeen, Scotland), or with IgG purified from mice immunized with human, rat, or huP42S MOG. MOG/anti-MOG complexes were then cross-linked with goat anti-mouse IgG (28).
Immunofluorescence Microscopy. To estimate antibody binding ability, live OLs were incubated with 100 μg/ml of either preimmune IgG, mAb 8-18C5 or IgG purified from mice immunized with human, rat or huP42S MOG. In some cases, cells were fixed (4% paraformaldehyde) and permeabilized (0.05% saponin) before incubation with anti-MOG antibodies. To examine changes in morphology, mature OLs were stained live with O4 antibody as described (27). The diameter of randomly chosen cells (100 cells per experiment) was determined by using a calibrated microscopic grid.
Preparation of Cell Lysate and Detergent Extraction. OLs were scraped into 150 mM NaCl/5 mM EDTA/25 mM Tris·Cl buffer (pH 7.5) containing 1 mM PMSF, 10 μg/ml leupeptin/aprotinin, 50 mM NaF, 10 mM NaP2O7, 1 mM Na o-Vanadate, and 1% Triton X-100, centrifuged to separate them into detergent insoluble pellet and detergent-soluble supernatant fractions, and processed for SDS/PAGE (27).
SDS/PAGE and Immunoblot Analysis. Samples of purified myelin (10 μg), recombinant mouse MOG (1 μg) or equal volumes of soluble and insoluble fractions of detergent extracts were solubilized in 50 mM Tris·HCl, pH 6.8/2.5% glycerol/5% SDS/4 M urea/0.01% bromophenol blue/10 mM DTT for SDS/PAGE (27), followed by immunoblot with 2 μg/ml of preimmune IgG, IgGs purified from human or rat MOG-immunized mice, anti-MOG 8-18C5 (1:3,000), or rabbit anti-myelin-associated glycoprotein (MAG) polyclonal antisera (1:5,000; J. Roder, University of Toronto, Toronto).
Results
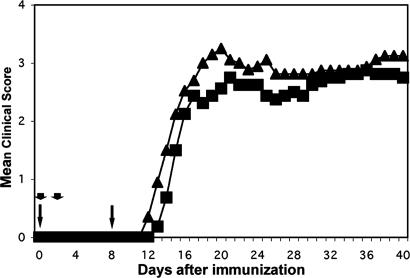
A Single Amino Acid Substitution Eliminates the B Cell Requirement for EAE to Human MOG Protein. Immunization of C57BL/6 mice with either rat MOG protein or rat MOG 35–55 peptide results in a B cell-independent disease, whereas human MOG protein generates B cell-dependent disease (21). Although human MOG protein differs from rodent MOG protein at a number of sites, several observations have identified the specific importance of position 42 (21). Human MOG peptide, with a proline at position 42, is only weakly encephalitogenic. Rat MOG peptide, with a serine at position 42, is highly encephalitogenic even in B cell-deficient mice. Substituting the serine in rat MOG protein with proline severely attenuates its encephalitogenicity (21, 24). Therefore, we reasoned that the reverse experiment, exchanging proline with serine at this site in human MOG protein by site directed mutagenesis, should result in a B cell-independent encephalitogenicity. In fact, the modified protein, termed huP42S, induced clinical signs of EAE in not only the wild-type but also B cell-deficient (μMT) mice, in which by all parameters the disease was, in fact, more intense (Fig. 1).
Fig. 1.
Substitution of a proline with serine in human MOG overcomes the requirement for B cells to induce EAE. Wild-type (WT; rectangles) or μMT (triangles) mice were immunized as indicated in Methods and evaluated for clinical signs for 40 days. All mice in both groups developed EAE with a mean day of onset of 14.6 (WT) and 13 (μMT). Mortality was 0 of 8 (WT) and 2 of 10 (μMT). Mean maximum score was 3.2 (2.5–3.5) for WT and 3.7 (2.5–5) for μMT. Day 40 disease index (sum of mean clinical scores/day of onset × 100) was 463 for WT and 593 for μMT. Arrows, 100-μg MOG plus complete Freund's adjuvant; arrowheads, 500-ng pertussis toxin
We then investigated whether antibodies against mouse MOG were generated when mice were immunized with huP42S, as they are with rodent MOG 35–55, rat MOG, and normal human MOG (21). When assayed by ELISA, pooled antisera from wild-type mice 21 days after a single immunization with huP42S reacted with recombinant mouse MOG (Table 1) in a manner similar to that obtained 14 days after immunization with either rat or normal human MOG (although human MOG generated higher IgG2c responses). We conclude that huP42S is immunogenic, as defined by ELISA, and that, consistent with our previous results, the amino acid at position 42 is an important factor in determining whether or not the disease is B cell dependent.
Table 1. Anti-MOG antisera titers against recombinant mouse MOG as determined by ELISA.
| HuP42S
|
||||
|---|---|---|---|---|
| Human | Rat | Day 14 | Day 21 | |
| IgG | 3,200 | 3,200 | < 100 | 12,800 |
| IgG1 | 800 | 800 | < 100 | 1,600 |
| IgG2c | 100 | 100 | < 100 | 1,600 |
C57BL/6 mice were immunized with the different MOG proteins in CFA. Pooled antisera from blood taken at day 14 or 21 after immunization were tested for reactivity to recombinant mouse MOG by ELISA.
Pathogenic and Nonpathogenic Antibodies Are Distinguished by Their Ability to Transfer EAE. Because a distinction could not be made among the various antisera by ELISA, we asked whether a biological assay might elucidate differences in the IgG elicited by immunization with rat, human, and huP42S MOG. Previous studies have shown that either anti-human MOG or anti-MOG mAb 8-18C5 can transfer EAE to recipient mice that have been first immunized (primed) with an antigen that produces a nonencephalitogenic T cell response to an endogenous CNS determinant (18, 29). Following this protocol, B cell-deficient mice primed with human MOG protein were injected with antisera generated in wild-type mice against either human, rat or huP42S MOG. In three separate experiments with two or three recipient mice per experiment, anti-human MOG antisera induced EAE with an incidence of six of eight mice (cumulative incidence), a mean day of onset of 13, and a mean maximum disease of those animals that developed EAE of 2.1 (range of 1–3). This disease was somewhat milder than that seen after active immunization of wild-type mice, in agreement with Lyons et al. (29). In contrast, despite similar or even higher titers by ELISA, neither anti-rat MOG (0 of 7) nor anti-huP42S (0 of 4) antisera induced EAE, indicating that this in vivo assay distinguishes between pathogenic and nonpathogenic antibodies. There was no EAE in recipients of anti-human MOG that were not primed or that were primed with ovalbumin (data not shown).
These observations are consistent with the requirement for a T cell component for antibody-mediated passive transfer of EAE (29). The exact mechanism by which T cells contribute to this effect is not known. One possibility is that activated T cells produce cytokines, leading to the opening of the blood–brain barrier, thus allowing the influx of antibodies, which, if pathogenic, then cause EAE through mechanisms investigated below. However, the T cell-induced signal is apparently either not sustained or is insufficient by itself to allow subsequent damage to myelin and OLs.
We conclude that anti-MOG antibodies generated by immunization with human, rat, or huP42S MOG, although of comparable titer when studied by ELISA, differ dramatically in pathogenicity and thus may recognize different antigenic determinants on MOG.
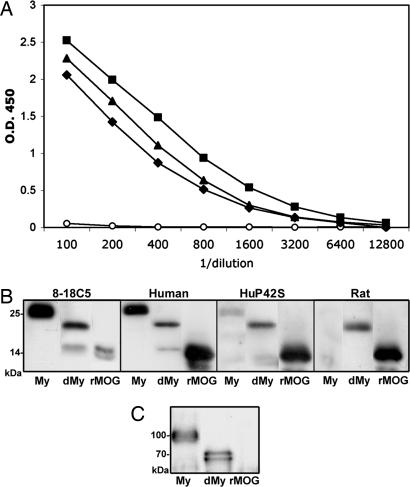
Pathogenic and Nonpathogenic Antibodies React Differentially with both Myelin and MOG Expressing Cells. The data described above indicate that a fairly cumbersome assay can distinguish between pathogenic and nonpathogenic antibodies generated in the course of EAE. To further evaluate the differences among the antibodies and determine their antigen recognition, we studied the reaction of these antibodies to MOG on purified myelin and on myelin-like membranes of cultured OLs by immunoblot analyses and immunofluorescence microscopy, respectively. Various purified IgGs of equivalent ELISA titers from pools of 10 mice (Fig. 2A) were tested by immunoblot for binding to purified recombinant MOG, MOG from untreated myelin, and myelin first treated with N-glycosidase (PNGase) to remove N-linked carbohydrate chains (Fig. 2B). The efficacy of the deglycosylation was demonstrated by treatment of another highly glycosylated myelin glycoprotein, myelin-associated glycoprotein (MAG); upon enzymatic treatment, the ≈100-kDa MAG was reduced to two nonglycosylated forms of 72 and 67 kDa, L- and S-MAG, respectively (35) (Fig. 2C). The positive control for these studies was anti-MOG mAb 8-18C5, which reacted at the predicted molecular mass of 25 kDa with MOG from untreated mouse myelin (no MOG dimer of 50 kDa was detected) and with recombinant mouse MOG. Anti-human MOG IgG bound both recombinant MOG and myelin MOG; in contrast, anti-rat MOG and -huP42S bound the former, but reacted only weakly with the latter (similar results were obtained with whole serum; data not shown). However, all three IgGs recognized the deglycosylated form of MOG (Fig. 2B). We conclude that of the IgGs examined, only the pathogenic monoclonal and anti-human MOG antibodies react with glycosylated MOG, the form most likely to resemble MOG on the surface of OLs and myelin.
Fig. 2.
IgGs from mice immunized with human, rat, or huP42S MOG, though equivalent by ELISA, differentially bind glycosylated MOG from purified myelin. (A) Purified IgG from pooled sera from mice 14 days after immunization with human (rectangles) or rat (diamonds) MOG protein, or 21 days after immunization with huP42S MOG protein (triangles), but not IgG from unimimmunized mice (circles), bound recombinant mouse MOG with approximately equivalent titer (ELISA; samples adjusted to same protein concentration). (B) Immunoblot of mouse myelin (My), deglycosylated myelin (dMy), or recombinant mouse MOG (rMOG): encephalitogenic mAb 8-18C5 and IgG from mice immunized with human, but not rat or huP42S, MOG protein bind MOG in purified myelin. In contrast, all four IgGs bind deglycosylated MOG in myelin and recombinant mouse MOG. (C) Immunoblot with anti-myelin associated glycoprotein (MAG): upon deglycosylation, ≈100-kDa MAG separates into bands of 72 (L-MAG) and 67 (S-MAG) kDa, respectively. Results are representative of three independent experiments
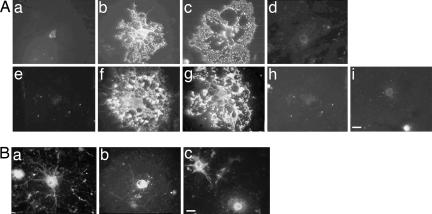
Anti-human MOG, but not anti-rat MOG, anti-huP42S, or IgG from nonimmunized mice, immunostained the surface of both mouse and rat live OLs in culture in a manner indistinguishable from that of mAb 8-18C5 (Fig. 3A). However, upon fixation and permeabilization of the cells, anti-human, -rat, and -huP42S MOG protein all stained internal antigens in the cell bodies of OLs (Fig. 3B). We conclude that purified IgG from mice immunized with human MOG, but not rat or huP42S MOG protein, binds to the surface of differentiated OLs in culture. The internal binding may represent a form that is not yet glycosylated.
Fig. 3.
IgGs from mice immunized with mAb 8-18C5 or human, but not rat or huP42S, MOG protein bind to live OLs. (A) Mouse (a–d) and rat (e–i) OL cultures were incubated with preimmune IgG (a and e), 8-18C5 (b and f), or IgG purified from mice immunized with human (c and g), rat (d and h), or huP42S (i) MOG protein. (Bar, 5 μm.) (B) IgGs from mice immunized with human (a), rat (b), or huP42S (c) MOG protein bind intracellular MOG in fixed/permeabilized OLs. (Bar, 5 μm.) Results are representative of three independent experiments
Cross-Linking of MOG on the Surface of Cultured OLs with Anti-Human MOG IgG Induces MOG Repartitioning into a Detergent-Insoluble Fraction and Morphological Alterations. Antibody binding per se does not necessarily indicate biological function. Conversely, the absence of binding as measured by immunoblotting and immunohistochemistry, although strongly arguing against high affinity antigen-antibody interactions, does not entirely preclude the possibility of transient, but biologically significant, roles. On this basis, we postulated that a demonstration of a direct correlation between antibody encephalitogenicity, binding capacity, and physiologically relevant effects on cells would be an important step in relating these data to antibody relevance in disease pathology.
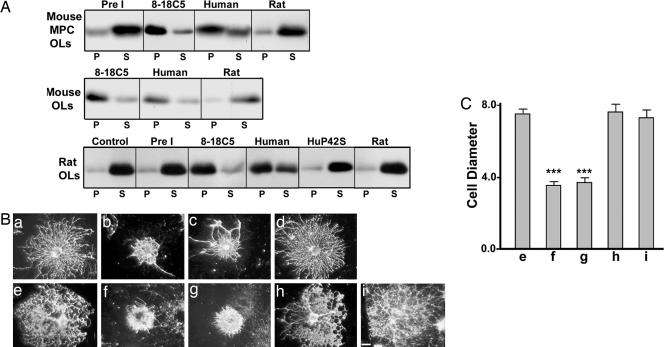
We have shown that, when mAb 8-18C5 bound to the surface of OLs in culture is cross-linked with a secondary antibody, MOG is rapidly repartitioned from a detergent-soluble to an insoluble fraction with the biochemical characteristics of lipid rafts; this is quickly followed by changes in the phosphorylation status of at least 10 proteins, culminating in dramatic changes in OL cytoarchitecture (27, 28). Here, we extended these observations to the polyclonal IgGs from mice immunized with various forms of human or rat MOG. We found that IgG from mice immunized with human MOG produced effects similar to those previously observed with mAb 8-18C5 (Fig. 4), including repartitioning of MOG into a detergent-insoluble fraction consistent with lipid rafts, and retraction of OL processes thought to correlate with changes in cytoskeletal stability (28). In contrast, no such changes in MOG repartitioning or morphological changes were seen with control IgG, or with IgG from mice immunized with either rat or huP42S MOG (Fig. 4).
Fig. 4.
MOG cross-linking with IgGs from mice immunized with human, but not rat or huP42S, MOG protein induces MOG repartitioning into a detergent insoluble fraction and morphological alterations in OLs. (A) MOG immunoblot of detergent soluble (S) or insoluble pellet (P) fractions from mixed primary or purified OL cultures incubated with media alone (Control), IgG from naïve mice (Pre I), mAb 8-18C5, or IgG from mice immunized with either human, huP42S, or rat MOG protein, followed by cross-linking with secondary antibodies. Treatment with mAb 8-18C5 or IgG raised against human MOG, but not huP42S or rat, MOG protein induces repartitioning of MOG into the detergent insoluble fraction. (B) Mouse (a–d) or rat (e–i) OLs were incubated with preimmune IgG (a and e), 8-18C5 (b and f), or IgG from mice immunized with human (c and g), rat (d and h), or huP42S (i) MOG protein, followed by cross-linking with anti-mouse IgG and stained with mAb O4 to visualize OL morphology. (Bar, 5 μm.) (C) Diameter (arbitrary units; mean ± SEM) of randomly chosen cells. IgG raised against human, but not rat or huP42S, MOG protein induces retraction of OL processes similar to 8-18C5 (***, P < 0.0001). Results are representative of three independent experiments
In summary, we conclude that the data strongly support the hypothesis that pathogenic (i.e., anti-human MOG), but not nonpathogenic (i.e., anti-rat or -huP42S MOG), antibodies bind to OL cell surfaces and induce membrane protein redistribution and dramatic changes in cell morphology that, based on our previous data obtained with a monoclonal antibody, reflect a striking change in the physiology of the cells. We propose that these considerations are at the heart of the varying B cell dependence in EAE induced by human vs. rat MOG.
Discussion
The data presented here support four principal conclusions based on antibodies generated in EAE (as summarized in Table 2). First, a single amino acid substitution in human MOG at position 42 changes the mechanism of encephalitogenicity from B cell dependent to independent. Second, although immunization with rat, human, or huP42S MOG generates antibodies against recombinant mouse MOG that are detectable by ELISA, only antibodies directed against human MOG, but not anti-rat or anti-huP42S MOG, are encephalitogenic in vivo. Third, antibodies to human MOG bind to glycosylated MOG in immunoblots and to live OLs in culture; anti-rat and anti-huP42S MOG antibodies require deglycosylation or permeabilized cells to be detectable by immunoblot or immunofluorescent microscopy, respectively. Fourth, upon cross-linking, antibodies to human MOG, but not rat or huP42S MOG, induce repartitioning of MOG into subdomains of OL membranes, a crucial target of MS, which is followed by dramatic morphological changes.
Table 2. Encephalitogenic and biologic properties of recombinant MOG proteins.
| MOG immugen
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Human MOG
|
Rat MOG
|
HuP42S
|
|||||||
| Result | Wild type | μMT | Wild type | μMT | Wild type | μMT | |||
| EAE | + | – | + | + | + | + | |||
| B cell dependence | Yes | No | No | ||||||
| ELISA | + | NA | + | NA | + | NA | |||
| Pathogenic antibodies | +* | +† | – | – | – | – | |||
NA, not applicable.
Determined by binding to OL surfaces and induction of MOG repartitioning and OL morphological alterations.
Determined by serum passive transfer experiments.
Previous authors using transfected fibroblasts have suggested that encephalitogenic antibodies recognize conformation-dependent epitopes (1, 26), although in none of these studies was the encephalitogenicity of the antibodies actually demonstrated, except in the case of mAb 8-18C5. The data presented here indicate that the epitopes previously recognized in transfected fibroblasts may be glycosylation dependent. We show that encephalitogenic anti-MOG mAb 8-18C5 and the encephalitogenic anti-human MOG antibodies bind to glycosylated MOG on immunoblots and surface MOG on live OLs in culture, and activate changes in OLs. It has previously been established that mAb 8-18C5 binds to discontinuous (i.e., conformation-dependent) determinants but not to linear MOG peptides (36, 37). The conclusion that encephalitogenic antibodies bind to nonlinear determinants is also supported by the studies of von Budingen et al. (8), who reported that antibodies raised in marmosets against rat MOG peptides could not transfer EAE to myelin basic protein primed animals, whereas antisera that included antibodies recognizing conformational determinants transferred a severe disease with regard to CNS lesions and demyelination. Bourquin et al. (26) demonstrated that B10.S, but not C57BL/6, mice developed EAE after immunization with rat MOG in incomplete Freund's adjuvant, arguing that the disease was B cell mediated and suggesting that the MHC dictated whether a pathogenic or nonpathogenic antibody was generated. Our previous data (21), and those provided here, indicate that the situation is considerably more complex. The MHC may censor the ability of H-2b mice to generate a pathogenic antibody to rat MOG; clearly it does not inhibit the ability to generate a pathogenic antibody to human MOG. On the basis of these correlated data, it is reasonable to conclude that nonpathogenic antibodies recognize linear MOG determinants and do not bind to OLs, but pathogenic antibodies recognize conformational, glycosylation-dependent determinants and bind to OLs.
Although anti-myelin antibodies are commonly found in MS, they are generally of limited diagnostic value because they are also found in a significant number of control patients and patients with nondemyelinating diseases (2, 12). Furthermore, it is possible that some MS patients generate antibody responses that reflect, rather than cause, the disease. The correlates we have shown here between antibody binding properties and pathogenesis suggest improved protocols for the identification of pathogenic antibodies in MS patients. We suggest three rapid diagnostic tools for the analysis of MS patient sera to distinguish between pathogenic (e.g., anti-human MOG) and nonpathogenic (e.g., anti-rat MOG) antibodies: screening by (i) immunoblot against native (glycosylated) vs. nonglycosylated MOG, (ii) immunofluorescent microscopy against live vs. fixed, permeabilized OLs, and (iii) light microscopic identification of rapid changes in OL morphology of cells treated with cross-linked IgG. These data provide a link between in vivo and in vitro observations (27, 28) and further strengthen a model for the biochemical mechanism for antibody-mediated demyelinating disease (27, 28). They also provide in vitro tools to determine whether an autoimmune antibody is pathogenic, and may be useful for evaluating the pathogenicity of antibodies in MS patients as an adjunct to diagnosis and treatment.
Acknowledgments
We thank Christopher Linington for the gift of plasmids encoding human and rat MOG and the 8-18C5 monoclonal antibody; Minetta Gardinier for the gift of the plasmid encoding mouse MOG; and Craig Pike, Myriam Hill, and Cheryl Bergman for outstanding technical assistance. This work was supported in part by National MS Society Grants RG 2394 (to N.H.R.) and FG1423A (to C.B.M.) and National Institutes of Health Grants NS10861 and NS41078 (to S.E.P.).
Author contributions: C.B.M., A.R.O., S.E.P., and N.H.R. designed research; C.B.M., A.R.O., R.A.S., and N.H.R. performed research; C.B.M., A.R.O., S.E.P., and N.H.R. analyzed data; and C.B.M., A.R.O., S.E.P., and N.H.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MS, multiple sclerosis; OL, oligodendrocyte; MOG, myelin oligodendrocyte glycoprotein; EAE, experimental autoimmune encephalomyelitis.
References
- 1.Haase, C. G., Guggenmos, J., Brehm, U., Andersson, M., Olsson, T., Reindl, M., Schneidewind, J. M., Zettl, U. K., Heidenreich, F., Berger, T., et al. (2001) J. Neuroimmunol. 114, 220-225. [DOI] [PubMed] [Google Scholar]
- 2.Lindert, R. B., Haase, C. G., Brehm, U., Linington, C., Wekerle, H. & Hohlfeld, R. (1999) Brain 122, 2089-2100. [DOI] [PubMed] [Google Scholar]
- 3.Genain, C. P., Cannella, B., Hauser, S. L. & Raine, C. S. (1999) Nat. Med. 5, 170-175. [DOI] [PubMed] [Google Scholar]
- 4.Lassmann, H. (2004) in Myelin Biology and Disorders, ed. Lazzarini, R. A. (Elsevier Academic, San Diego), Vol. 2, pp. 733-762. [Google Scholar]
- 5.Lucchinetti, C., Bruck, W., Parisi, J., Scheithauer, B., Rodriguez, M. & Lassmann, H. (2000) Ann. Neurol. 47, 707-717. [DOI] [PubMed] [Google Scholar]
- 6.Mathey, E., Breithaupt, C., Schubart, A. S. & Linington, C. (2004) Eur. J. Immunol. 34, 2065-2071. [DOI] [PubMed] [Google Scholar]
- 7.Storch, M. K., Stefferl, A., Brehm, U., Weissert, R., Wallstrom, E., Kerschensteiner, M., Olsson, T., Linington, C. & Lassmann, H. (1998) Brain Pathol. 8, 681-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Budingen, H. C., Hauser, S. L., Ouallet, J. C., Tanuma, N., Menge, T. & Genain, C. P. (2004) Eur. J. Immunol. 34, 2072-2083. [DOI] [PubMed] [Google Scholar]
- 9.Kieseier, B. C. & Hartung, H. P. (2003) Semin. Neurol. 23, 133-146. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Villoslada, P., Shih, A., Shao, L., Genain, C. P. & Hauser, S. L. (1999) J. Neuroimmunol. 99, 36-43. [DOI] [PubMed] [Google Scholar]
- 11.Berger, T., Rubner, P., Schautzer, F., Egg, R., Ulmer, H., Mayringer, I., Dilitz, E., Deisenhammer, F. & Reindl, M. (2003) N. Engl. J. Med. 349, 139-145. [DOI] [PubMed] [Google Scholar]
- 12.Lampasona, V., Franciotta, D., Furlan, R., Zanaboni, S., Fazio, R., Bonifacio, E., Comi, G. & Martino, G. (2004) Neurology 62, 2092-2094. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias, A., Bauer, J., Litzenburger, T., Schubart, A. & Linington, C. (2001) Glia 36, 220-234. [DOI] [PubMed] [Google Scholar]
- 14.Eugster, H. P., Frei, K., Kopf, M., Lassmann, H. & Fontana, A. (1998) Eur. J. Immunol. 28, 2178-2187. [DOI] [PubMed] [Google Scholar]
- 15.Cross, A. H., Lyons, J. A., San, M., Keeling, R. M., Ku, G. & Racke, M. K. (1999) Eur. J. Immunol. 29, 3140-3147. [DOI] [PubMed] [Google Scholar]
- 16.Hjelmstrom, P., Juedes, A. E., Fjell, J. & Ruddle, N. H. (1998) J. Immunol. 161, 4480-4483. [PubMed] [Google Scholar]
- 17.Kerlero de Rosbo, N., Honegger, P., Lassmann, H. & Matthieu, J. M. (1990) J. Neurochem. 55, 583-587. [DOI] [PubMed] [Google Scholar]
- 18.Schluesener, H. J., Sobel, R. A., Linington, C. & Weiner, H. L. (1987) J. Immunol. 139, 4016-4021. [PubMed] [Google Scholar]
- 19.Linington, C., Bradl, M., Lassmann, H., Brunner, C. & Vass, K. (1988) Am. J. Pathol. 130, 443-454. [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons, J. A., San, M., Happ, M. P. & Cross, A. H. (1999) Eur. J. Immunol. 29, 3432-3439. [DOI] [PubMed] [Google Scholar]
- 21.Oliver, A. R., Lyon, G. M. & Ruddle, N. H. (2003) J. Immunol. 171, 462-468. [DOI] [PubMed] [Google Scholar]
- 22.Mendel, I. K. d. R., N. & Ben-Nun, A. (1996) Eur. J. Immunol. 26, 2470-2479. [DOI] [PubMed] [Google Scholar]
- 23.Petersen, T. R., Bettelli, E., Sidney, J., Sette, A., Kuchroo, V. & Backstrom, B. T. (2004) Eur. J. Immunol. 34, 165-173. [DOI] [PubMed] [Google Scholar]
- 24.Albouz-Abo, S., Wilson, J. C., Bernard, C. C. & von Itzstein, M. (1997) Eur. J. Biochem. 246, 59-70. [DOI] [PubMed] [Google Scholar]
- 25.Brehm, U., Piddlesden, S. J., Gardinier, M. V. & Linington, C. (1999) J. Neuroimmunol. 97, 9-15. [DOI] [PubMed] [Google Scholar]
- 26.Bourquin, C., Schubart, A., Tobollik, S., Mather, I., Ogg, S., Liblau, R. & Linington, C. (2003) J. Immunol. 171, 455-461. [DOI] [PubMed] [Google Scholar]
- 27.Marta, C. B., Taylor, C. M., Coetzee, T., Kim, T., Winkler, S., Bansal, R. & Pfeiffer, S. E. (2003) J. Neurosci. 23, 5461-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marta, C. B., Montano, M. B., Taylor, C. M., Taylor, A. L., Bansal, R. & Pfeiffer, S. E. (2005) J. Biol. Chem. 280, 8985-8993. [DOI] [PubMed] [Google Scholar]
- 29.Lyons, J. A., Ramsbottom, M. J. & Cross, A. H. (2002) Eur. J. Immunol. 32, 1905-1913. [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer, S. E., Warrington, A. E. & Bansal, R. (1993) Trends Cell Biol. 3, 191-197. [DOI] [PubMed] [Google Scholar]
- 31.Bansal, R., Kumar, M., Murray, K., Morrison, R. S. & Pfeiffer, S. E. (1996) Mol. Cell. Neurosci. 7, 263-275. [DOI] [PubMed] [Google Scholar]
- 32.Bottenstein, J. E. & Sato, G. H. (1979) Proc. Natl. Acad. Sci. USA 76, 514-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gard, A. L. & Pfeiffer, S. E. (1989) Development (Cambridge, U.K.) 106, 119-132. [DOI] [PubMed] [Google Scholar]
- 34.Menon, K., Rasband, M. N., Taylor, C. M., Brophy, P., Bansal, R. & Pfeiffer, S. E. (2003) J. Neurochem. 87, 995-1009. [DOI] [PubMed] [Google Scholar]
- 35.Pedraza, L., Frey, A. B., Hempstead, B. L., Colman, D. R. & Salzer, J. L. (1991) J. Neurosci. Res. 29, 141-148. [DOI] [PubMed] [Google Scholar]
- 36.Breithaupt, C., Schubart, A., Zander, H., Skerra, A., Huber, R., Linington, C. & Jacob, U. (2003) Proc. Natl. Acad. Sci. USA 100, 9446-9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litzenburger, T., Fassler, R., Bauer, J., Lassmann, H., Linington, C., Wekerle, H. & Iglesias, A. (1998) J. Exp. Med. 188, 169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]