Abstract
Background
Hepatic fibrosis (HF) continues to be a significant global health concern, substantially contributing to morbidity and mortality due to the absence of effective therapeutic options. This study examines the pharmacological effectiveness and underlying mechanisms of Notoginsenoside R2 (R2) in mitigating HF, aiming to find a new multifunctional candidate for therapeutic application.
Methods
An integrative methodology utilizing network pharmacology, molecular docking, and experimental validation was implemented. In vitro models (HSC-T6), in vivo systems (zebrafish), and microinjection of morpholinos were employed to corroborate the antifibrotic effects of R2 and transcription 3 (STAT3)-dependent processes.
Results
Network pharmacology identified 32 common targets between R2 and HF, with a particular emphasis on pathways critical for the activation of HSCs. Molecular docking confirmed strong interactions between R2 and signal transducer and activator of STAT3. In vitro, R2 inhibited HSCs proliferation and decreased the expression of α-SMA, COL-I, Desimin and TIMP1. In vivo, R2 mitigated thioacetamide-induced fibrosis in zebrafish, leading to decreased collagen deposition and suppression of pro-inflammatory cytokines. Mechanistically, R2 induced senescence in HSCs via the STAT3 pathway, characterized by increased expression of cyclin-dependent kinase inhibitor 2A (CDKN2A/p16) and cyclin-dependent kinase inhibitor 1A (CDKN1A/p21), as well as components of the senescence-associated secretory phenotypes (SASPs).
Conclusion
This study identified R2 as a regulator of STAT3 with dual antifibrotic effects: reduction of the inflammatory microenvironment and induction of senescence. These findings position R2 as a viable treatment candidate for HF, necessitating additional clinical investigation.
Keywords: Notoginsenoside R2 (R2), Hepatic fibrosis (HF), Hepatic stellate cells (HSCs), Senescence, Signal transducer and activator of transcription 3 (STAT3)
Graphical abstract

1. Introduction
Hepatic fibrosis (HF) is a significant unmet medical need that contributes to global morbidity and mortality [1]. Although timely intervention can reverse fibrotic progression [2], there are currently no approved therapeutic strategies specifically targeting HF [3,4]. Chronic inflammation and persistent activation of hepatic stellate cells (HSCs) are the primary pathogenic characteristics of HF [[5], [6], [7]]. Activated HSCs are responsible for the excessive deposition of extracellular matrix (ECM) components, leading to the disruption of hepatic architecture and function, alterations in intrahepatic blood flow, and eventually HF [8]. Therefore, the targeted modulation of HSCs activation and function has emerged as a crucial strategy for reversing HF.
Panax notoginseng (PNS) exhibits significant antifibrotic potential via its active constituents, including panax saponins, polysaccharides, and flavonoids, by multi-targeted modulation of HSCs activity, suppression of inflammatory signaling pathways, and mitigation of oxidative damage [9,10]. Diverse elements of the PNS exhibited distinct anti-HF strategies. Notoginsenoside R1 inhibits fibrogenesis by targeting the peroxisome proliferator-activated receptor γ pathway [11], while Ginsenoside-Rg3 mitigates disease development by triggering ferroptosis [12]. R2, a member of the PNS family, exhibits structural similarities to identified ginsenosides [13]. Recent research highlights the anti-inflammatory, antioxidant, and cardioprotective characteristics of R2, indicating its potential as a therapeutic candidate for metabolic and inflammatory illnesses [[14], [15], [16]]. The structural homology and mechanistic enrichment provide a compelling rationale for the ability of R2 to ameliorate HF. Moreover, our previous study identified R2 as a principal bioactive compound in Panax notoginseng within Baoganning Decoction [17], a formulation with demonstrated anti-fibrotic efficacy. Nonetheless, the therapeutic potential and pharmacological profile of R2 remain unexamined.
The fundamental pathology of HF involves the aberrant transformation of HSCs from a quiescent to an activated state [18]. Hence, inducing senescence in HSCs may represent a novel strategy to reverse fibrosis by halting their proliferation and modulating the microenvironment [19,20]. Potential strategies for inducing senescence in HSCs are regulation of senescence-related signaling pathways, the targeting of metabolic reprogramming, epigenetic regulation, and the manipulation of SASPs [[21], [22], [23], [24]]. Herbal medications and their active constituents exhibit distinct benefits by promoting the senescence of HSCs and suppressing the SASPs via multi-targeting mechanisms [25,26]. Numerous studies have examined the therapeutic potential and cellular mechanisms of PNS in preventing aging and disorders associated to cellular senescence [27,28]. Nevertheless, there is a deficiency of research focusing on activated HSCs. This study examined the inhibitory effect of R2 on HF by investigating its impact on the senescence of activated HSCs.
STAT3, as a key signal transduction molecule, plays a central role in the development of HF through the regulation of inflammatory responses, activation of HSCs, and cell proliferation and apoptosis [29]. The investigation of the mechanisms by which Chinese medicine monomers target STAT3 for the treatment of HF is a significant focus in the modernization of traditional Chinese medicine[[30], [31], [32]]. Herbal monomers demonstrate distinct benefits in the treatment of HF by targeting STAT3 signaling[[33], [34], [35]]. Consequently, the combined strategy of targeting STAT3 and inducing senescence in HSCs provides a new concept of efficient and low-toxicity treatment for HF by synergistically suppressing activation and microenvironmental remodeling [36].
In this study, the therapeutic potential of R2 in HF was systematically investigated, employing a multimodal approach to dissect its roles in hepatic inflammation, antioxidative processes, and HSCs senescence. Mechanistic studies integrating pathway enrichment analysis and morpholino-mediated STAT3 knockdown reveal that R2 induces senescence in activated HSCs through selective modulation of STAT3 signaling. This senescence-driven mechanism, confirmed by the downregulation of fibrotic markers and the reduction of inflammatory mediators, establishing R2 as a pioneering therapeutic drug capable of impeding HF. In summary, these findings position R2 as a multifunctional candidate for HF intervention, offering an innovative approach to mitigate fibrotic remodeling by targeting STAT3-dependent HSCs senescence and inflammatory signaling.
2. Materials and methods
2.1. Materials
R2 (Purity≥ 98 %, #HY-N0909) was provided by MedChemExpress (MCE) (City of Rahway, New Jersey, USA). The splice-blocking Morpholino oligonucleotides (MO) against STAT3 and a standard control MO were designed and manufactured by Gene Tools (South San Francisco, California, USA). COL-Ⅰ (#14695-1-AP, 1:2000), α-SMA (#67735-1-Ig), TIMP1 (#26847-1), P53 (#10442-1) and P21 (#10355-1-AP) antibodies were purchased from Proteintech (Wuhan, China). P16 antibody (#ab51243) was obtained from Abcam (Cambridge, England). STAT3 (#9139T), P-STAT3 (#9145), JAK (#3344T), P-JAK (#74192), mTOR (#2983), P- mTOR (#5536) and HSP90 (#4877) antibodies were provided by Cell Signaling Technology (CST) (Boston, Massachusetts, USA). Desmin (#HY-P80103) antibodies were purchased from MCE. Cell-Counting-Kit-8 (#C6005) Kit was purchased from New Cell and Molecular Biotech (NCM) (Jiangsu, China). Thioacetamide (TAA) (#163678) was provided by MERCK (Darmstadt, Germany). Reactive Oxygen Species Assay Kit (#CA1420) was obtained from Solarbio (Beijing, China). The FastPure® Cell/Tissue Total RNA Isolation Kit (#RC112) was purchased from Vazyme (Nanjing, China). PrimeScriptTM RT Master Mix (#RR036A) and TB Green® Premix Ex TaqTM Ⅱ (#RR820A) were provided by Takara (Osaka, Japan).
2.2. Zebrafish and maintenance
The wild-type (AB strain) and transgenic Tg (lfabp10α: eGFP) zebrafish utilized in this investigation were obtained from Southern Medical University in Guangzhou, China. Adult zebrafish were housed in recirculating aquaculture systems at 28 °C with regulated photoperiods (14 h light/10 h dark), utilizing local tap water adjusted to a pH of 7.2–7.6 and a salinity of 0.03–0.04 %. The Ethics Committee of Southern Medical University approved all experimental protocols (Approval No. SMUL202403002, approval date: April 9, 2024).
2.3. Cell line
The HSC-T6 line and AML12 hepatocyte cell line were provided by the Hepatology Center of Nanfang Hospital (Guangzhou, China). HSC-T6 was transformed from SV40 transfected cultured SD rat HSCs for 15 ds and its phenotype of activated HSC is an immortalized cell lineage [[37], [38], [39]].
2.4. Prediction of targeted genes and HF therapeutic targets
R2 was input into SwissTarget Prediction to predict potential targets. To systematically discover therapeutic targets for HF, the keywords “HF” and “Liver Fibrosis” were searched across databases.
2.5. Network analysis
To investigate the potential mechanisms of R2 against HF, A Venn diagram was created to identify the shared targets between the expected targets of the chemical and the therapeutic targets of HF. The resulting protein-protein interaction data were imported into Cytoscape 3.9.0 for network visualization.
2.6. Functional enrichment analysis
Pathway enrichment analysis utilizing the Kyoto Encyclopedia of Genes and Genomes was conducted to uncover dysregulated biological pathways, with statistically significant outcomes illustrated through specialized bioinformatics visualization tools. Simultaneously, Gene Ontology analysis systematically classified differentially expressed target proteins organized functional domains. Results were additionally corroborated via repeated permutation testing to reduce false discovery rates.
2.7. Molecular docking validation
The tertiary structures of the protein receptors of relevance were obtained from the Protein Data Bank, prioritizing entries with high-resolution crystallographic data (≤2.5 Å) and validated biological activity annotations. Molecular docking simulations were performed using the AutoDock Vina algorithm, employing a Lamarckian genetic algorithm to predict receptor-ligand binding affinities (kcal/mol).
2.8. CCK-8 assay to select the safe concentration of R2
The cytotoxicity profile of R2 was assessed using CCK-8 assays. The HSC-T6 and AML12 cells were cultured in 96-well plates and treated with varying concentrations of R2 (0, 10, 20, 40, 80, 100 μM). Following a 24-h exposure, 10 μL of CCK-8 reagent was added to each well. After a 2-h incubation at 37 °C, absorbance values were measured using the microplate reader.
2.9. Experimental animals
The zebrafish toxicity assessment examined critical characteristics such as survival rate, hatching success, heart rhythm, somatic growth, and morphological integrity. Five groups of 2-d post-fertilization larvae were randomly assigned: a control group, a 0.06 % TAA-treated group, a low dose group (6.25 μM), a medium dose group (12.5 μM), and a high dose group (25 μM). All intervention groups received 0.06 % TAA continuously. After 72-h TAA preconditioning, zebrafish received R2 at the prescribed concentrations. A detailed schematic of zebrafish modelling and drug administration is shown in Supplementary Fig. 2.
2.10. Microinjection of morpholinos
A targeted antisense morpholino oligonucleotide (MO) method was employed to downregulate STAT3 expression, utilizing a sequence-specific MO from Gene Tools, LLC, Philomath, OR, USA. The morpholino sequence (5′-CCT-CTT-ACC-TCA-GTT-ACA-ATT-TAT-A-3′) was engineered to complement the STAT3 mRNA translation initiation site or splice junctions, hence facilitating selective suppression. Morpholino solutions were formulated at varying concentrations (0, 1, 2, 4, and 8 mg L−1) in nuclease-free water. Approximately 1 nL of fluid was microinjected into the yolk of zebrafish embryos at the one-to two-cell developing stage with a Pneumatic PicoPump. STAT3 inhibition was measured using quantitative polymerase chain reaction. A schematic of the detailed microinjection of morpholinos process and operation is shown in Supplementary Fig. 3.
2.11. Measurement of Reactive oxygen species (ROS)
Staining with DCFH-DA at a 1:1000 dilution was conducted under specific conditions: HSC-T6 cells underwent incubation at 37 °C for 30 min, whereas zebrafish specimens necessitated 60 min of incubation at 28.5 °C. Following PBS washing, the samples were examined via fluorescence microscopy.
2.12. Immunofluorescence staining
Liver sections were stabilized in 4 % paraformaldehyde in phosphate-buffered saline for 45 min, thereafter, undergoing permeabilization with 0.4 % Triton X-100 for 10 min. Primary antibodies directed against critical fibrosis and senescence markers were diluted in an antibody dilution buffer and administered to the sections. The slides were incubated overnight at 4 °C in a humidified room. Following three washes with PBS, sections were incubated with species-specific Alexa Fluor-conjugated secondary antibodies for 45 min at room temperature, shielded from light. Fluorescence images were obtained via a Zeiss Axio Scan.Z1 automated slide scanner fitted with a 20 × Plan-Apochromat objective.
2.13. H&E staining
Zebrafish were paraffin-fixed and sectioned to a thickness of 14 μm. Hematoxylin and eosin staining was used to evaluate fibrosis accumulation in zebrafish liver tissue.
2.14. Senescence-associated β-galactosidase assay
The growth media was aspirated, and the cells were washed once with PBS, thereafter, fixed with 1 mL of β-galactosidase staining fixative at 25 °C for 15 min. Subsequent to the removal of the fixative, 1 mL of the staining working solution was introduced to each well and incubated at 37 °C overnight.
2.15. Western blotting
A 10 % SDS-PAGE gel was created with 20 μg of protein per lane. Electrophoresis began at 80 V for 30 min and increased to 120 V for 90. Proteins were transferred to PVDF membranes for 120 min at 300 mA. After 2 h in 5 % skim milk at 25 °C, the membranes were sectioned by target protein molecular weight. Primary antibodies targeting was incubated overnight at 4 °C. HRP-conjugated secondary antibodies were then incubated at 25 °C for 2 h. ImageJ software was used to quantify relative expression levels of protein bands identified by enhanced chemiluminescence (ECL).
2.16. RNA extraction and qRT-PCR analysis
Total RNA was extracted from harvested cells using Trizol reagent according to the manufacturer's protocol. For zebrafish samples, RNA was isolated using the same method, with pooled RNA from 30 zebrafish per group. The expression data were normalized to the β-actin gene. Relative mRNA expression levels of target genes were calculated using the 2ˆ−ΔΔCt method. Primer sequences for all analyzed genes are provided in Supplementary Table 1.
2.17. Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 10.3 (GraphPad Software, San Diego, CA, USA). Data normality was assessed using the Shapiro–Wilk test, and homogeneity of variances was evaluated using Levene's test. An unpaired two-tailed Student's t-test was used for comparisons between two independent groups. One-way analysis of variance (ANOVA) was used for comparisons involving three or more groups, followed by Tukey's post hoc test. If the assumption of homogeneity of variances was violated, Welch's ANOVA was applied, followed by the Games–Howell post hoc test for pairwise comparisons. A p-value of less than 0.05 was considered statistically significant.
3. Result
3.1. Extraordinary anti-fibrotic potential of Notoginsenoside R2
R2 was characterized via ultra-performance liquid chromatography coupled with high-resolution mass spectrometry (Fig. S1A). To determine its potential therapeutic targets, an extensive bioinformatic analysis was performed utilizing the Swiss Target Prediction database (Fig. S1B). After consolidating the results and eliminating duplicates, 33 candidate targets of R2 were identified (Supplementary Table 1). A Venn diagram illustrated 32 overlapping targets between R2 and HF (Fig. S1C). A protein-protein interaction network for the common targets was constructed utilizing STRING (Fig. S1D), with nodes organized by degree centrality. Kyoto encyclopedia of genes and genomes pathway enrichment analysis identified 6 key signaling pathways associated with the therapeutic effects of R2 (Fig. S1E–F). To validate target engagement, molecular docking simulations were performed between R2 and key hub targets: STAT3, HSP90, and MTOR (Fig. S1G–I). The findings highlight the ability of R2 to influence key pathways involved in hepatic fibrogenesis, establishing R2 as a potential therapeutic candidate for HF.
3.2. In vitro antifibrotic evaluation of Notoginsenoside R2
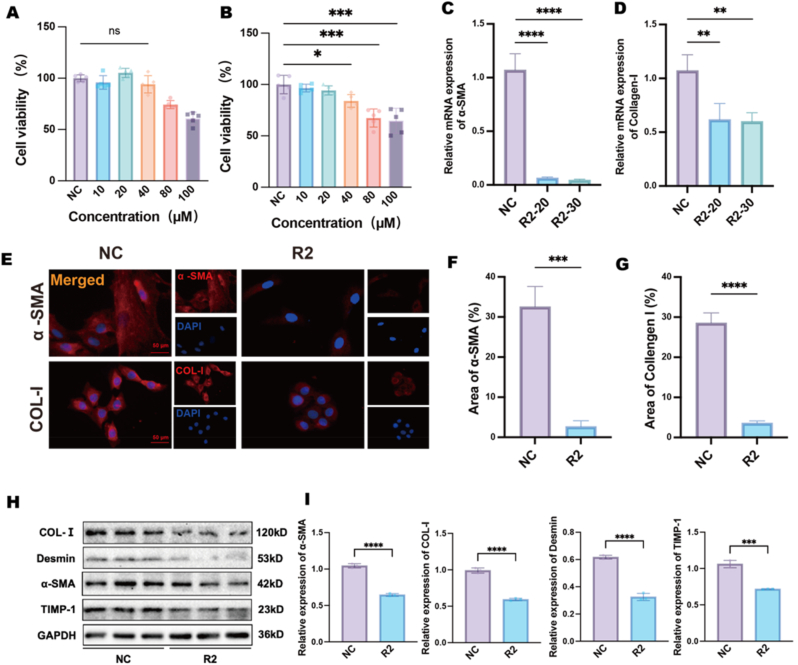
The antifibrotic efficacy of R2 was evaluated using activated HSC-T6 as a model for HF. CCK-8 viability assays conducted on AML12 hepatocytes indicated that R2 displayed concentration-dependent cytoprotection within the range of 10–100 μM (Fig. 1A), thereby confirming its hepatocyte-preserving properties at non-toxic doses. The subsequent CCK-8 analysis of HSC-T6 cells demonstrated a dose-dependent (40–100 μM) inhibition of proliferation (Fig. 1B). RT-PCR was performed to assess transcriptional alterations in essential fibrotic markers, thereby clarifying the antifibrotic effect of R2. R2 in concentration of 20 μM treatment markedly reduced mRNA expression of α-SMA and COL-I (Fig. 1C and D), aligning with the inhibition of stellate cell activation. The findings were validated using multiple modalities, including immunofluorescence staining, which revealed decreased intracellular levels of α-SMA and COL-I in R2-treated HSC-T6 cells (Fig. 1E–G). Complementary Western blot analysis verified that HSC-T6 cells exhibited elevated baseline levels of α-SMA, collagen I, Desmin, and TIMP1 expression, whereas R2 severely disrupted the fibrotic markers and extracellular matrix homeostasis (Fig. 1H and I). These data collectively demonstrate that R2 inhibits HSCs activation by modulating both pro-fibrotic markers (a-SMA, Desmin) and regulators of ECM homeostasis (TIMP-1, COL-I).
Fig. 1.
In vitro antifibrotic effects of R2 in HSC-T6. A: CCK-8 viability assay in AML12 hepatocytes treated with R2 (10–100 μM, 24 h). B: Dose-dependent suppression of HSC-T6 cells proliferation by R2 (10–100 μM, 24 h). C–D: RT-PCR analysis showing R2 (20 and 30 μM, 24 h)-mediated downregulation of α-SMA (C) and collagen I (D) mRNA levels in HSC-T6 cells. E–G: Immunofluorescence staining of α-SMA (E, F) and collagen I (E, G) in R2 (20 μM, 24 h)-treated HSC-T6 cells. H–I: Western blot quantification of α-SMA, Col-I, Desmin and TIMP-1 expression in R2 (20 μM, 24h)-treated HSC-T6 cells. Scale bar = 50 μm, Data are presented as mean ± SD (n = 3 independent cell cultures), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001 vs. normal control (One-way ANOVA with Tukey's post hoc test, or Welch's ANOVA with Games–Howell post hoc test where variance homogeneity was violated).
3.3. R2 induces HSCs senescence and inhibits inflammatory microenvironment
Considering the established role of inflammatory signaling in sustaining HSCs activation, the ability of R2 to regulate inflammatory pathways and senescence in HSC-T6 cells were assessed. RT-PCR analysis indicated that R2 significantly reduced mRNA levels of pro-inflammatory mediators, such as Interleukin-6 (IL-6), Interleukin-1β (IL-1β), Chemokine ligand 2 (CCL2), Interleukin-8 (IL-8), and Tumor Necrosis Factor alpha (TNF-α) (Fig. 2A), highlighting its strong anti-inflammatory effects in the fibrotic microenvironment. Intracellular ROS levels were quantified to evaluate oxidative stress mitigation, while R2 caused a considerable decrease in ROS accumulation (Fig. 2B and C), Senescence-associated β-galactosidase (SA-β-gal) staining demonstrated a notable increase in senescent HSC-T6 cells after R2 exposure for 24h (Fig. 2D and E). Western blot analysis revealed increased protein expression of senescence regulators p16, p21 and p53(Fig. 2F–I), and qPCR indicated upregulation of components associated with the SASPs (Fig. 2J). The increase in p53 correlated with elevated p21 and p16, confirming activation of the p53-p21 axis as part of the senescence program. The findings collectively identify R2 as a regulator of HSCs senescence, a process associated with fibrotic resolution. Western blotting demonstrated that R2 mediates suppression of the JAK2/STAT3 signaling pathway, as indicated by decreased phosphorylation of JAK and STAT3 (Fig. 2K and L). R2 suppressed the expression of p-mTOR/mTOR and HSP90, while the inhibition of mTOR synergizes with STAT3/HSP90 targeting to suppress fibro-genic pathways (Fig. S5). By disrupting HSP90-STAT3 binding, R2 promotes STAT3 degradation, amplifying the direct inhibition of STAT3 phosphorylation. The dual actions of inhibiting activation (JAK2-STAT3 phosphorylation) and diminishing stability (HSP90 inhibition) elucidate the robust regulation of STAT3 signaling.
Fig. 2.
R2 (20 μM, 24h) attenuates inflammation and oxidative stress and promotes senescence in HSC-T6 cells. A: qPCR analysis of pro-inflammatory cytokines (IL-6, IL-1β, CCL2, IL-8, TNF-α) in R2 (20 μM, 24h)-treated HSC-T6 cells. B–C: Intracellular ROS levels measured by DCFH-DA fluorescence. D–E: SA-β-gal staining of senescent HSC-T6 cells for 24h of R2 treatment. F–I: Western blot analysis of senescence markers p16, p21 and p53. J: qPCR analysis of SASP components (P16, P21, p53, MMP10) in R2 (20 μM, 24h)-treated cells. K–L: Western blot analysis of p-JAK/JAK and p-STAT3/STAT3 phosphorylation. Scale bar = 200 μm, Data are presented as mean ± SD (n = 3 independent cell cultures). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001 vs. normal control (unpaired two-tailed Student's t-test).
3.4. R2 attenuated TAA-induced hepatic fibrosis in zebrafish
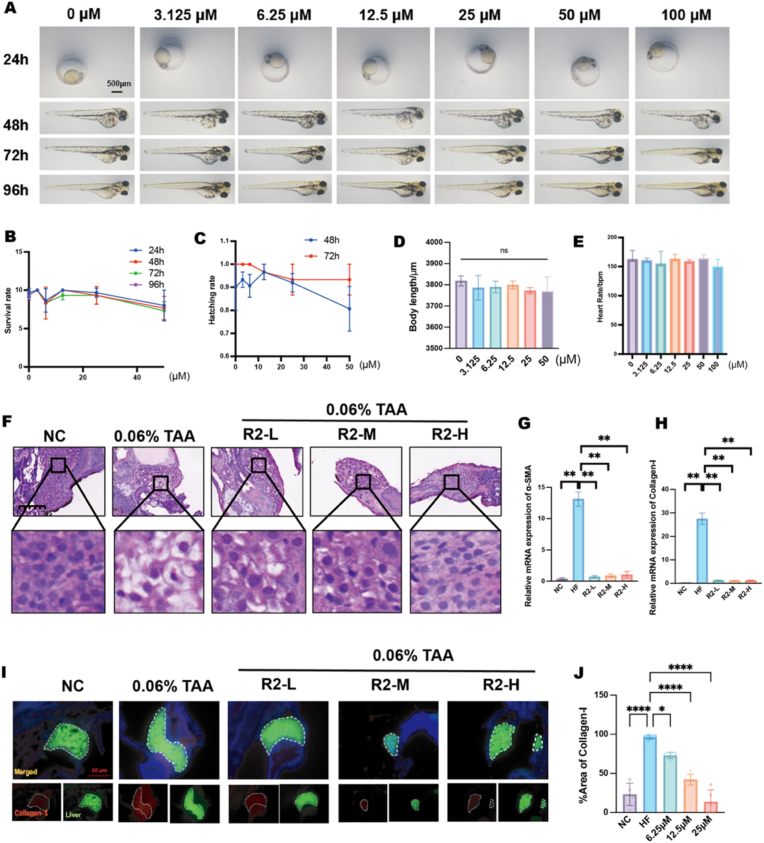
To evaluate the antifibrotic efficacy and safety profile of R2 in vivo, zebrafish were employed as a vertebrate model. No notable morphological abnormalities, developmental delays, or differences in hatching rates were detected in R2-treated larvae (6.25–25 μM) when compared to untreated controls (Fig. 3A–E). H&E staining indicated that R2 significantly mitigated TAA-induced hepatic damage, as shown by decreased intercellular gaps, reduced hepatocellular enlargement, and inhibited vacuolation (Fig. 3F). PCR demonstrated a dose-dependent reduction in fibrosis-associated markers, with mRNA levels of α-SMA and COL-I significantly decreased in R2-treated larvae (Fig. 3G and H). Collagen deposition, indicative of fibrotic progression, was significantly reduced in the 25 μM R2 group relative to TAA-exposed controls (Fig. 3I). The findings collectively demonstrate R2 exhibits substantial antifibrotic activity in vivo, all while preserving developmental integrity.
Fig. 3.
In vivo safety and antifibrotic efficacy of R2 in a zebrafish hepatic fibrosis model. A: Developmental toxicity assessment of R2 (3.125–100 μM) at 24–96 hpf. B–E: Quantitative analysis of (B) survival rate, (C) hatching rate (48–72 hpf), (D) body length, and (E) heart rate in R2-treated larvae. F: H&E staining of zebrafish livers treated with R2 (6.25 12.5, 25 μM). G–H: qPCR analysis of fibrotic markers (G) α-SMA and (H) COL-I mRNA levels in R2 (6.25 12.5, 25 μM)-treated larvae. I–J: Immunofluorescence analysis of collagen-I deposition in larval livers following R2 (6.25 12.5, 25 μM) treatment. Scale bar = 50 μm, Data are presented as mean ± SD, (n = 10 zebrafish per group) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001 vs. normal control (One-way ANOVA with Tukey's post hoc test, or Welch's ANOVA with Games–Howell post hoc test where variance homogeneity was violated).
3.5. R2 decreased oxidative stress and decreased cellular senescence in TAA-induced zebrafish hepatic fibrosis
The ability of R2 was examined to regulate oxidative stress, inflammatory signaling, and senescence in vivo through a zebrafish model. R2 significantly reduced ROS generation compared to the fibrosis-induced control group (Fig. 4A and B). RT-PCR analysis demonstrated a dose-dependent reduction of pro-inflammatory mediators (Fig. 4C), indicating the capacity of R2 to mitigate inflammation-induced HSCs activation. IF staining (Fig. 4D and E) and transcriptional profiling (Fig. 4F and G) validated the suppression of the JAK/STAT3 pathway. IF and RT-PCR analyses revealed a significant upregulation of senescence biomarkers p16 and p21, observed at both protein (Fig. 4H–J) and mRNA levels (Fig. 4K). Furthermore, components of the SASPs such as p16, p21 and p53, exhibited transcriptional elevation, thereby reinforcing the senescence-inducing effects of R2. The findings indicate that R2 inhibites HSCs activation through antioxidative and anti-inflammatory pathways while inducing a senescent state in fibrogenic cells.
Fig. 4.
R2 attenuates oxidative stress and inflammation and induces senescence in zebrafish hepatic stellate cells. A–B: Intracellular ROS levels in hepatic tissues measured by DCFH-DA fluorescence in R2 (6.25 12.5, 25 μM)-treated zebrafish. C: qPCR analysis of pro-inflammatory cytokines in R2 (25 μM)-treated zebrafish. D–E: Immunofluorescence staining of STAT3 in hepatic tissues of R2 (25 μM)-treated zebrafish. F–G: qPCR analysis of JAK/STAT3 pathway target genes in R2 (25 μM)-treated zebrafish. H–J: Immunofluorescence analysis of senescence markers in R2 (25 μM)-treated zebrafish. K: qPCR analysis of SASP components in R2 (25 μM)-treated zebrafish. Scale bar = 50 μm, Data are presented as mean ± SD (n = 10 zebrafish per group), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001 vs. normal control (One-way ANOVA with Tukey's post hoc test, or Welch's ANOVA with Games–Howell post hoc test where variance homogeneity was violated).
3.6. Knock-down of STAT3 abolished Notoginsenoside R2-mediated suppression of fibrosis in zebrafish
To elucidate the molecular reliance of R2 on STAT3 signaling in HF, a STAT3-specific morpholino oligonucleotide (MO-STAT3) was utilized to transcriptionally inhibit STAT3 in zebrafish. qPCR validated significant STAT3 knockdown (Fig. S2A), with 2 μg/L MO-STAT3 chosen for subsequent investigations owing to its superior knockdown efficacy. H&E staining revealed that R2's pharmacological effectiveness in mitigating fibrotic pathology, including hepatic vacuolation and tissue disorganization, depended on intact STAT3 signaling. Notably, the reduction of STAT3 almost completely eliminated the therapeutic efficacy of R2 (Fig. S2B). IF examination indicated that R2 inhibited the activation of HSCs (Fig. S2C and D). Concurrent qPCR tests verified that α-SMA nor COL-I mRNA levels exhibited substantial reduction in STAT3-knockdown groups relative to controls (Fig. S2E and F).
3.7. Knock-down of STAT3 eliminated Notoginsenoside R2-mediated HSCs senescence and inflammatory microenvironment in zebrafish
To clarify the role of STAT3 in the pharmacological activity of R2, zebrafish with STAT3 knockdown were examined for oxidative stress and senescence-related outcomes. In models deficient in STAT3, the antioxidative efficacy of R2 markedly diminished, as evidenced by a reduced suppression of ROS production (Fig. 5A and B). Similarly, the anti-inflammatory effectiveness of R2 was compromised, as demonstrated by a decreased inhibition of the pro-inflammatory cytokines IL-6 and TNF-α (Fig. 5C). Notably, JAK2 protein levels remained unchanged in STAT3-knockdown fibrotic models (Fig. 5D). The initiation of cellular senescence, a hallmark of R2's antifibrotic effect, relied on STAT3 signaling. The ability of R2 to increase the expression of senescence biomarkers p16 and p21 in hepatic tissues was removed by STAT3 knockdown, according to IF analysis (Fig. 5E–G). Additionally, components of the SASPs were significantly diminished in STAT3-deficient zebrafish (Fig. 5H). The findings suggest that STAT3 is central to the multifaceted antifibrotic pharmacology of R2, which orchestrates antioxidant, anti-inflammatory and pro-aging activities to inhibit HSCs-mediated fibrosis.
Fig. 5.
STAT3 is indispensable for the antioxidative, anti-inflammatory, and pro-senescent activities of R2 (25 μM) treatment. A–B: Intracellular ROS levels in STAT3-knockdown zebrafish. C: qPCR analysis of pro-inflammatory cytokines. D: qPCR analysis of JAK mRNA levels in STAT3-knockdown fibrotic models. E–G: Immunofluorescence staining of senescence markers H: qPCR analysis of SASP components in R2 (25 μM)-treated zebrafish. Scale bar = 50 μm, Data are presented as mean ± SD (n = 10 zebrafish per group), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001 vs. normal control (One-way ANOVA with Tukey's post hoc test, or Welch's ANOVA with Games–Howell post hoc test where variance homogeneity was violated).
4. Discussion
This study provides a comprehensive analysis of the therapeutic potential of R2 in HF, demonstrating its ability to attenuate fibrogenesis through STAT3-dependent modulation of HSCs activation, oxidative stress, inflammation, and senescence. R2 is a novel multi-target medication that promotes cellular senescence and inhibits the deposition of HSCs-ECM deposition.
Recent investigations have increasingly focused on R2 due to its potential bioactivities [9]. In this study, network pharmacology indicaties the potential dual therapeutic of R2 involvement in both the prevention and treatment of HF by modulating shared signaling pathways. Further in vitro and in vivo validation experiments explored R2 as a potent inhibitor of HSCs activation and extracellular matrix deposition. This study addresses a critical gap by providing the first comprehensive evaluation of antifibrotic properties of R2.
The activation of HSCs represents a pivotal event in the pathogenesis of HF, with the inflammatory microenvironment serving as a critical driver of their sustained activation[[40], [41], [42]]. By engaging these pathways, the inflammatory milieu not only promotes the transition of HSCs to a profibrogenic phenotype but also facilitates ECM deposition, thereby establishing itself as a central therapeutic target in HF [43]. Given the pivotal role of the inflammatory microenvironment in sustaining HSCs activation, we assessed the therapeutic efficacy of R2 in regulating oxidative stress and inflammatory signaling through in vitro and in vivo methodologies. Our findings indicate that R2 attenuates fibrotic development by interrupting the feedforward loop among oxidative stress, inflammation, and HSCs activation.
The activation and senescence of HSCs maintain a dynamic equilibrium throughout the evolution of HF, with the deliberate stimulation of HSCs senescence becoming a pivotal approach for antifibrotic treatment [44]. Nonetheless, a significant shortcoming of these methodologies is their neglect of the SASPs, in which senescent HSCs release pro-inflammatory and profibrotic factors that intensify local inflammation and fibrosis via paracrine signaling. To address this dual challenge, R2 has emerged as a promising bifunctional therapeutic agent capable of simultaneously inducing HSCs senescence and SASPs-mediated inflammatory cascades. Notably, R2 significantly modified the SASPs profile by selectively attenuating the secretion of pro-inflammatory cytokines while retaining its capacity to promote senescence. This dual mechanism conferred robust antioxidant and anti-inflammatory properties, synergistically amplifying its antifibrotic efficacy in mitigating HF.
STAT3, a crucial modulator of cytokine signaling, exhibits context-dependent functions in the advancement and resolution of HF [32]. Temporary suppression of STAT3 in activated HSCs induces cellular senescence through the overexpression of p53/p21, hence arresting proliferation and facilitating extracellular matrix remodeling [34,36,45]. Similarly, the explorations in this study revealed that STAT3 plays a key role in fiber formation, orchestrating inflammatory cascades and cellular senescence pathway. The findings demonstrated that R2 exerts anti-inflammatory effects and induces HSCs senescence by decreasing STAT3, hence preventing the progression of HF. The STAT3-dependent mechanism underlying the therapeutic efficacy of R2 was conclusively established through in vivo loss-of-function experiments. Genetic inhibition of STAT3 abolished the ability of R2 to mitigate HSCs activation markers, alleviate oxidative stress, and modulate pro-inflammatory cytokine production. Notably, STAT3 suppression by R2 activated the p53-p21 axis, triggering HSCs senescence. This aligns with evidence that STAT3 represses p53 transcriptionally, suggesting crosstalk between these pathways in fibrosis resolution. Collectively, these results definitively characterize R2 as a novel STAT3-targeting therapeutic agent that simultaneously suppresses HSCs activity and reprograms HSCs into a senescent state.
This study identifies STAT3 as a unique mediator of the antifibrotic actions of R2, establishing a link between the resolution of inflammation and the induction of senescence. Our findings advance the mechanistic comprehension of STAT3 in liver pathology and underscore the therapeutic potential of targeting senescence pathways in fibrotic disorders. Nonetheless, despite the similarities in fibrotic pathways between zebrafish and mammals [46,47], it is essential to conduct translational validation in rat models and human-derived HSCs to establish clinical significance. Furthermore, the interaction between STAT3 and other regulators of senescence remains to be explored, and proteomics or single-cell RNA sequencing may elucidate the underlying mechanisms. Future research should prioritize translational studies to assess the efficacy of R2 in preclinical mammalian models and clarify its specific molecular interactions with STAT3.
Authorship statement
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the Journal of Ginseng Research.
Authorship contributions
Please indicate the specific contributions made by each author (list the authors’ initials followed by their surnames, e.g., Y.L. Cheung). The name of each author must appear at least once in each of the three categories below.
Category 1
Conception and design of study: Kaili Deng, Min Li; Yuanyuan Li
acquisition of data: Kaili Deng, Min Li; Yuanyuan Li; Yuhua Wang; Hechen Shi; Jiayi Cheng
analysis and/or interpretation of data: Kaili Deng, Min Li; Yuanyuan Li; Liangliang Xiang.
Category 2
Drafting the manuscript: Kaili Deng, Min Li; Yuanyuan Li; Liangliang Xiang
revising the manuscript critically for important intellectual content: Liangliang Xiang; Sha Huang; Zhiping Lv.
Category 3
Approval of the version of the manuscript to be published (the names of all authors must be listed).
Financial support statement
The project was financially supported by the National Natural Science Foundation of China (82374339, 82405073 and 82174168), the Natural Science Foundation of Guangdong Province (2025A1515012710 and 2023A1515110616), Science and Technology Program of Guangzhou (2024A04J4789), Guangdong Province Traditional Chinese Medicine Research Project (20251254 and 20223008),and Lv Zhiping-National Famous Traditional Chinese Medicine Inheritance Studio.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2025.05.007.
Contributor Information
Sha Huang, Email: hstcm2018@163.com.
Zhiping Lv, Email: lzp48241@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Sepanlou S.G., Safiri S., Bisignano C., Ikuta K.S., Merat S., Saberifiroozi M., et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya C., Bajaj J.S. Chronic liver diseases and the microbiome : translating our. Gastroenterology (New York, N Y, 1943) 2020:1–17. doi: 10.1053/j.gastro.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginès P., Castera L., Lammert F., Graupera I., Serra-Burriel M., Allen A.M., et al. Population screening for liver fibrosis: toward early diagnosis and intervention for chronic liver diseases. Hepatology (Baltim, Md) 2022;75:219–228. doi: 10.1002/hep.32163. [DOI] [PubMed] [Google Scholar]
- 4.Roehlen N., Crouchet E., Baumert T.F. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9 doi: 10.3390/cells9040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammerich L., Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633–646. doi: 10.1038/s41575-023-00807-x. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T., Friedman S.L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisseleva T., Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 8.Hu M., Wang Y., Liu Z., Yu Z., Guan K., Liu M., et al. Hepatic macrophages act as a central hub for relaxin-mediated alleviation of liver fibrosis. Nat Nanotechnol. 2021;16:466–477. doi: 10.1038/s41565-020-00836-6. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y., Wang C. Herb–drug interactions between Panax notoginseng or its biologically active compounds and therapeutic drugs: a comprehensive pharmacodynamic and pharmacokinetic review. J Ethnopharmacol. 2023;307 doi: 10.1016/j.jep.2023.116156. [DOI] [PubMed] [Google Scholar]
- 10.Liu H., Lu X., Hu Y., Fan X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol Res. 2020;161 doi: 10.1016/j.phrs.2020.105263. [DOI] [PubMed] [Google Scholar]
- 11.Guo C., Lai L., Ma B., Huang Q., Wang Z. Notoginsenoside R1 targets PPAR-γ to inhibit hepatic stellate cell activation and ameliorates liver fibrosis. Exp Cell Res. 2024;437 doi: 10.1016/j.yexcr.2024.113992. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y., Lang Z., Li X., Lin L., Li Y., Zhang R., et al. Ginsenoside Rg3 promotes hepatic stellate cell ferroptosis by epigenetically regulating ACSL4 to suppress liver fibrosis progression. Phytomedicine (Stuttg) 2024;124 doi: 10.1016/j.phymed.2023.155289. [DOI] [PubMed] [Google Scholar]
- 13.Guo X., Yang L., An X., Hu M., Shen Y., Wang N., et al. Protective effects of Notoginsenoside R2 on reducing lipid accumulation and mitochondrial dysfunction in diabetic nephropathy through regulation of c-Src. Chinese Medicine (United Kingdom) 2025;20 doi: 10.1186/s13020-024-01057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai X., Liu Y., Liu T., Zhang Y., Wang S., Xu T., et al. SiJunZi decoction ameliorates bone quality and redox homeostasis and regulates advanced glycation end products/receptor for advanced glycation end products and WNT/β-catenin signaling pathways in diabetic mice. J Ethnopharmacol. 2024;319 doi: 10.1016/j.jep.2023.117167. [DOI] [PubMed] [Google Scholar]
- 15.Wang P., Gao Y., Yang G., Zhao Y., Zhao Z., Gao G., et al. Enhancing the inhibition of cell proliferation and induction of apoptosis in H22 hepatoma cells through biotransformation of notoginsenoside R1 by Lactiplantibacillus plantarum S165 into 20(S/R)-notoginsenoside R2. RSC Adv. 2023;13:29773–29783. doi: 10.1039/d3ra06029b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González-Burgos E., Fernandez-Moriano C., Gómez-Serranillos M.P. Potential neuroprotective activity of ginseng in Parkinson's disease: a review. J Neuroimmune Pharmacol. 2015;10:14–29. doi: 10.1007/s11481-014-9569-6. [DOI] [PubMed] [Google Scholar]
- 17.Deng K., Li M., Xiang L., Wang Y., Li Y., Wen J., et al. Integrated UHPLC-Q-exactive orbitrap HRMS and serum pharmacochemistry for the investigation of anti-hepatic fibrosis effect of Baoganning Decoction. Phytomedicine (Stuttg) 2025;137 doi: 10.1016/j.phymed.2025.156363. [DOI] [PubMed] [Google Scholar]
- 18.Puche J.E., Saiman Y., Friedman S.L. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 19.Huang S., Wang Y., Xie S., Lai Y., Mo C., Zeng T., et al. Isoliquiritigenin alleviates liver fibrosis through caveolin-1-mediated hepatic stellate cells ferroptosis in zebrafish and mice. Phytomedicine (Stuttg) 2022;101 doi: 10.1016/j.phymed.2022.154117. [DOI] [PubMed] [Google Scholar]
- 20.Yang L., Han B., Zhang M., Wang Y.H., Tao K., Zhu M.X., et al. Activation of BK channels prevents hepatic stellate cell activation and liver fibrosis through the suppression of TGFβ1/SMAD3 and JAK/STAT3 profibrotic signaling pathways. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M., Serna-Salas S., Damba T., Borghesan M., Demaria M., Moshage H. Hepatic stellate cell senescence in liver fibrosis: characteristics, mechanisms and perspectives. Mech Ageing Dev. 2021;199 doi: 10.1016/j.mad.2021.111572. [DOI] [PubMed] [Google Scholar]
- 22.Zhao D., Gao Y., Su Y., Zhou Y., Yang T., Li Y., et al. Oroxylin A regulates cGAS DNA hypermethylation induced by methionine metabolism to promote HSC senescence. Pharmacol Res. 2023;187 doi: 10.1016/j.phrs.2022.106590. [DOI] [PubMed] [Google Scholar]
- 23.Li F., Huangyang P., Burrows M., Guo K., Riscal R., Godfrey J., et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat Cell Biol. 2020;22:728–739. doi: 10.1038/s41556-020-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi X., Song A., Ma M., Wang P., Zhang X., Lu C., et al. Curcumol inhibits ferritinophagy to restrain hepatocyte senescence through YAP/NCOA4 in non-alcoholic fatty liver disease. Cell Prolif. 2021;54 doi: 10.1111/cpr.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H. Advances in anti hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula. J Ethnopharmacol. 2020;251 doi: 10.1016/j.jep.2019.112442. [DOI] [PubMed] [Google Scholar]
- 26.Xing Y., Zhong W., Peng D., Han Z., Zeng H., Wang Y., et al. Chinese herbal formula ruangan granule enhances the efficacy of entecavir to reverse advanced liver fibrosis/early cirrhosis in patients with chronic HBV infection: a multicenter, randomized clinical trial. Pharmacol Res. 2023;190 doi: 10.1016/j.phrs.2023.106737. [DOI] [PubMed] [Google Scholar]
- 27.Tao A., Zhang Y., Gan Z., Yin C., Tian Y., Zhang L., et al. Isolation, structural features, and bioactivities of polysaccharides from Panax notoginseng: a review. Int J Biol Macromol. 2024;280 doi: 10.1016/j.ijbiomac.2024.135765. [DOI] [PubMed] [Google Scholar]
- 28.Feng S., Cheng H., Xu Z., Shen S., Yuan M., Liu J., et al. Thermal stress resistance and aging effects of Panax notoginseng polysaccharides on Caenorhabditis elegans. Int J Biol Macromol. 2015;81:188–194. doi: 10.1016/j.ijbiomac.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 29.Xiang D.M., Sun W., Ning B.F., Zhou T.F., Li X.F., Zhong W., et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2018;67:1704–1715. doi: 10.1136/gutjnl-2016-313392. [DOI] [PubMed] [Google Scholar]
- 30.Deng Y.R., Ma H Di, Tsuneyama K., Yang W., Wang Y.H., Lu F.T., et al. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis bythe protein kinase inhibitor sorafenib. J Autoimmun. 2013;46:25–34. doi: 10.1016/j.jaut.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Cao S., Zhu C., Feng J., Zhu L., Yin J., Xu Y., et al. Helicobacter hepaticus infection induces chronic hepatitis and fibrosis in male BALB/c mice via the activation of NF-κB, Stat3, and MAPK signaling pathways. Helicobacter (Camb, Mass) 2020;25 doi: 10.1111/hel.12677. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z., Li J., Xiao W., Long J., Zhang H. The STAT3 inhibitor S3I-201 suppresses fibrogenesis and angiogenesis in liver fibrosis. Lab Invest. 2018;98:1600–1613. doi: 10.1038/s41374-018-0127-3. [DOI] [PubMed] [Google Scholar]
- 33.Cui Y., Wang Q., Zhang X., Yang X., Shi Y., Li Y., et al. Curcumin alleviates Aflatoxin B1-induced liver pyroptosis and fibrosis by regulating the JAK2/NLRP3 signaling pathway in ducks. Foods. 2023;12 doi: 10.3390/foods12051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z., Zheng Y., Zhang L., Xu E. Cryptotanshinone alleviates liver fibrosis via inhibiting STAT3/CPT1A-dependent fatty acid oxidation in hepatic stellate cells. Chem Biol Interact. 2024;399 doi: 10.1016/j.cbi.2024.111119. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X ling, yan Zhang X., qun Ge X., xuan Liu M. Mangiferin prevents hepatocyte epithelial-mesenchymal transition in liver fibrosis via targeting HSP27-mediated JAK2/STAT3 and TGF-β1/Smad pathway. Phytother Res. 2022;36:4167–4182. doi: 10.1002/ptr.7549. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda T., Koiwa M., Yonemura A., Miyake K., Kariya R., Kubota S., et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108779. [DOI] [PubMed] [Google Scholar]
- 37.Vogel S., Piantedosi R., Frank J., Lalazar A., Rockey D.C., Friedman S.L., et al. vol. 41. 2000. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro Supplementary key words hepatic cells • Ito cells • fat-storing cells • vitamin A • oleic acid • cellular retinol-binding protein, type I (CRBP-I) • retinol-binding protein. (RBP) • transthyretin (TTR) • Hepa-1 hepatocytes • retinoid nuclear receptor). [PubMed] [Google Scholar]
- 38.Bruck R., Genina O., Aeed H., Alexiev R., Nagler A., Avni Y., et al. Halofuginone to prevent and treat thioacetamide-induced liver fibrosis in rats. Hepatology (Baltim, Md) 2001;33:379–386. doi: 10.1053/jhep.2001.21408. [DOI] [PubMed] [Google Scholar]
- 39.Herrmann J., Gressner A.M., Weiskirchen R. Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function? J Cell Mol Med. 2007;11:704–722. doi: 10.1111/j.1582-4934.2007.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dou J.Y., Zhou M.J., Xuan M.Y., Guo J., Liu S.H., Lian L.H., et al. Astilbin alleviates hepatic fibrosis through PXR-PINK1/Parkin pathway: a new strategy by regulating hepatic stellate cells-macrophage crosstalk. Phytomedicine (Stuttg) 2024;135 doi: 10.1016/j.phymed.2024.156144. [DOI] [PubMed] [Google Scholar]
- 41.Wan L., Xia T., Du Y., Liu J., Xie Y., Zhang Y., et al. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: a role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J. 2019;33:8530–8542. doi: 10.1096/fj.201802675R. [DOI] [PubMed] [Google Scholar]
- 42.YeQiannan PingJian X. Advances in the regulation of functions of hepatic stellate cells by exosomes. Chin J Hepatol. 2020;28:981–984. doi: 10.3760/cma.j.cn501113-20190412-00123. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y., Zhang L., Ma Y., Xie L., Yang Y.Y., Jin C., et al. Secretome of senescent hepatic stellate cells favors malignant transformation from nonalcoholic steatohepatitis-fibrotic progression to hepatocellular carcinoma. Theranostics. 2023;13:4430–4448. doi: 10.7150/thno.85369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wijayasiri P., Astbury S., Kaye P., Oakley F., Alexander G.J., Kendall T.J., et al. Role of hepatocyte senescence in the activation of hepatic stellate cells and liver fibrosis progression. Cells. 2022;11 doi: 10.3390/cells11142221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madaro L., Passafaro M., Sala D., Etxaniz U., Lugarini F., Proietti D., et al. Denervation-activated STAT3–IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol. 2018;20:917–927. doi: 10.1038/s41556-018-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X., Copmans D., De Witte P.A.M. Molecular sciences using zebrafish as a disease model to study fibrotic disease. 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.