Abstract
Background
Aging is a complex and inevitable biological process that involves the decline of function in multiple systems and organs, and it is possible to delay aging process and improve health conditions through diet. Ginsenosides, the major active compounds in Panax ginseng Meyer, exhibit anti-oxidant, anti-cancer, and anti-aging properties. However, the relationship between bioactivities and structures of ginsenoside derivatives with same molecular formula remain unclear.
Methods
Using Caenorhabditis elegans (C. elegans) model, we evaluated the anti-aging activities of 4 ginsenoside derivatives (Rg5, Rg6, Rk1, and F4), which differ in glycoside composition and double bond position. Their effects on lifespan, physiological functions, locomotion ability, lipofuscin accumulation, stress resistance, and acetylcholinesterase (AChE) activity were assessed.
Results
Four ginsenoside derivatives showed different activities of delaying aging by improving muscle function, enhancing anti-oxidant stress, and reducing AChE activity in C. elegans. Particularly, Rg5 and Rk1, which contain two glucose residues, demonstrated superior activity compared to Rg6 and F4, which possess glucose-(2-1)-rhamnose residues. Meanwhile, Rg5 and F4, with a double bond at Δ20(22) had better effects than Rk1 and Rg6 with a double bond at Δ20(21). Molecular docking analysis showed that Rg5 and Rk1 formed more hydrogen bonds and hydrophobic interactions with amino acid residues at the AChE active site compared to Rg6 and F4, Rg5 exhibited the most favorable binding energy, while Rg6 formed only a hydrogen bond and F4 showed no hydrogen bonding; both had the same binding energy.
Conclusion
These findings suggest that glycoside types and double bond position are key structural determinants of the anti-aging activities of ginsenoside derivatives. This provides a theoretical foundation for the development of ginsenoside-based therapeutics for aging and aging-related chronic diseases.
Keywords: Ginsenosides, Structure-activity relationship, Anti-oxidant, Acetylcholinesterase, Molecular docking
Graphical abstract
Highlights
-
•
Four derivatives of ginsenoside (Rg5, Rg6, Rk1, and F4) extend the lifespan of C. elegans.
-
•
Ginsenosides delay aging by improving muscle function and enhancing anti-oxidant stress.
-
•
Ginsenosides with two glucose residues have better anti-aging bioactivities than one glucose residue.
-
•
Ginsenosides with double bond at Δ20(22) have better anti-aging bioactivities than those at Δ20(21).
1. Introduction
According to world population data released by the United Nations, the elderly population (aged 65 and above) is projected to exceed 20 % of the total population by 2050 [1], a trend that underscores the accelerating demographic aging worldwide. Aging is a complex, time-dependent biological process [2], characterized by a progressive decline in the functionality of cells, tissues, and organs [3], the incidence and even mortality of multiple diseases, including cardiovascular diseases, diabetes, neurodegenerative diseases, and cancer, are positively correlated with age [4]. Chronic exposure to adverse conditions that trigger biological stress responses has been linked to aging [5]. These findings have led to the hypothesis that the acceleration of biological aging processes drives phenomena such as decreased muscle function, lipofuscin accumulation, and increased oxidative stress [6].
Acetylcholinesterase (AChE) is a key enzyme in the cholinergic system, with increasing age, there is a gradual decline in cholinergic function, resulting in decreased acetylcholine level as well as increased AChE level [7]. Several studies have shown that inhibiting AChE activity alleviate the cognitive impairments in D-galactose-induced aging mice [8,9]. Moreover, AChE is implicated in locomotion ability and stress responses [10,11], playing a crucial role in spontaneous movement activities [12]. Efforts to delay aging have garnered widespread attention, and in addition to genetics, such as diets, physical activity, environmental factors, and lifestyle having a more significant impact on the aging process [13]. Many studies have focused on the anti-aging effects of specific diets, especially those supplemented with active natural plant compounds [14,15].
Panax ginseng Meyer (P. ginseng) is an herbaceous plant, celebrated globally for its dual role as both food and medicinal herb [16]. Ginsenosides, the primary active compounds of P. ginseng, include over 150 identified types [17,18], which can be divided based on their glycosyl chemical structure: 20(S)-protopanaxadiol (PPD)-type (such as Rb1, Rh2, Rg5, and Rk1), the glycosidic bond position attached to C-3 and C-20; and 20(S)-protopanaxatriol (PPT)-type (such as Re, Rg6, Rh1, and F4), the glycosidic bond position attached to C-6 and C-20 [[19], [20], [21]]. It have been found that 0.2 mg/mL total ginsenosides significantly extended the lifespan of C. elegans by 14.02 % without affecting their growth or development [22]. Ginsenoside Re increased the residence time of target quadrant and the number of crossing platforms of water maze, enhanced the autonomous alternating ability and the number of entering new arms of Y maze, and improved the cognitive decline by regulating oxidative stress in mice [23].
Moreover, structural variations in ginsenosides, including the type, number, and position of sugar moieties attached to glycosidic bond, as well as the position of double bond, can significantly affect their bioactivities [24,25]. Rare ginsenosides with a double bond exhibited stronger anti-inflammatory activity than those without a double bond, and 20(S)-ginsenosides had better anti-inflammatory activity than 20(R)-ginsenosides [26]. Regarding the ginsenoside isomers Rg6 and F4, with different C-20 double bond position, this distinction results in a faster metabolic rate of F4 (Δ20(22)) than Rg6 (Δ20(21)) in zebrafish [20]. Nevertheless, the structure-activity relationship of ginsenoside derivatives with same molecular formula, especially the contribution of glycosidic bond and/or double bond position on anti-aging bioactivities, remain unclear.
C. elegans is a widely used model organism in aging studies because of short and easily monitored lifespan [27,28] and absence of ethical issues [29]. As shown in Fig. 1, ginsenoside derivatives Rg5, Rg6, Rk1, and F4 have the molecular formula C42H70O12, Rg5 and Rk1 are PPD-type, two glucose residues attached at the C-3 position; Rg6 and F4 are PPT-type, with glycosides of glucose-(2-1)-rhamnose linked to the C-6 position; the C-20 double bond position of Rg5 and F4 are at Δ20(22), and Rg6 and Rk1 are at Δ20(21) [20,21]. Here, we investigated the effects of the ginsenoside derivatives (Rg5, Rg6, Rk1, and F4) on the lifespan, physiological functions, locomotion ability, lipofuscin accumulation, stress resistance, and AChE activity of C. elegans. Additionally, the interactions between ginsenoside derivatives and human AChE were simulated using molecular docking analysis, and the binding energies of these compounds for AChE were evaluated. Our work will provide new insights into the glycoside types and double bond position contributions of ginsenoside derivatives to structure-activity relationship and their applications in improving muscle function, enhancing anti-oxidant stress, and reducing AChE activity in food and medicine.
Fig. 1.
The chemical structural formulas of ginsenoside derivatives, including Rg5 (A), Rg6 (B), Rk1 (C), and F4 (D).
2. Materials and methods
2.1. Reagents
Ginsenoside derivatives (Rg5, Rg6, Rk1, and F4; purity ≥ 95 %) were gifted from the Biomedicine Institute of Northwest University (Shaanxi, China). Dimethyl sulfoxide (DMSO, Solarbio, Beijing, China), hydrogen peroxide (Sinopharm, Shanghai, China), levamisole (Sigma-Aldrich, USA) and TRIzol (Vazyme, Beijing, China) were obtained from commercial suppliers.
2.2. C. elegans strains and culture conditions
The wild-type C. elegans strain Bristol N2 and standard feed Escherichia coli OP50 (E. coli OP50) were obtained from the Caenorhabditis Genetics Center (https://cgc.umn.edu/). The CL2166 (dvIs19 [(pAF15) gst-4p::GFP::NLS] III) and CF1553 (muIs84 [(pAD76) sod-3::GFP + rol-6 (su1006)]) mutant strains were gifted from Huazhong Agricultural University (Wuhan, China).
E. coli OP50 was incubated in Luria-Bertani (LB) broth at 37 °C for 12 h with shaking at 225 rpm. Four ginsenoside derivatives (Rg5, Rg6, Rk1, and F4) were dissolved in DMSO at 500 mM. To prepare bacterial plates for worms feeding, 500 mM ginsenosides were gradiently diluted with E. coli OP50 to a final concentration of 0.05 mM, and 40 μL bacterial solution was inoculated on 60 mm nematode growth medium (NGM) plate.
Worms were maintained at 20 °C on NGM agar [30] plates inoculated with pre-prepared E. coli OP50 according to standard method [31]. Hermaphrodites were synchronized by isolating eggs and transferred to NGM plates seeded with E. coli OP50 containing DMSO (0.2 %, control) or ginsenoside derivatives (Rg5, Rg6, Rk1, and F4) [32].
2.3. Lifespan assay
Lifespan analyses were performed at 20 °C as previously described [30]. Late L4 stage worms were measured at t = 0 for the lifespan assay and transferred to fresh plates every other day until all worms died. For each group, 90 worms were tested on 3 plates (30 per plate). Survival of the worms was monitored and recorded daily. Worms were considered death if they ceased pharyngeal pumping and not respond to gentle mechanical stimulation using a platinum wire picker [33,34]. Worms that extruded organs, matricidal hatching or accidently lost were eliminated from lifespan analysis [35].
2.4. Life cycle, brood size, and body size assays
The assays were performed according to previous method [36]. To assess the life cycle of the worms, 20 eggs were placed on 20 plates, with the life cycle analysis initiated at t = 0. The completion of the life cycle was marked by the time when the worm laid its first egg. To determine the brood size, 20 synchronized hermaphrodites (one per plate) at the late L4 stage were transferred to fresh plates daily until reproduction ceased. The total number of offspring produced by each worm was tallied. To measure the body size of the worms, 20 worms were selected from Days 2–6, placed on 2 % agarose pads, anesthetized with 10 mM levamisole, photographed with stereomicroscope (Olympus CX23), and analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). The body size was quantified by calculating the projected area of the worms.
2.5. Preference choice assays
The preference of the worms was determined according to previous methods [35,37]. Worms were grown on E. coli OP50 to late L4 stage and washed 3 times with M9 buffer (5 g NaCl, 3 g KH2PO4, 6 g Na2HPO4, 1 mL 1 M MgSO4). For the first experiment, 200 worms were placed in the center of plate equidistant from the control, Rg5, Rg6, Rk1, or F4. For the second experiment, E. coli OP50 or control, E. coli OP50 or Rg5, E. coli OP50 or Rg6, E. coli OP50 or Rk1, and E. coli OP50 or F4 were seeded on the edges of each end of plate, and 100 worms were placed equidistant from both ends in the center of the plate. The worms were permitted to move freely for 3 h, after which the number of worms that migrated to each lawn was counted.
2.6. Aging-related biomarkers assays
The pharyngeal pumping rate of worms was measured according to previous method [33]. Days 8–16 worms were selected, and the number of pharyngeal constrictions in the terminal bulb of pharynx was counted in 20 s using stereomicroscope (Motic SMZ-168).
The motility of worms was examined as previous method [38]. Worms from Days 8–16 were selected, and classified according to differences in movement activity. Worms exhibiting spontaneous and/or rhythmical sinusoidal movement were categorized as class “A” (normal locomotion); worms with irregular and/or uncoordinated movement were classified as class “B” (uncoordinated/sluggish); worms moved merely their head and/or tail in response to gentle touch with a platinum wire picker were assigned to class “C” (cannot move body); and dead worms were categorized as class “D”. At least 90 worms were evaluated in each group.
Autofluorescence of intestinal lipofuscin was detected according to previous method [39]. Day 15 worms were washed 3 times with M9 buffer, placed on 2 % agarose pads, and anesthetized with 10 mM levamisole. Images of lipofuscin autofluorescence were captured using laser confocal scanning microscopy (Olympus FV1200) with blue excitation light at 405 nm. The accumulation of lipofuscin was assessed by quantifying the fluorescence intensity using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.7. Stress resistance assays
Stress assays were performed according to previous method [34]. For thermotolerance assessment, Day 10 worms were subjected to 34 °C for 12, 18, and 24 h, and their survival was documented. To evaluate anti-oxidant stress capacity, Day 5 worms were transferred to NGM plates supplemented with 10 mM H2O2 and incubated at 20 °C. Survival was monitored at various time points, as detailed in the “Lifespan assay”. On Day 10, gst-4p::GFP and sod-3::GFP worms were placed on 2 % agarose pads and anesthetized with 10 mM levamisole. Fluorescence intensity images were captured using laser confocal scanning microscope (Olympus FV1200) with green excitation light at 473 nm. GFP fluorescence intensity was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.8. RNA isolation and real-time quantitative PCR (RT-qPCR) analyses
Total RNA was isolated using TRIzol, 500 Day 10 worms were washed 3 times with M9 buffer, 1 mL of TRIzol was added, and the worms were homogenized by repeated freezing and thawing using liquid nitrogen. RNA was isolated according to the manufacturer's instructions (Vazyme, Beijing, China). RNA quantity was detected using NanoDrop One spectrophotometer (Thermo Fisher Scientific, USA), and RNA integrity was assessed by agarose gel electrophoresis. For gene expression analysis, complementary DNA (cDNA) was synthesized using reverse transcriptase kit according to the manufacturer's instructions (TIANGEN, Beijing, China). Gene expression levels were detected by RT-qPCR using SYBR Green SuperReal PreMix Plus (TIANGEN, Beijing, China) in QuantStudio™ 1 RT-qPCR instrument (Thermo Fisher Scientific, USA). The relative expression levels of mRNA were calculated using the 2−ΔΔCt method and normalized to that of act-1 as an internal control [40]. The sequences of primers used for RT-qPCR are shown in Table S1.
2.9. AChE assays
The AChE activity of C. elegans was determined using AChE assay kit. Briefly, 500 Day 10 worms were washed 3 times with M9 buffer, and the worms were placed into extracting solution and ground with a glass grinder on ice. The worm lysates were centrifuged at 8000 × g for 10 min at 4 °C, and the supernatant was taken for determination of AChE activity according to the manufacturer's instructions (Solarbio, Beijing, China).
2.10. Molecular docking analysis
Structures of the ginsenosides were created using ChemDraw software. The human AChE proteins, crystal structure of recombinant human acetylcholinesterase in complex with (−)-galantamine (PDB: 4EY6) and donepezil (PDB: 4EY7) were obtained from Protein Data Bank (https://www.rcsb.org/) [41], and molecular docking analysis was performed using AutoDock Vina software (version 1.5.6) [42,43]. The binding sites of the co-crystalized ligands were selected for docking simulations, and their coordinates were determined using AutoDock Tools software, and the hydrogen bonds and charges of the protein were calculated. The top-scoring binding sites with RMSD values less than 2 Å were selected and visualized using PyMOL software [44].
2.11. Statistical analysis
Statistical analyses were conducted using GraphPad Prism software version 10.0 (La Jolla, California, USA). Data are presented as means ± standard error of the mean (SEM). Lifespan data were performed using Log-rank (Mantel-Cox) test. For other quantitative comparisons, two-group comparisons were analyzed using Student's t-test, while comparisons involving three or more groups were assessed by one-way ANOVA followed by Tukey's post hoc test. In all cases, p < 0.05 was considered significant.
3. Results
3.1. Ginsenoside derivatives extend the healthspan of C. elegans
To investigate the lifespan-extending effects of ginsenosides on C. elegans, late L4 stage worms were seeded with E. coli OP50 containing either DMSO (control) or 4 ginsenoside derivatives (Rg5, Rg6, Rk1, and F4; 0.05 mM) with same molecular formula, different glycosides and double bond position, and survival was monitored daily until all worms had died. Compared with the control, feeding with ginsenosides Rg5, Rg6, Rk1, or F4 significantly extended the lifespan of worms by 11.39 %, 13.86 %, 15.90 %, or 13.32 %, respectively. However, there was no significant difference in the effect of extending the lifespan of worms among those 4 ginsenosides, indicating that they have comparable effects on extending the lifespan of nematodes (Fig. 2A, Table S2). To eliminate the possibility that ginsenosides might affect the growth of E. coli OP50, we generated growth curves of E. coli OP50 in LB liquid medium supplemented with 0.05 mM of each ginsenoside derivative. We found that 4 ginsenoside derivatives had no effect on the proliferation or inhibition of E. coli OP50 (Fig. 2B).
Fig. 2.
Ginsenoside derivativesextend the healthspan of C. elegans. (A). Ginsenosidederivatives (Rg5, Rg6, Rk1, and F4, 0.05 mM) dissolved inE. coliOP50 solution significantly extended the lifespan of wild-type N2 worms (p< 0.01, Log rank test). Lifespan was tested in at least 3 biologically independent experiments, and 90 worms were tested in each group, the results from a single representative experiment are shown.(B).The growth curve ofE. coliOP50 withginsenosidederivativesin vitroculture experiment, demonstrated that there was no significant difference inE. coliOP50 orE. coliOP50 containingginsenosidederivatives at OD600(N = 3 biological experiments,p> 0.05).
It is believed that delaying growth and development may extend the lifespan of C. elegans [37,45]. Upon investigating the effects of 4 ginsenoside derivatives on the growth and development of C. elegans, we found that these derivatives did not influence the nematodes to reach reproductive age, reproductive cycle or body size (Fig. S1A–D). To exclude the possibility of worm preferences, we conducted choice assays to determine whether the worms exhibited avoidance behavior. Worm eggs were cultured to the late L4 stage on E. coli OP50, and then transferred to plates containing control, Rg5, Rg6, Rk1, or F4 (Fig. S2A) for 3 h, the results showed that the number of worms on each lawn was similar (Fig. S2B). Similarly, there was no significant difference in the number of worms on each lawn after 3 h of feeding by a binary selection analysis (Fig. S2C and D). Taken together, those data suggest that 4 ginsenoside derivatives can delay the aging process and extend the lifespan and healthspan of C. elegans.
3.2. Comparison of improvement effects of ginsenoside derivatives on aging-related biomarkers in C. elegans
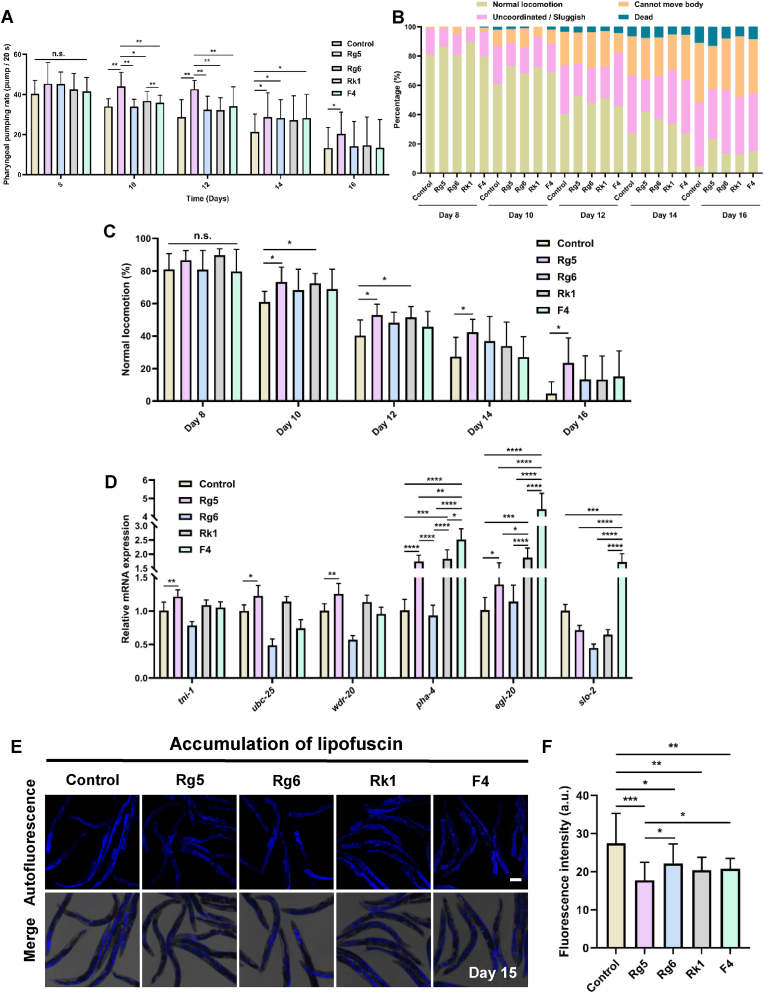
In C. elegans, not only lifespan but also muscle function deterioration and lipofuscin accumulation are important age-related phenotypes that progressively worsen with age [46]. We next measured related biomarkers, including pharyngeal pumping rate, locomotion ability and lipofuscin accumulation. With increasing age, the pharyngeal pumping rate decreased progressively in all treatment groups, but the rate was slower with the treatment of 4 ginsenoside derivatives than that of the control on Days 10–16 of adulthood (Fig. 3A). Notably, Rg5 significantly delayed the decline in the pharyngeal pumping rate on Days 10–16 of adulthood, showing a more pronounced effect than the other 3 ginsenoside derivatives, particularly on Days 10 and 12. Additionally, Rg6 and F4 exhibited a significant difference in the pharyngeal pumping rate on Day 14 compared to the control. However, Rk1 feeding increased the pharyngeal pumping rate but not significantly (Fig. 3A). In parallel with the observed decline in pharyngeal pumping rate, the locomotion ability of worms fed with the 4 ginsenoside derivatives also exhibited a gradual decrease during Days 10–16 of adulthood (Fig. 3B and C). Qualification of normal locomotion ability showed that among the ginsenoside treatments, ginsenoside derivatives Rg5 improved most in locomotion ability, Rk1-fed only significantly improved the normal locomotion on Days 10 and 12. Whereas both Rg6 and F4 showed a trend of enhancing normal locomotion ability on Days 10–16 of adulthood, but no significant differences were observed compared to the control (Fig. 3C).
Fig. 3.
Comparison of improvement effects of ginsenoside derivatives on aging-related biomarkers inC. elegans. (A). The pharyngeal pumping rate of wild-type N2 worms grown onginsenosidederivatives was increased than that of the control on Days 8–16 (N = 20 worms,p< 0.05). (B). The locomotion ability of wild-type N2 worms grown on the control andginsenosidederivatives was measured on Days 8–16. According to their locomotion, the worms were divided into 4 classes: Class A, normal locomotion, spontaneous and/or rhythmical sinusoidal movement; Class B, cannot move body, irregular and/or uncoordinated movement; Class C, uncoordinated/sluggish, moved merely their head and/or tail in response to a moderate touch with a platinum wire picker; and Class D, dead (N ≥ 90 worms,p< 0.05). (C) On Days 10–16,ginsenosidederivative Rg5 feeding significantly increased the normal locomotion ability of the worms than those of Rg6, Rk1, or F4 (N = 3 biological replicates,p< 0.05). (D) Quantification of the mRNA levels of muscle function related genes inC. elegans(N = 6 biological replicates,p< 0.05). (E, F). The lipofuscin accumulation in wild-type N2 worms decreased followingginsenosidederivatives feeding. Lipofuscin accumulation was detected by autofluorescence under FV1200 confocal microscope on Day 15 in worms; scale bar, 20 μm (E), and fluorescence intensity was measured using ImageJ software (F). The results are shown in arbitrary units (a.u.) (N = 20 worms,p< 0.05). In A, C, D, and F, the values are presented as the mean ± SEM.
Furthermore, to confirm the effect of the 4 ginsenoside derivatives on the muscle function of C. elegans, we examined the mRNA expression levels of motor-related genes, tni-1, ubc-25, wdr-20, pha-4, egl-20, and slo-2, which are involved in the regulation of muscle contraction and are crucial for the coordinated locomotion behavior of nematodes [[47], [48], [49]]. Rg5 significantly increased the mRNA expression levels of tni-1, ubc-25, wdr-20, pha-4, and egl-20, compared with the control (Fig. 3D), consistent with the results of the pharyngeal pumping rate and normal locomotion ability (Fig. 3A–C). Furthermore, the mRNA expression levels of pha-4 and egl-20, was significantly increased in Rk1 than that of the control, but no significant difference was observed between the control and Rg6 (Fig. 3D). Interestingly, the mRNA expression levels of pha-4 and egl-20 and slo-2, were significantly increased in F4, compared with the control (Fig. 3D), suggests that the observed locomotoion enhancement results from coordinated polygenic regulation rather than single-gene effects.
In addition, we found that the accumulation of lipofuscin, a lipid peroxidation product that accumulates in aging cells, was significantly reduced in worms fed with 4 ginsenoside derivatives than that of control on Day 15 of adulthood (Fig. 3E and F). Moreover, ginsenoside derivative Rg5 feeding significantly reduced lipofuscin accumulation in worms better than Rg6 or F4 feeding, but these was no difference between Rg5 and Rk1 (Fig. 3F). These findings indicated that, compared with ginsenoside derivatives Rg6 and F4, Rg5 and Rk1 support the maintenance of higher muscle mass and enhance physical function in worms, and those effects can be attributed to the two glucose residues of Rg5 and Rk1, which may contribute to decreasing the aging rate of C. elegans.
3.3. Comparison of effects of ginsenoside derivatives stress on resistance in C. elegans
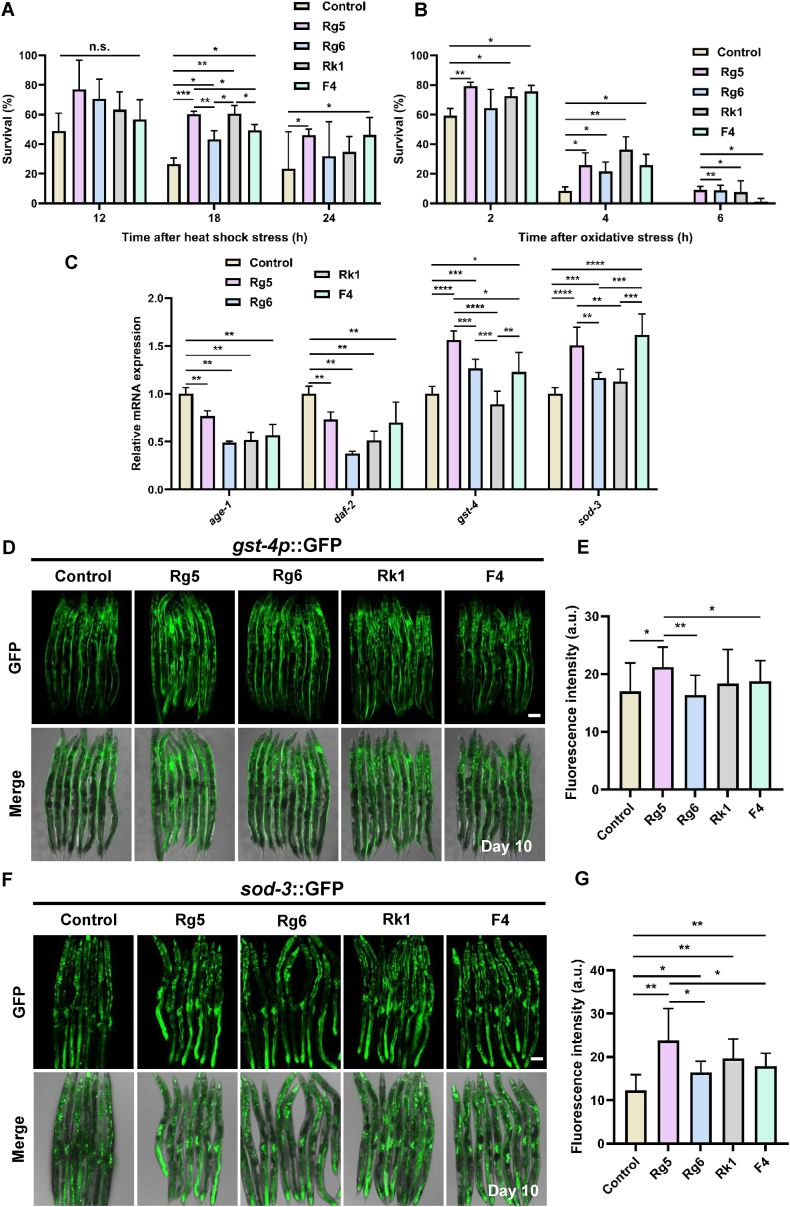
Longevity is closely linked to the ability to survive under conditions of physical or chemical stresses [35]. We investigated the effects of ginsenosides on resistance to physical stress (heat) and chemical stress (H2O2) in C. elegans. Worms fed with 4 ginsenoside derivatives exhibited higher survival rates, than that of the control, following 12, 18, or 24 h of incubation at 34 °C (Fig. 4A). Compared with the control, ginsenoside derivatives Rg5 and F4 significantly increased the survival of worms treated at 34 °C for 18 and 24 h, Rg6 and Rk1 feeding increased survival only at 18 h (Fig. 4A). Moreover, ginsenoside derivatives Rg5 and Rk1 increased the survival of worms treated at 34 °C for 18 h better than Rg6 and F4 (Fig. 4A). In addition, after 10 mM H2O2-induced oxidative stress, ginsenoside derivative Rg5 treatment significantly increased the survival rate of nematodes and maintained activity for 6 h, while Rk1 and F4 increased survival only for 2 and 4 h, and Rg6 increased survival for 4 and 6 h, meanwhile, Rg5 and Rk1 increased the survival of worms for 4 h better than Rg6 and F4 (Fig. 4B).
Fig. 4.
Comparison of effects of ginsenoside derivatives stress on resistance inC. elegans. (A, B). Under heat stress (A) and H2O2-induced stress (B), wild-type N2 worms grown on ginsenoside derivatives increased the survival rate compared with the control (N = 3 biological replicates,p< 0.05). (C). Quantification of the mRNA levels of stress-related genes inC. elegans(N = 6 biological replicates,p< 0.05). (D, F). The stress response reportersgst-4p::GFP (D) andsod-3::GFP (F) were used to measure GST-4 and SOD-3, respectively. (E, G). The fluorescence intensity of GFP was quantified using ImageJ software. Thegst-4p::GFP (E) andsod-3::GFP (G) worms grown on ginsenoside derivative Rg5 showed increased fluorescence intensity (N = 20 worms,p< 0.05). In D and F, scale bar, 20 μm. In E and G, the results are shown in arbitrary units (a.u.). In A-C, E and G, the values are presented as the mean ± SEM.
To uncover the anti-stress mechanisms of ginsenoside derivatives in C. elegans, we examined the insulin/insulin-like growth factor 1 (IGF-1) signaling (IIS) pathway, which plays a crucial role in regulating aging and stress responses [50]. We found that the mRNA expression levels of daf-2 and age-1, two key upstream components of IIS, were significantly down-regulated in nematodes fed with ginsenoside derivatives Rg5, Rg6, Rk1, and F4, compared with the control (Fig. 4C), revealing that the anti-stress effects of ginsenoside derivatives in C. elegans. Therefore, we next examined the role of other oxidative stress-induced genes related to the IIS pathway in ginsenoside derivatives-mediated resistance mechanism. The gene expression level of gst-4, a coding factor regulating the enzyme activity of GSH-px, was significantly up-regulated in ginsenoside derivatives Rg5, Rg6 and F4 feeding compared with the control (Fig. 4C). Superoxide dismutase 3 (SOD-3) encodes the protein that regulates superoxide dismutase (SOD) enzyme activity, and was also significantly increased in ginsenoside derivatives Rg5, Rg6, and F4 feeding than in the control (Fig. 4C).
To further confirm whether 4 ginsenoside derivatives feeding increased the expression of gst-4 and sod-3, we investigated two GFP reporters gst-4p::GFP and sod-3::GFP to reflect the stress resistance of GST-4 and SOD-3, respectively. Results showed that Rg5 feeding significantly increased the fluorescence intensity of gst-4p::GFP in C. elegans compared with the control (Fig. 4D and E). The fluorescence intensity of ginsenoside derivative Rg5-fed was significantly increased than those of Rg6 and F4 (Fig. 4D and E). Similarly, compared with the control, the fluorescence intensity of sod-3::GFP of worms fed with ginsenoside derivative Rg5 was significantly increased, exhibiting a more pronounced effect than those of Rg6 or F4, but there was no significant difference between Rg5 and Rk1 (Fig. 4F and G). Taken together, these results indicated that ginsenoside derivative Rg5 with two glucose and double bond position at Δ20(22) increased resistances to heat stress and oxidative stress better than Rk1 with the same glycosides but double bond position at Δ20(21), whereas, Rg6 with glucose-(2-1)-rhamnose and double bond position at Δ20(21) showed the least effect on markers of stress, possibly due to the different glycosides and double bond position of ginsenoside derivatives.
3.4. Comparison of reducing effect of ginsenoside derivatives on AChE activity
AChE is implicated in stress responses, locomotion ability, and aging [10]. Therefore, we examined the effect of 4 ginsenoside derivatives on AChE activity in C. elegans. Compared with the control, ginsenoside derivatives Rg5 feeding reduced AChE activity most than Rk1 or F4 feeding, but no difference was found between Rg6 and control (Fig. 5A). Furthermore, to confirm the structure-activity relationship between the ginsenosides and AChE, ginsenoside derivatives Rg5, Rg6, Rk1, and F4 were subjected to flexible docking analysis against human AChE. The results revealed that the bond energies of ginsenoside derivatives Rg5, Rg6, Rk1, and F4 were approximately −10 kcal/mol (Table S3), which indicates the stability of the complex and the inhibition of AChE activity by the 4 ginsenoside derivatives were better. Galantamine, donepezil, Rg5, Rg6, Rk1, and F4 interact with AChE by forming 3, 3, 4, 1, 4, and 0 hydrogen bonds as shown in Table S3. Meanwhile, the binding poses of ginsenoside derivatives Rg5 and Rk1 were comparable to those of the co-crystalized ligands galantamine and donepezil, sharing more hydrogen bonds and hydrophobic interactions, such as TYR-124, TRP-86, TRP-286, TYR-337, PHE-338, and SER-293 (Fig. 5B–D and F, Fig. S3A-C and E). Especially, Rg5 and galantamine interacted with TYR-337 residue by hydrophobic interaction; Rg5 and donepezil interacted with SER-293 residue by hydrogen bond, with PHE-338, TRP-86, and TYR-337 residues by hydrophobic interactions (Fig. 5B–D, Fig. S3A–C). Rg6 and donepezil interacted with PHE-338 and TRP-286 residues by hydrophobic interactions (Fig. 5C and E, Fig. S3B and D). Additionally, Rk1 and galantamine interacted with TYR-124 residue by hydrogen bond, with TYR-337 residue by hydrophobic interaction; Rk1 and donepezil interacted with PHE-338 and TYR-337 residues by hydrophobic interactions (Fig. 5B, C and F, Fig. S3A, B and E). However, F4 and donepezil only interacted with TRP-286 residue by hydrophobic interaction (Fig. 5C and G, Fig. S3B and F). In summary, ginsenoside derivatives Rg5 and Rk1 significantly reduced AChE activity, and the interactions (hydrogen bonds and hydrophobic interactions) and binding energies with co-crystalized ligands galantamine and donepezil were stronger than Rg6 and F4, which may be related to their glycosides linking two glucose residues, meanwhile, Rg5 with double bond at Δ20(22) has better effect than Rk1 with double bond at Δ20(21).
Fig. 5.
Comparison of reducing effect of ginsenoside derivatives on AChE activity. (A). Compared with the control, the AChE activity in the wild-type N2 worms was inhibitedfollowing ginsenosidederivatives feeding (N = 3 biological replicates,p< 0.05). (B–G). Binding modes of the top-scoring co-crystalized ligands andginsenosidederivatives inside the active site of AChE:galantamine (B), donepezil (C), Rg5 (D), Rg6 (E), Rk1 (F), and F4 (G).
4. Discussion
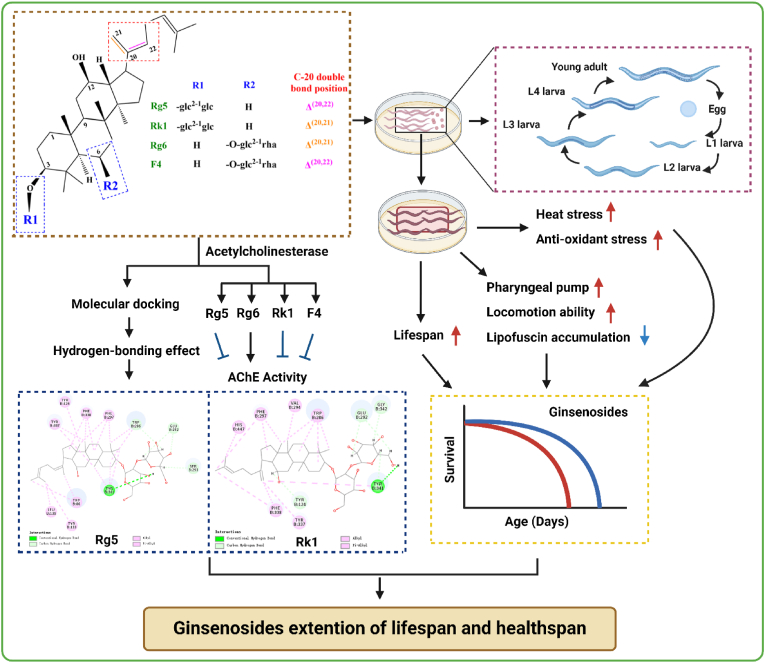
This study demonstrated that feeding ginsenoside derivatives Rg5, Rg6, Rk1, and F4 with same molecular formula, different glycosides and double bond position, have anti-aging effects on the model organism C. elegans. Ginsenoside derivatives Rg5 and Rk1 with two glucose residues had better anti-aging bioactivities by improving muscle function, enhancing anti-oxidant stress, and reducing AChE activity than those of Rg6 and F4 with one glucose residue and one rhamnose residue; furthermore, Rg5 and F4 with double bond at Δ20(22) exhibited better those effects than Rk1 and Rg6 with double bond at Δ20(21), respectively (Fig. 6). These findings show that structural differences of glycoside types and double bond position of ginsenosides lead to significant variations in their anti-aging activities, highlighting the importance of specific structural elements in determining the efficacy of compounds.
Fig. 6.
Schematic representation of ginsenoside derivatives-AChE-Aging. Ginsenosidederivativesfeedingmaydelay agingby improving muscle function, enhancing anti-oxidant stress, and reducing AChE activity inC. elegans. Meanwhile, molecular docking analysis also showed that ginsenoside derivatives Rg5 and Rk1 shared hydrogen bonds and hydrophobic interactions with amino acid residues at the AChE active site, and the binding energy of Rg5 was better than Rk1.
Aging is characterized by the progressive decline of multiple physiological functions within an organism [51], and is closely associated with diminished muscle function and increased lipofuscin accumulation [52]. Our results revealed that 4 ginsenoside derivatives Rg5, Rg6, Rk1, and F4 enhanced the pharyngeal pumping rate, normal locomotion ability and motor-related proteins, and reduced lipofuscin accumulation in older worms (Days 10–16). This finding is similar to previous report that total ginsenosides increased locomotion ability and decreased lipofuscin accumulation in middle (Day 8) and older (Day 12) worms [22].
Moreover, the hallmarks of aging may also be exacerbated by physical and chemical stresses [34,53,54]. Our data suggested that, after heat stress and H2O2-indeced oxidative stress, 4 ginsenoside derivatives treatment increased the survival rate of C. elegans. Consistent with our findings, Yu et al. showed that total ginsenosides extended the lifespan of worms by enhancing resistance to heat stress and paraquat-indeced oxidative stress [55]. Additionally, we also found that 4 ginsenoside derivatives increased the mRNA expression levels of gst-4 and sod-3, as well as the fluorescence intensity of gst-4p::GFP and sod-3::GFP in C. elegans. Consistent with our results, Wang et al. verified that total ginsenosides up-regulated the mRNA expression levels of gst-4 and sod-3 and increased the fluorescence intensity of gst-4p::GFP and sod-3::GFP in C. elegans [22].
Our data indicated that, compared to two glucose residues ginsenoside derivatives Rg5 and Rk1 (PPD-type), Rg6 and F4 (PPT-type) with glucose-(2-1)-rhamnose residues had a lower ability to enhance muscle function and anti-oxidant stress. Similar to our findings, Ying et al. showed that two pairs of geometric isomers, Rg5/Rk1 (PPD-type) and Rk3/Rh4 (PPT-type), both contribute to restoring erectile function, but Rg5 is more effective because it inhibits the phenotypic transformation of corpus cavernosum smooth muscle cells in hypoxic environments [25], suggesting that the glycosides linking two glucose residues seem to enhance their bioactivities [56].
Our results also revealed that ginsenoside derivative Rg5 with two glucose residues and double bond position at Δ20(22), improved muscle function and enhanced the resistance to oxidative stress better than Rk1, with same glycosides but double bond position at Δ20(21); and ginsenoside derivative F4 with glucose-(2-1)-rhamnose residues and double bond position at Δ20(22), improved muscle function and enhanced the resistance to oxidative stress better than Rg6, with same glycosides but double bond position at Δ20(21). Consistent with our data, Ma et al. indicated that ginsenosides with double bond position at Δ20(22) had better pharmacokinetic characteristics than those with the same glycosylated double bond position at Δ20(21)-ginsenosides [21]. Similarly, Shen et al. showed that ginsenoside isomers with the same glycosides but F4 double bond position at Δ20(22), and Rg6 double bond position at Δ20(21), might lead to a faster metabolic rate of F4 than Rg6 in zebrafish [20]. In view of the above data, it can be concluded that ginsenoside derivatives Rg5 and Rk1, which are linked to two glucose residues, and their anti-aging bioactivities by improving muscle function, enhancing anti-oxidant stress, and reducing AChE activity are superior to those of ginsenoside derivatives Rg6 and F4 with glucose-(2-1)-rhamnose residues, meanwhile, Rg5 and F4 with double bond at Δ20(22) have better effects on those markers than Rk1 and Rg6 with double bond at Δ20(21), respectively.
In addition, we found that the AChE activity of the worms fed with ginsenoside derivative Rg5 was significantly lower than those of the Rk1 or F4, while there was no difference between the Rg6 and control. Consistent with the findings of previous, Rg5 (5, 10 or 20 mg/kg) might enhance the learning and memory ability and decrease the AChE activity in streptozotocin-induced learning and memory impairments in rats [57]. Similarly, An et al. revealed that ginsenoside isomers Rg5 and Rk1 ameliorated memory impairment by inhibiting AChE activity leading to increased acetylcholine level in the brain of mice induced by scopolamine [58]. Additionally, molecular docking analysis showed that ginsenoside isomers Rg5 and Rk1 shared hydrogen bonds and hydrophobic interactions with amino acid residues at the AChE active site, and the binding energy and interaction of Rg5 were better than that of Rk1, which was consistent the results of the decreased of AChE activity in worms, possibly due to the ginsenoside Rg5 glycoside linking two glucose residues and the double bond at Δ20(22). However, such inference needs further experimental verification. We will be interested in verifying in an aging mice model, and combining multi-omics analyses to demonstrate the molecular mechanisms by which ginsenosides health-promoting and longevity extension. Although challenging, future studies should focus on the effects of structure-activity relationships of ginsenosides on the anti-aging bioactivities, providing essential information for the clinical application of ginsenosides in delaying aging and aging-related diseases.
CRediT authorship contribution statement
Juntao Zhang: Writing – Original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Weimin Wang: Molecular docking analysis, Investigation, Visualization, Validation, Supervision. Daidi Fan: Materials. Jianjun Deng: Writing & Editing, Resources. Haixia Yang: Writing & Editing, Supervision, Funding acquisition, Conceptualization, All authors have checked and agreed to the final version of manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this paper.
Acknowledgments
This research is supported by National Natural Science Foundation of China (32422069) and National Key Research and Development Program (2022YFF1100205).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2025.05.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Dogra S., Dunstan D.W., Sugiyama T., Stathi A., Gardiner P.A., Owen N. Active aging and public health: evidence, implications, and opportunities. Annu Rev Publ Health. 2022;43(1):439–459. doi: 10.1146/annurev-publhealth-052620-091107. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Khan S.S., Singer B.D., Vaughan D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16(4):624–633. doi: 10.1111/acel.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh T., Newman A.B. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polsky L.R., Rentscher K.E., Carroll J.E. Stress-induced biological aging: a review and guide for research priorities. Brain Behav Immun. 2022;104:97–109. doi: 10.1016/j.bbi.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole S.W. The conserved transcriptional response to adversity. Curr Opin Behav Sci. 2019;28:31–37. doi: 10.1016/j.cobeha.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schliebs R., Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221(2):555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Liu Y., Guo Y., Xu L., Wang H. Phlorizin exerts potent effects against aging induced by D-galactose in mice and PC12 cells. Food Funct. 2021;12(5):2148–2160. doi: 10.1039/d0fo02707c. [DOI] [PubMed] [Google Scholar]
- 9.Sha A., Liu Y., Qiu X., Xiong B. Polysaccharide from Paris polyphylla improves learning and memory ability in D-galactose-induced aging model mice based on antioxidation, p19/p53/p21, and Wnt/β-catenin signaling pathways. Int J Biol Macromol. 2023;251:126311–126322. doi: 10.1016/j.ijbiomac.2023.126311. [DOI] [PubMed] [Google Scholar]
- 10.Sklan E.H., Berson A., Birikh K.R., Gutnick A., Shahar O., Shoham S., Soreq H. Acetylcholinesterase modulates stress-induced motor responses through catalytic and noncatalytic properties. Biol Psychiatry. 2006;60(7):741–751. doi: 10.1016/j.biopsych.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 11.Sun C., Yin Z., Chen J., Wang W., Zheng G., Li J., Chen L., Zhang Q. Dihydromyricetin improves cognitive impairments in D-galactose‐induced aging mice through regulating oxidative stress and inhibition of acetylcholinesterase. Mol Nutr Food Res. 2021;66(4):e2101002–e2101010. doi: 10.1002/mnfr.202101002. [DOI] [PubMed] [Google Scholar]
- 12.Giacobini E., Cuello A.C., Fisher A. Reimagining cholinergic therapy for Alzheimer's disease. Brain. 2022;145(7):2250–2275. doi: 10.1093/brain/awac096. [DOI] [PubMed] [Google Scholar]
- 13.Wang K., Liu H., Hu Q., Wang L., Liu J., Zheng Z., Zhang W., Ren J., Zhu F., Liu G. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct Targeted Ther. 2022;7(1):374–395. doi: 10.1038/s41392-022-01211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song L., Zhang S. Anti-aging activity and modes of action of compounds from natural food sources. Biomolecules. 2023;13(11):1600–1655. doi: 10.3390/biom13111600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo C., Huang Q., Wang Y., Yao Y., Li J., Chen J., Wu M., Zhang Z., E M., Qi H., et al. Therapeutic application of natural products: NAD+ metabolism as potential target. Phytomedicine. 2023;114:154768–154796. doi: 10.1016/j.phymed.2023.154768. [DOI] [PubMed] [Google Scholar]
- 16.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9(1):23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 17.Baek S.H., Bae O.N., Park J.H. Recent methodology in ginseng analysis. J Ginseng Res. 2012;36(2):119–134. doi: 10.5142/jgr.2012.36.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali M.Y., Zaib S., Jannat S., Khan I. Inhibition of angiotensin-I converting enzyme by ginsenosides: structure-activity relationships and inhibitory mechanism. J Agric Food Chem. 2021;69(21):6073–6086. doi: 10.1021/acs.jafc.1c01231. [DOI] [PubMed] [Google Scholar]
- 19.Hu C., Lau A.J., Wang R., Chang T.K.H. Comparative analysis of ginsenosides in human glucocorticoid receptor binding, transactivation, and transrepression. Eur J Pharmacol. 2017;815:501–511. doi: 10.1016/j.ejphar.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Shen W., Zhang H., Qiu S., Wei Y., Zhu F., Wang J., Wang D., Jia X., Tang D., Chen B. Biotransformation of ginsenosides F4 and Rg6 in zebrafish. J Asian Nat Prod Res. 2017;20(7):686–696. doi: 10.1080/10286020.2017.1307184. [DOI] [PubMed] [Google Scholar]
- 21.Ma C., Lin Q., Xue Y., Ju Z., Deng G., Liu W., Sun Y., Guan H., Cheng X., Wang C. Pharmacokinetic studies of ginsenosides Rk1 and Rg5 in rats by UFLC-MS/MS. Biomed Chromatogr. 2021;35(8):e5108–e5116. doi: 10.1002/bmc.5108. [DOI] [PubMed] [Google Scholar]
- 22.Wang H., Zhang S., Zhai L., Sun L., Zhao D., Wang Z., Li X. Ginsenoside extract from ginseng extends lifespan and health span in Caenorhabditis elegans. Food Funct. 2021;12(15):6793–6808. doi: 10.1039/d1fo00576f. [DOI] [PubMed] [Google Scholar]
- 23.Kim J.M., Park C.H., Park S.K., Seung T.W., Kang J.Y., Ha J.S., Lee D.S., Lee U., Kim D.O., Heo H.J. Ginsenoside Re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet-induced C57BL/6 mice. J Agric Food Chem. 2017;5(13):2719–2729. doi: 10.1021/acs.jafc.7b00297. [DOI] [PubMed] [Google Scholar]
- 24.Feng R., Liu J., Wang Z., Zhang J., Cates C., Rousselle T., Meng Q., Li J. The structure-activity relationship of ginsenosides on hypoxia-reoxygenation induced apoptosis of cardiomyocytes. Biochem Biophys Res Commun. 2017;494(3–4):556–568. doi: 10.1016/j.bbrc.2017.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying A., Yu Q., Guo L., Zhang W., Liu J., Li Y., Song H., Li P., Qi L., Ge Y., et al. Structural-activity relationship of ginsenosides from steamed ginseng in the treatment of erectile dysfunction. Am J Chin Med. 2018;46(1):137–155. doi: 10.1142/S0192415X18500088. [DOI] [PubMed] [Google Scholar]
- 26.Ye X., Zhang H., Li Q., Ren H., Xu X., Li X. Structural-activity relationship of rare ginsenosides from Red Ginseng in the treatment of Alzheimer's disease. Int J Mol Sci. 2023;24(10):8625. doi: 10.3390/ijms24108625. 8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finch C.E., Ruvkun G. The genetics of aging. Annu Rev Genom Hum Genet. 2001;2(1):435. doi: 10.1146/annurev.genom.2.1.435. 435. [DOI] [PubMed] [Google Scholar]
- 28.Heintz C., Mair W. You are what you host: microbiome modulation of the aging process. Cell. 2014;156(3):408–411. doi: 10.1016/j.cell.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irazoqui J.E., Urbach J.M., Ausubel F.M. Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol. 2010;10(1):47–58. doi: 10.1038/nri2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gusarov I., Gautier L., Smolentseva O., Shamovsky I., Eremina S., Mironov A., Nudler E. Bacterial nitric oxide extends the lifespan of C. elegans. Cell. 2013;152(4):818–830. doi: 10.1016/j.cell.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sulston J.E., Brenner S. The DNA of C. elegans. Genetics. 1974;77:95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han B., Sivaramakrishnan P., Lin C., Neve I., He J., Li W., Sowa J.N., Sizovs A., Du G., Wang J. Microbial genetic composition tunes host longevity. Cell. 2017;169(7):1249–1262.e13. doi: 10.1016/j.cell.2017.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donato V., Ayala F.R., Cogliati S., Bauman C., Costa J.G., Leñini C., Grau R. Bacillus subtilis biofilm extends Caenorhabditis elegans longevity through downregulation of the insulin-like signalling pathway. Nat Commun. 2017;8:14332–14346. doi: 10.1038/ncomms14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J., Zhao Y., Sun Z., Sun T. Lacticaseibacillus rhamnosus Probio-M9 extends the lifespan of Caenorhabditis elegans. Commun Biol. 2022;5(1):1139–1154. doi: 10.1038/s42003-022-04031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keith S.A., Amrit F., Ratnappan R., Ghazi A. The C. elegans healthspan and stress-resistance assay toolkit. Methods. 2014;68(3):476–486. doi: 10.1016/j.ymeth.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Fan S., Yan Y., Xia Y., Zhou Z., Luo L., Zhu M., Han Y., Yao D., Zhang L., Fang M., et al. Pregnane X receptor agonist nomilin extends lifespan and healthspan in preclinical models through detoxification functions. Nat Commun. 2023;14(1):3368–3390. doi: 10.1038/s41467-023-39118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosono R., Sato Y., Aizawa S.I., Mitsui Y. Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegan. Exp Gerontol. 1980;15(4):285–289. doi: 10.1016/0531-5565(80)90032-7. [DOI] [PubMed] [Google Scholar]
- 39.Lee J., Kwon G.L.Y. Elucidating the mechanism of Weissella-dependent lifespan extension in Caenorhabditis elegans. Sci Rep. 2015;5:17128–17141. doi: 10.1038/srep17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chun L., Gong J., Yuan F., Zhang B., Liu H., Zheng T., Yu T., Xu X.Z.S., Liu J. Metabotropic GABA signalling modulates longevity in C. elegans. Nat Commun. 2015;6(1):8828–8837. doi: 10.1038/ncomms9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung J., Rudolph M.J., Burshteyn F., Cassidy M.S., Gary E.N., Love J., Franklin M.C., Height J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem. 2012;55:10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- 42.Seeliger D., Groot B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des. 2010;24(5):417–422. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whittle M., Willett P., Klaffke W., Noort P. Evaluation of similarity measures for searching the dictionary of natural products database. J Chem Inf Comput Sci. 2003;43:449–457. doi: 10.1021/ci025591m. [DOI] [PubMed] [Google Scholar]
- 44.DeLano W.L. Pymol: an open-source molecular graphics tool. CCP4 Newsletter on protein crystallography. 2002;40:82–92. [Google Scholar]
- 45.Jasienska G. Reproduction and lifespan: trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. Am J Hum Biol. 2009;21(4):524–532. doi: 10.1002/ajhb.20931. [DOI] [PubMed] [Google Scholar]
- 46.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Félix A.A., Williams E.G., Jha P., Lo Sasso G., Huzard D., et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22(8):879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 47.Schulze E., Altmann M.E., Adham I.M., Schulze B., Fröde S., Engel W. The maintenance of neuromuscular function requires UBC-25 in Caenorhabditis elegans. Biochem Biophys Res Commun. 2003;305(3):691–699. doi: 10.1016/s0006-291x(03)00824-6. [DOI] [PubMed] [Google Scholar]
- 48.Barnes D.E., Hwang H., Ono K., Lu H., Ono S. Molecular evolution of troponin I and a role of its N-terminal extension in nematode locomotion. Cytoskeleton. 2016;73(3):117–130. doi: 10.1002/cm.21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahlberg C.L., Juo P. The WD40-repeat proteins WDR-20 and WDR-48 bind and activate the deubiquitinating enzyme USP-46 to promote the abundance of the glutamate receptor GLR-1 in the ventral nerve cord of Caenorhabditis elegans. J Biol Chem. 2014;289:3444–3456. doi: 10.1074/jbc.M113.507541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altintas O., Park S., Lee S., Jae V. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016;49(2):81–92. doi: 10.5483/BMBRep.2016.49.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ham S., Lee S.J.V. Advances in transcriptome analysis of human brain aging. Exp Mol Med. 2020;52(11):1787–1797. doi: 10.1038/s12276-020-00522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pincus Z., Slack F.J. Developmental biomarkers of aging in Caenorhabditis elegans. Dev Dyn. 2010;239(5):1306–1314. doi: 10.1002/dvdy.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westerheide S.D., Anckar J., Stevens S.M., Sistonen L., Morimoto R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morley J.F., Morimoto R.I. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu X., Li H., Lin D., Guo W., Xu Z., Wang L., Guan S. Ginsenoside prolongs the lifespan of C. elegans via lipid metabolism and activating the stress response signaling pathway. Int J Mol Sci. 2021;22(18):9668–9685. doi: 10.3390/ijms22189668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo B., Jiang J., Fang Y., Yang F., Yin M., Zhang B., Zhao R., Shao J. The effects of ginsenosides on platelet aggregation and vascular intima in the treatment of cardiovascular diseases: from molecular mechanisms to clinical applications. Pharmacol Res. 2020;159:105031–105086. doi: 10.1016/j.phrs.2020.105031. [DOI] [PubMed] [Google Scholar]
- 57.Chu S., Gu J., Feng L., Liu J., Zhang M., Jia X., Liu M., Yao D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int Immunopharmacol. 2014;19(2):317–326. doi: 10.1016/j.intimp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 58.An K.S., Choi Y.O., Lee S.M., Ryu H.Y., Kang S.J., Yeon Y., Kim Y.R., Lee J.G., Kim C.J., Lee Y.J., et al. Ginsenosides Rg5 and Rk1 enriched cultured wild ginseng root extract bioconversion of Pediococcus pentosaceus HLJG0702: effect on scopolamine-induced memory dysfunction in mice. Nutrients. 2019;11(5):1120–1134. doi: 10.3390/nu11051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.